Abstract
BACKGROUND
Identification of risk factors for BKV replication may improve transplant outcome. We investigated the impact of immunosuppressive drugs on the prevalence of BKV replication in recipients of human renal allografts.
METHODS
One hundred twenty renal allograft recipients were studied prospectively at one, three, and six months post-transplantation to identify risk factors for BKV replication. BKV replication was quantified by measurement of urinary cell BKV VP1 mRNA levels using BKV specific primers and TaqMan® probe in a real-time quantitative PCR assay. Levels of urinary cell mRNA for granzyme B, CD103 and TGF-β1 were measured to ascertain whether BKV replication is associated with an inflammatory signature.
RESULTS
The prevalence of BKV replication increased over time and was highest at six-months compared to 1 or 3 months post-transplantation (P<0.001). A logistic regression model analysis demonstrated that steroid maintenance therapy (odds ratio: 8.3, P= 0.003) and induction with rabbit anti-human thymocyte globulin (ATG) (odds ratio: 5.8, P= 0.008) were independent risk factors for BKV replication. Neither mycophenolate mofetil dose nor tacrolimus dose or trough levels were different between those with or without BKV replication. The development of acute rejection or anti-rejection treatment with methylprednisolone did not increase the risk of BKV replication. BKV replication was associated with heightened levels of urinary cell mRNA for granzyme B (P<0.002), CD103 (P<0.005) but not for TGF-β1 (P>0.05).
CONCLUSIONS
Steroid maintenance therapy and induction with ATG are independent risk factors for BKV replication in renal allograft recipients treated with tacrolimus and mycophenolate mofetil.
INTRODUCTION
BK virus (BKV) is a member of the human polyomavirus family (1) and infection with the virus is common and a majority of adult population is seropositive for the virus (2). BKV resides dormant in uroepithelial cells and is not known to cause tissue damage in immunocompetant individuals (3). The virus, however, can become reactivated in the setting of immunodeficiency (e.g., secondary to HIV infection or immunosuppressive medications), and result in cellular damage and organ dysfunction (4-6). Clinical manifestations of active BKV disease include hemorrhagic cystitis and nephritis, with or without renal allograft dysfunction. BKV nephritis is an emerging cause of renal allograft failure and the rate of renal allograft loss in the setting of BKV nephritis varies from 10-80% (7-9).
A major unresolved puzzle is the reason for the recent emergence of BKV as a clinically significant pathogen. Newer immunosuppressive drugs (e.g., calcineurin inhibitors, mycophenolate mofetil) and anti-rejection therapy with methylprednisolone pulse therapy have been implicated in BKV replication and nephropathy (8-11). However, the contribution of heightened immunosuppression including the role of corticosteroids to BKV reactivation has not been fully resolved (5, 12).
Moens et al have investigated the effect of steroid hormones on BKV infection in vitro and reported that physiologic concentrations of dexamethasone increased BKV (Gardner) infectivity of permissive Vero cells (ATCC CCL81) and enhanced viral transcript levels; furthermore a glucocorticoid and/or progesterone response element (GRE/PRE) was identified in the late leader sequence of the BKV genome (13).
In the current investigation, we examined the risks conferred by immunosuppressive therapy including steroid maintenance therapy on BKV replication. We used BKV VP1 mRNA real time quantitative PCR assay developed in our laboratory (14) to quantify BKV replication in renal allograft recipients induced with or without rabbit anti-thymocyte globulin and treated with or without steroid maintenance therapy. We have ascertained the prevalence of BKV replication in sequential urine specimens, and examined as well whether BKV replication is associated with an increased expression of urinary cell mRNAs for granzyme B, CD103 and TGF- β1. We have reported that renal allograft status can be predicted noninvasively by measurement of urinary cell levels of mRNA for granzyme B and CD103 (15, 16).
METHODS
Study Cohort
This is a prospective single center study of 120 renal allograft recipients who received their renal transplants between July 2001 and April 2003, and who were enrolled in our Weill Cornell IRB approved protocol entitled “Use of PCR to Evaluate Renal Allograft Status.” The median age of the 120 renal allograft recipients was 48.5 years (range: 22 to 77). There were 58 females (48.3%) and 62 males (51.7%). Thirty-three (27.5%) of the 120 recipients were African-Americans. Forty-six patients (38.3%) received deceased donor kidneys and 74 patients (61.7%) received living donor kidneys.
Immunosuppressive Regimens
The study participants were treated with either a steroid maintenance protocol (N=71 subjects) or an early steroid withdrawal protocol (N=49 subjects). The initial steroid dosage in either group was similar, and both groups received 500 mg IV methylprednisolone (MP) on day 1, 250 mg IV on day 2, 125mg IV on day 3, 60 mg IV on days 4 and 5 post-transplantation. The patients managed with steroid maintenance protocol received 20 mg prednisone by oral route on days 5-14 and the prednisone maintenance dose was reduced to 5 mg per day by 3-months post-transplantation. Corticosteroids were stopped on day 5 in the early steroid withdrawal cohort.
Among the 71 recipients treated with the steroid maintenance protocol, 15 received basiliximab as induction therapy, 15 received a five day course of rabbit anti-human thymocyte globulin (Thymoglobulin®, 1.5 mg per kilogram of bodyweight per day for five days, full dose ATG), and 19 received a single dose (1.5mg/kg/day) of ATG. Among the 49 recipients treated with the early steroid withdrawal protocol, 4 received basiliximab induction, and the remaining 45 received full dose ATG induction therapy. Among the entire study cohort of 120 recipients, all but 5 recipients (97%) received tacrolimus and all but 8 recipients (93%) were maintained on mycophenolate mofetil (MMF).
Sixteen (13.3%) of the 120 recipients received anti-rejection treatment for clinical acute rejection during the first six months post-transplantation, and 15 of the 16 clinical acute rejections were biopsy confirmed. All 16 recipients received methylprednisolone pulse therapy (250 mg IV MP, twice a day for three consecutive days), as their initial anti-rejection therapy, and 4 received additional therapy with ATG, and one patient received IVIG / plasmapheresis / anti-CD20 monoclonal antibody (Rituximab®).
Collection of Urine Samples and Measurement of mRNA Levels
We collected urine specimens from 120 renal-allograft recipients at 1, 3, and 6 months post-transplantation, and a total of 360 urine specimens were collected for measurement of urinary cell levels of BKV VP1 mRNA and transcripts for granzyme B, CD103, TGF-β1 and 18s rRNA. Total RNA was extracted from the urine cell pellet using RNeasy Minikit® (Qiagen) and reverse transcribed to cDNA (14).
BKV replication was quantified using real-time quantitative PCR assay using BKV specific primers and TaqMan® probe designed, developed and validated in our laboratory (14). In the current study, a subject was classified as BKV replication positive if the urinary cell BKV VP1 copy number was greater than >1 BKV VP1 mRNA copy / one picogram (pg) of total RNA.
Urinary cell mRNA levels of granzyme B, CD103, and TGF-β1 were measured using a pre-amplification enhanced real-time quantitative PCR assay developed in our laboratory (17). We measured urinary cell levels of 18S rRNA as the reference gene and transcript levels were measured using an ABI Prism 7700® system and using the standard curve method, as previously described (14).
Statistical Analysis
Risk factors for BKV replication and the associated inflammatory signature were analyzed using the statistical software packages, STATA® version 9.2 and GraphPad Prism® version 4.0 for Windows. Categorical variables were compared using the chi-square test and continuous variables using the nonparametric Mann-Whitney test. Variables thought to be risk factors for BKV replication were entered into a multivariate logistic regression model and a stepwise backward elimination technique was used to identify independent risk factors for BKV replication. Since only 37 of the 120 recipients reached the primary endpoint (positive for BKV replication) at 6 months post-transplantation, we limited the number of variables and entered only six variables related to immunosuppressive exposure into the logistic regression model. Since the number of recipients with acute rejection was small (N=16), a Fisher’s exact test with Yates correction was used to analyze the contingency table consisting of acute rejection therapy versus BKV replication. Urinary cell levels of mRNA for granzyme B, CD103, TGF-β1 and 18S rRNA deviated significantly from a normal distribution, but a log transformation substantially reduced the skew. All P-values were calculated using a two-tailed analysis with alpha value of 0.05.
RESULTS
Prevalence of BKV replication
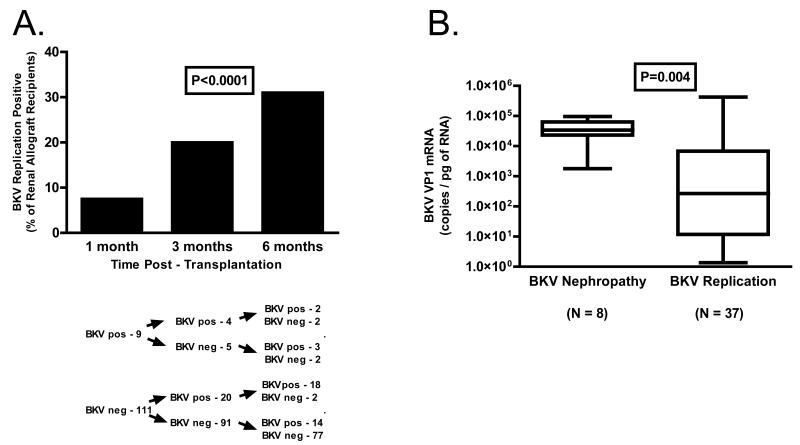
Measurement of urinary cell BKV VP1 mRNA levels in urine specimens, collected longitudinally at 1, 3 and 6 months from 120 renal allograft recipients, demonstrated that the prevalence of BKV replication increases from 7.5% at one month post-transplantation to 20% at 3-months and 31 % by six months (P<0.0001, Chi Squared test for Trend, Fig.1A). The median (25 and 75 percentiles) BKV VP1 mRNA copy number was lower in the 9 subjects who became positive at one month compared to the 34 subjects who became positive at 3 or 6 months (21 [2.4, 5854] copies/pg RNA vs. 761 [21.4, 20400] copies/pg RNA, P=0.04). The prevalence of BKV replication appeared to peak by 6 months post-transplantation since viral replication was evident in 28.3% of the urine specimens collected at 12 months from 106 of the 120 individuals available for study.
Figure 1. Prevalence of BKV Replication in Sequential Urine Samples from Renal Allograft Recipients.
Urine samples were collected at 1 month, 3 months and 6 months post-transplantation from 120 renal allograft recipients, and urinary cell BKV VP1 mRNA levels were measured using BKV specific oligonucleotide primers and TaqMan® probes in the real time quantitative PCR assay. Panel A. The prevalence of BKV replication increased from 7.5% at one month post-transplantation to 20% at three months and 31 % by six months (P<0.0001 The median (25 and 75 percentiles) BKV VP1 mRNA copy number was lower in the 9 subjects who became positive at one month compared to the 34 subjects who became positive at 3 or 6 months (21 [2.4, 5854] copies/ pg RNA vs. 761 [21.4, 20400] copies/pg RNA, P=0.04, Mann Whitney test). Panel B. Box and whisker plots illustrate the 10%, 25%, 50% (median), 75% and 90% percentile values in the subjects with BKV replication at 6 months post-transplant, and in the previously reported 8 patients with biopsy confirmed BKV nephropathy (14). The median BKV VP1 mRNA copy number was significantly lower in the BKV replication positive group compared to those with BKV nephritis (267 vs. 34,000; P=0.004, Mann-Whitney test).
We have reported that measurement of BKV VP1 mRNA levels in urinary cells offers a noninvasive means of diagnosing BKB nephropathy (14). The median (25 and 75 percentiles) BKV VP1 copy number in the BKV positive group was significantly lower compared to the previously reported 8 renal allograft recipients with biopsy confirmed BKV nephropathy (267 [12, 6805] BKV VP1 copies/pg RNA vs. 34,000 [1780, 63800], P=0.004, Fig. 1B).
Demographic and Transplant Variables
The demographic and transplant variables of the 37 renal allograft recipients with BKV replication at 6-months and the 83 recipients without BKV replication are summarized in Table 1. Age, gender, and race were not different between the two groups (P>0.05). The transplant variables, the type of donor kidney (deceased donor vs. living donor), number of HLA mismatches, incidence of DGF or the frequency of ureteral stent usage were also not different between the two groups (P>0.05).
Table l. Demographics and Transplant Related Variables in BKV Replication Positive or Negative Group.
| Variable | BKV Positive N=37 (31%) |
BKV Negative N=83 (69%) |
P Value* |
|---|---|---|---|
| Age (mean ±SD) | 46.8 ± 12.8 | 47.4 ± 13.5 | 0.9 |
| Gender (% females) | 16 (43%) | 42 (51%) | 0.4 |
| Race (% African-Americans) | 12 (32%) | 21 (25%) | 0.4 |
| Deceased Donor Grafts (N=46) | 17 (46%) | 29 (35%) | 0.3 |
| Number of HLA Mismatches (median) | 3 | 3 | 0.9 |
| DGF (N=11) | 4 (11%) | 7 (8%) | 0.7 |
| Ureteral Stent (N=52) | 17 (46%) | 35 (42%) | 0.7 |
| Acute Rejection Treatment | |||
| Within the First 6 months Post-Transplantation (N=16) | 4 (11%) | 12 (14%) | 0.6 |
Urine specimens were collected from 120 renal-allograft recipients at 6 months post-transplantation and urinary cell levels of BKV VP1 mRNA were quantified using BKV specific primers and TaqMan® probe in the real-time quantitative PCR assay (14). A subject was classified as BKV replication positive if the urinary cell BKV VP1 copy number was greater than >1 BKV VP1 mRNA copy / one pg total RNA.
P Values for categorical variables were derived using the chi-square test and for continuous variables using the Mann-Whitney test.
Immunosuppressive Therapy and BKV Replication
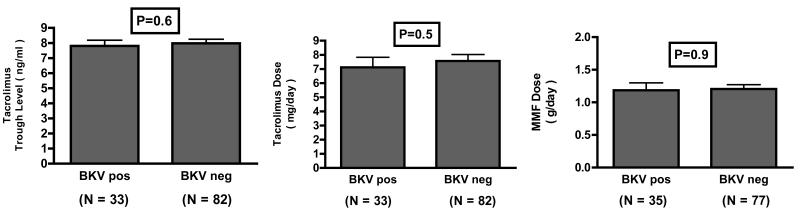
Sixteen of the 120 subjects received anti-rejection therapy for acute rejection, and the frequency of anti-rejection treatment was not different between the BKV replication positive group and the negative group (P=0.6, Table 1). Among the 120 recipients, 115 received tacrolimus as their calcineurin inhibitor drug and 5 received cyclosporine, and 112 of the 120 received mycophenolate mofetil, and 8 received sirolimus. We examined whether tacrolimus trough levels or dosage was different between BKV replication positive or negative group. The mean (±SE) tacrolimus trough level at six months post-transplantation was 7.8 ±0.4ng/ml in patients with BKV replication (N=33) and 8.0 ±0.3 ng/ml in those without (N=82) (P=0.6, Fig.2A); the mean (±SE) tacrolimus dose was also not significantly different between the two groups (7.1±0.7 mg/day vs. 7.6±0.5, P=0.5, Fig.2B). We also found that the mean (±SE) dose of MMF was not different between the two groups; 1.2±0.1 grams per day in the patients with BKV replication (N=35), and 1.2±0.1 grams per day in the patients without (N=77) (P=0.9, Fig. 2C).
Figure 2. Tacrolimus and Mycophenolate Mofetil Dosage in Renal Allograft Recipients with or without BKV Replication at 6 Months Post-Transplantation.
Panel AOne hundred fifteen of the 120 renal allograft recipients received tacrolimus as their calcineurin inhibitor drug. The mean (±SE) tacrolimus trough level was 7.8±0.4 ng/ml in the 33 patients with BKV replication (BKV Positive) and 8.0±0.3 ng/ml in the 82 patients without BKV replication (BKV Negative) (P=0.6) Panel B. The mean (±SE) tacrolimus dose was 7.1±0.7mg/day in the 33 patients with BKV replication and 7.6±0.5mg/day in the 82 patients without BKV replication (P=0.5). Panel C. one hundred twelve of the 120 received mycophenolate mofetil (MMF). The mean (±SE) MMF dose was 1.2±0.1 grams per day in the 35 patients with BKV replication (BKV Positive) and 1.2±0.1 in the 77 patients without BKV replication (BKV Negative) (P=0.9). P values were calculated using Mann-Whitney test.
Risk Factors for BKV Replication
Seventy one of 120 recipients (59%) patients were managed with the steroid maintenance regimen, and 49 (41%) were managed with an early steroid withdrawal protocol. Steroid maintenance therapy was significantly more frequent in the BKV positive group compared to the BKV negative group (73% in the BKV positive group vs. 53% in the BKV negative group, P=0.03). The odds ratio for developing BKV replication in allograft recipients on steroid maintenance compared to early steroid withdrawal was 2.4 (95% CI 1.04, 5.4; P<0.04) by univariate analysis.
Forty-five of 49 recipients managed with the early steroid withdrawal protocol received full dose rabbit ATG induction therapy (5 doses of 1.5mg/kg/day) and 4 received basiliximab induction whereas the 71 subjects managed with steroid maintenance therapy received either no induction (N=22), basiliximab induction (N=15), a single dose of ATG induction (N=19) or full dose ATG induction (N=15). In order to control for potential confounders, we used a multivariate logistic regression model and a stepwise backward elimination approach to identify independent risk factors for BKV replication. We limited the number of variables that were entered into the logistic regression analysis since relatively small number patients (N=37) reached our primary endpoint of BKV replication. Our selection of variables to be included in the logistic regression model was informed by biological plausibility and included parameters related to immunosuppression: ATG induction therapy, steroid maintenance therapy, tacrolimus trough levels, tacrolimus dose, mycophenolate mofetil dose and treatment for acute rejection. In our final model, we incorporated only variables that had P value < 0.10. This resulted in tacrolimus trough level (P=0.37), tacrolimus dose (P=0.20) and mycophenolate mofetil dose (P=0.72) being removed from the final model.
Our multivariable logistic regression analysis using stepwise backward elimination identified steroid maintenance (odds ratio: 8.27, 95% CI: 2.10 - 32.66, P=0.003, Table 2) as an independent risk factor for BKV replication at 6-months post-transplantation. Our analyses also identified full dose ATG induction therapy as an independent risk factor (odds ratio: 5.83, 95% CI: 1.60-21.35, P=0.008, Table 2) at this time point. Logistic regression analysis also showed that prednisone maintenance (OR=6.1, CI-1.68, 22.2; P=0.006) and full-dose thymoglobulin induction therapy (OR=3.8, CI- 1.17, 12.33; P=0.03) increased risk for BKV replication at three months post-transplantation. Similar analysis could not be performed at one month post-transplantation secondary to the prevalence being only 7.5%.
Table 2. Risk Factors for BKV Replication in Renal Allograft Recipients.
| Variable | Odds Ratio |
95 Percent Confidence Intervals |
P Value * |
|---|---|---|---|
| Steroid Maintenance (N=71) |
8.27 | 2.01, 32.86 | 0.003 |
| Full Dose ATG Induction (N=60) |
5.83 | 1.60, 21.35 | 0.008 |
| Anti-Rejection Treatment (N=16) |
0.22 | 0.04, 1.29 | 0.09 |
A multivariate logistic regression model and a stepwise backward elimination approach were used to identify risk factors for BKV replication. The selection of variables to be included in the logistic regression model was based on biological plausibility and the number of variables to be included was influenced by the number of subjects reaching the primary end-point (BKV replication). In our final model, the variables that had P value of less than 0.10 were only included. Thus, tacrolimus trough level (P=0.37), tacrolimus dose (P=0.20), and mycophenolate mofetil dose (P=0.72) were removed from the final model. Multivariable logistic regression analysis using stepwise backward elimination identified steroid maintenance therapy and full dose ATG induction as risk factors for BKV replication. Steroid maintenance and full dose ATG induction remained significant risk factors after adjusting for acute rejection therapy (P=0.006).
Anti-rejection treatment within the first six months post-transplantation was not associated with an increased risk of BKV replication (odds ratio: 0.22, 95% CI: 0.04-1.29, P=0.09, Table 2). Steroid maintenance and full dose ATG induction remained significant risk factors after adjusting for anti-rejection therapy (P=0.006).
We examined whether BKV VP1 mRNA copy numbers in sequential urine specimens was influenced by steroid maintenance therapy. Seventeen of the 37 renal allograft recipients had greater than a two log increase in their BKV copy number from three months to six months post-transplantation (Group 1); 14 had less than a two log increase (Group 2); and 6 had greater than a two log decrease in their BKV VP1 mRNA copy number (Group 3). Our analysis showed that the number of recipients managed with steroid maintenance therapy was significantly higher in Group 1 (13 of 17 recipients, 76%) compared to Group 2 (6 of 14, 43%) or Group 3 (1 of 6, 17%) (P=0.007, Chi-Square test for Trend). Our analysis also showed that other variables such as the type of donor graft, the development of DGF, ureteral stent placement, acute rejection therapy within the first six-months or full ATG induction therapy were not different among these three groups.
We did additional subgroup analysis since 15 of the 60 patients who received full dose ATG induction therapy were managed with steroid maintenance therapy due to increased immune risk (e.g., PRA > 50%, repeat transplant) and the remaining 45 were managed with the early steroid withdrawal protocol. The subgroup analysis showed that steroid maintenance therapy increased the risk of BKV replication by 38% in those who received full dose ATG induction (Risk Ratio: 2.7, 95% CI: 1.36-5.36, P=0.007, Table 3A).
Table 3. Sub-Group Analysis for Risk Factors for BKV Replication.
| A. Rabbit Anti-Human Thymocyte Globulin Induction Cohort | ||||
|---|---|---|---|---|
| Steroid Maintenance Sub-Group (N=15) |
Early Steroid Withdrawal Sub-Group (N=45) |
Risk Ratio (95% CI) |
P Value* | |
|
|
||||
| BKV Replication | 9 (60%) | 10 (22%) | 2.7 (1.36, 5.36) | 0.01 |
| No BKV Replication | 6 (40%) | 35 (78%) | ||
| B. Steroid Maintenance Cohort | ||||
|
Anti-Rejection Treatment
Sub-Group (N=15) |
No Anti-Rejection
Treatment Sub-Group (N=56) |
Risk Ratio
(95% CI) |
P Value | |
|
|
||||
| BKV Replication | 4 (27%) | 23 (41%) | 0.65 (0.27, 1.59) | 0.38 |
| No BKV Replication | 11 (73%) | 33 (59%) | ||
Sub-group analysis for risk factors was performed using as study cohort 60 patients who all received full dose ATG induction therapy (Table 3A, Rabbit Anti-Thymocyte Induction Cohort) or all 71 patients managed with steroid maintenance therapy (Table 3B, Steroid Maintenance Cohort). The analysis showed that steroid maintenance therapy increased the risk of BKV replication by 38% (95% CI 0.10, 0.65) in those who received full dose ATG induction; the sub-group analysis also showed that anti-rejection therapy for acute rejection (AR) did not significantly alter the risk of BKV replication. The risk ratio and ninety-five percent confidence intervals (95% CI) are shown.
P values were derived using two-sided Fisher’s Exact Test.
Sixteen of the 120 patients were treated for clinical acute rejection (15 of 16 acute rejection episodes were biopsy confirmed) within the first six months of transplantation; all 16 received methylprednisolone pulse therapy (250 mg IV twice per day for 3 days) as their initial anti-rejection therapy, 4 received additional therapy with ATG, and one patient received IVIG / plasmapheresis / anti-CD20 antibody (Rituximab®) therapy.
Fifteen of the 16 patients with an episode of acute rejection belonged to the steroid maintenance group. We therefore performed a subgroup analysis of patients who were all maintained on steroid to see whether acute rejection therapy within the first six months in the steroid maintenance group increased the risk of BKV replication in the steroid maintenance group. Our analysis showed that the risk of BKV replication in those with acute rejection therapy (N=15) compared to those without acute rejection therapy (N=56) varied from a decrease of 40% to an increase of 11% and that the anti-rejection therapy did not increase the risk of BKV replication (Risk Ratio 0.65, 95% CI: 0.27-1.59, P=0.38, Table 3B).
Urinary Cell mRNA Profiles of Patients with or without BKV Replication
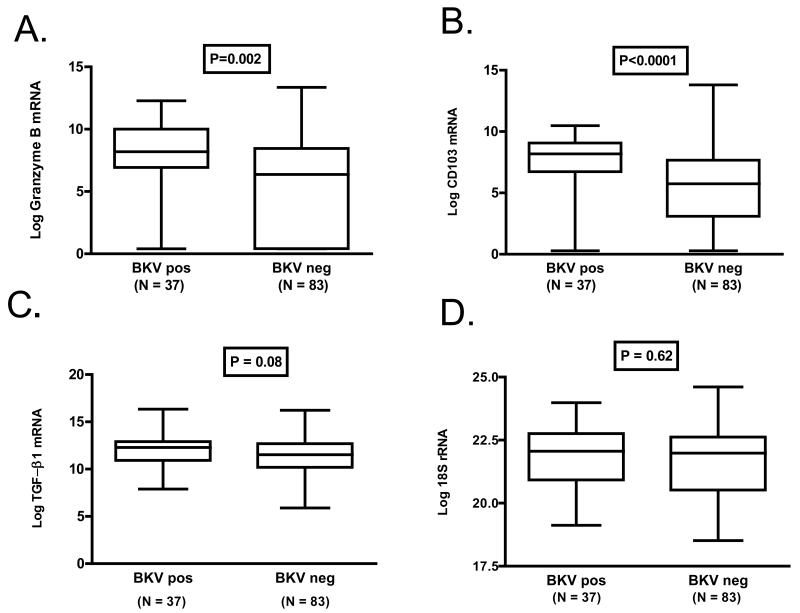
Levels of mRNA for granzyme B and CD103 were higher in urinary cells from patients with BKV replication than in those without BKV replication (Fig. 3). The log-transformed mean (±SE) level of granzyme B mRNA was 7.7±0.5 copies/microgram of RNA in patients with BKV replication and 5.6±0.4 copies/microgram of RNA in patients without BKV replication (P=0.002, Fig. 3A). The level of CD103 mRNA was 7.4±0.4 copies/microgram of RNA in patients with BKV replication and 5.1±0.3 mRNA copies/microgram of RNA in patients without BKV replication (P<0.0001, Fig 3B). Levels of TGF-β1 mRNA were also higher in the BKV replication group, and the difference was marginally significant (12.0±0.3 vs. 11.3±0.2, P=0.08, Fig. 3C). As expected, the levels of the reference gene 18s rRNA were not different between the two groups (21.8±0.2 vs. 21.6±0.2, P=0.62, Fig. 3D).
Figure 3. Levels of mRNA in Urinary Cells from Renal Allograft Recipients with or without BKV Replication.
Box and whisker plots show the 10th, 25th, 50th (median) 75th, and 90th percentile values for granzyme B mRNA (Panel A), CD103 mRNA (Panel B) and TGFβ1 mRNA (Panel C) and mRNA for 18S rRNA (Panel D) in urine samples collected at 6 months post-transplantation from renal allograft recipients with BKV replication (BKV Positive) or without BKV replication (BKV Negative). Levels of mRNA for granzyme B and CD103 were higher in urinary cells from patients with BKV replication than in those without BKV replication. The log-transformed mean (±SE) level of granzyme B mRNA was 7.7±0.5 copies/microgram of RNA in patients with BKV replication and 5.6±0.4 copies/microgram of RNA in patients without BKV replication (P=0.002, Fig. 3A). The level of CD103 mRNA was 7.4±0.4 copies/microgram of RNA in patients with BKV replication and 5.1±0.3 mRNA copies/microgram of RNA in patients without BKV replication (P<0.0001, Fig 3B). Levels of TGFβ1 mRNA were also higher in the BKV replication group, and the difference was marginally significant (12.0±0.3 vs. 11.3±0.2, P=0.08, Fig. 3C). The levels of the reference gene 18s rRNA were not different between the two groups (21.8±0.2 vs. 21.6±0.2, P=0.62, Fig. 3D). In all cases log-transformed values are shown. P values were calculated using the Mann-Whitney test.
DISCUSSION
Our longitudinal study has identified that both steroid maintenance therapy and full dose ATG induction independently increase the risk for BKV replication in adult recipients of human renal allografts. Our urinary cell mRNA profiling suggests that viral replication is associated with trafficking of granzyme B expressing cytopathic cells and CD103+ T cells to the renal allograft.
BK VP1 protein is central for viral entry into human cells (18). Our molecular surveillance of renal allograft recipients demonstrate that BKV replication can be detected as early as one month post-transplantation and that the prevalence peaks by 6 months. The 31% prevalence rate we observed is similar to the prevalence noted in earlier studies using decoy-cells in urine as the indicator of viral replication (10) or by quantification of viral DNA in urine (19-21).
Decoy-cell shedding in urine, BK viruria and viremia have all been reported to precede the development of BKV nephropathy (10, 20-22). Our findings support the clinical approach of serial monitoring for BKV replication in the first year post-transplantation in all recipients of renal allografts. However, whether urine or plasma should be assayed, and whether BKV should be measured at the DNA or mRNA level remain unaddressed. Our approach, quantification of levels of BKV VP1 mRNA rather than BKV VP1 DNA, may have the added advantage of documenting ongoing transcription/replication of the BKV genome in the uroepithelial cells. However, the BKV genome lacks introns, and the potential contribution of BKV VP1 DNA to the BKV VP1 copy numbers we observed remains unresolved.
Moens et al. identified a functional glucocorticoid response element in the late leader region of the BKV genome and reported that exposure to dexamethasone results in an increase in the number of cells expressing BKV capsid proteins (13). Basse et al. noted that those who developed BK viremia during the first year post-transplant had received larger cumulative dose of steroids from day 0 to 7 compared to those who did not (22). In accord with idea that corticosteroids enhance the risk for BKV replication, early withdrawal of corticosteroids in our study was associated with a reduced the risk of BKV replication and the beneficial impact of early steroid withdrawal was also evident in our subjects treated with full dose ATG therapy (Table 3A). Our observations confirm and extend the findings reported in an abstract by Pavalakis et al (23) and need further investigation using a randomized controlled trial design comparing steroid maintenance vs. early steroid withdrawal/avoidance.
We also identified that a full dose ATG induction regimen (5 doses of ATG, 1.5mg/kg/day) is an independent risk factor for BKV replication (odds ratio=5.83, P=0.008, Table 2). Recently, it was reported that all 9 of the 145 SPK recipients diagnosed with BKV nephropathy received induction therapy with either ATG or OKT3 (24). Steroid avoidance however may reduce the risk associated with antibody therapy mediated T cell depletion. Gupta et al. reported a decrease in the incidence of BKV nephropathy when ATG induction therapy was combined with a steroid avoidance protocol for their SPK recipients (25).
Hirsch et al. found that renal allograft recipients who had decoy-cell shedding or BKV viremia (but not BKV nephropathy) had received a higher number of anti-rejection treatments with corticosteroid pulses compared to those without decoy-cell shedding in urine (10). In our study, neither acute rejection nor anti-rejection therapy was associated with an increased risk of BKV replication. Although the low incidence of acute rejection in our study cohort (16 of 120 subjects were treated with ant-rejection therapy) may have contributed to the lack of association, we hypothesize that those who experienced an acute rejection episode were not over-immunosuppressed and had preserved immunity, and the non-compromised immune response constrained BKV replication in these subjects.
In our study, neither the tacrolimus dose nor drug trough levels were significantly different between the BKV replication positive and the BKV replication negative group. We also found that the MMF dose was not different between the two groups. In a randomized study of 200 renal allograft recipients induced with ATG, Brennan et al. noted that the incidence of BKV viruria was higher with the tacrolimus/MMF regimen compared to cyclosporine/MMF, but tacrolimus, MMF or cyclosporine was not independently associated with viruria or viremia (19). In a retrospective analysis of 575 patients who received ATG induction and maintenance therapy with tacrolimus (high dose or low dose)/MMF/prednisone, the low dose group had a lesser incidence of BKV nephropathy (26).
BKV nephropathy with graft dysfunction is associated with intragraft inflammation, and is currently identified by percutaneous biopsy of the allograft. Our study suggests that noninvasive assessment of the inflammation associated with BKV replication is feasible. It is interest that urinary cells from patients with stable graft function and BKV replication express significantly higher levels of granzyme B and CD103 compared to patients without BKV replication. Granzyme B, a serine peptidase, is an integral member of lytic machinery of cytotoxic T cells (CTLs) (27). CD103 is expressed by alloreactive CD8+ CTLs and binds E-cadherin expressed on tubular epithelial cells, and CD103+ cells have been associated with tubulitis (28, 29).
BKV nephropathy resulting in allograft failure is distinguished by extensive fibrosis. Neither the mechanisms for allograft fibrosis nor the basis for BKV nephropathy resulting in end stage renal disease (ESRD) remains unknown (5). Because TGF- β1 is a fibrogenic cytokine and an excess of TGF- β1 is associated with renal interstitial fibrosis including chronic allograft nephropathy (30, 31), we investigated the hypothesis that BKV replication is associated with a heightened expression of mRNA for TGF- β1. Our study showed that urinary cell levels of mRNA for TGF-β1 were higher in the BKV replication positive group compared to the negative group, albeit not being statistically significant. It is worth noting that our subjects did not manifest graft dysfunction. We hypothesize that BKV nephropathy associated with graft dysfunction would be distinguished by heightened expression of TGF- β1 mRNA, and that the patients who progress to ESRD would exhibit pro-fibrotic molecular signature. Future studies may help clarify whether advanced BKV nephropathy (PVAN C) that progresses to ESRD is indeed associated a pro-fibrotic profile.
In the current study, none of the patients exhibited graft dysfunction; thus the issue of distinguishing BKV nephropathy from other causes of graft dysfunction (e.g., ATN or clinical acute rejection) did not arise. In our earlier study, analysis involving the receiver –operating- characteristic curve identified that BKV nephropathy can be predicted with a sensitivity of 94 percent and a specificity of 94 percent with the use of a cutoff value of 6,500 BKV VP1 mRNA copy number per pg of RNA isolated from urinary cells (14). We have also reported that urinary cell mRNA encoding cytotoxic attack proteins granzyme B and perforin are not hyperexpressed during ATN, and are expressed at a significantly level during an episode of acute rejection compared to those without acute rejection (15). Our urinary cell mRNA profiling studies support the idea that a urinary cell mRNA profile that includes measurement of BKV VP1 mRNA and mRNA for perforin and granzyme B would distinguish BKV nephropathy from ATN or acute rejection.
Age, gender, race, the type of donor kidney, and number of HLA antigen mismatches, incidence of DGF or the frequency of ureteral stent usage was not associated with BKV replication in our study. A similar lack of association between BKV replication/nephropathy and several of the demographic and transplant variables has also been reported (10). However, Thomas et al. reported that ureteral stent placement was more frequent in renal allograft recipients with BKV nephropathy compared to those without BKV nephropathy (32). Additional studies with a larger study cohort may help clarify the independent risk engendered by ureteral stent placement.
In summary, our longitudinal study of 120 renal allograft recipients suggests that both ATG induction and steroid maintenance therapy are independent risk factors for BKV replication. Our gene expression profiling of urinary cells suggests that BKV replication, even in the absence of allograft dysfunction, is associated with increased urinary cell levels of granzyme B expressing cytopathic T cells and CD103+ T cells. Randomized controlled trials to investigate whether early steroid withdrawal is associated with a reduced risk of BKV replication, and to ascertain the independent risk conferred by the type of induction therapy on BKV replication, are worthy of consideration, and may inform future clinical practice. Urinary cell mRNA profiling, by elucidating BKV associated inflammation, may help identify allografts at risk for graft dysfunction, as well as guide modifications in immunosuppressive therapy.
ACKNOWLEDGEMENT
Supported by an award (NIH RO1 AI 60706) from the National Institute of Allergy and Infectious Diseases to MS. DD is a recipient of the American Society of Transplantation-Fujisawa Clinical Fellowship award. The authors are grateful to Ms. Linda Stackhouse and Ms. Maria Trantino for the meticulous preparation of the manuscript.
References
- 1.Imperiale MJ, Major EO. Polyomaviruses. In: Knipe David, Howley Peter., editors. Fields Virology. 5th ed. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2007. p. 2263. [Google Scholar]
- 2.Shah KV. Polyomaviruses. In: Fields Bernard, Knipe David, Howley Peter., editors. Fields Virology. 3rd ed. Vol. 2. Lippincott-Raven Publishers; Philadelphia: 1996. p. 2027. [Google Scholar]
- 3.Boldorini R, Veggiani C, Barco D, Monga G. Kidney and urinary tract polyomavirus infection and distribution: molecular biology investigation of 10 consecutive autopsies. Arch Pathol Lab Med. 2005;129(1):69. doi: 10.5858/2005-129-69-KAUTPI. [DOI] [PubMed] [Google Scholar]
- 4.Jin L, Gibson PE, Booth JC, Clewley JP. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol. 1993;41(1):11. doi: 10.1002/jmv.1890410104. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79(10):1277. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 6.Nebuloni M, Tosoni A, Boldorini R, et al. BK virus renal infection in a patient with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1999;123(9):807. doi: 10.5858/1999-123-0807-BVRIIA. [DOI] [PubMed] [Google Scholar]
- 7.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67(1):103. doi: 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 8.Vasudev B, Hariharan S, Hussain SA, Zhu YR, Bresnahan BA, Cohen EP. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005;68(4):1834. doi: 10.1111/j.1523-1755.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 9.Trofe J, Hirsch HH, Ramos E. Polyomavirus-associated nephropathy: update of clinical management in kidney transplant patients. Transpl Infect Dis. 2006;8(2):76. doi: 10.1111/j.1399-3062.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347(7):488. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 11.Ginevri F, De Santis R, Comoli P, et al. Polyomavirus BK infection in pediatric kidney-allograft recipients: a single-center analysis of incidence, risk factors, and novel therapeutic approaches. Transplantation. 2003;75(8):1266. doi: 10.1097/01.TP.0000061767.32870.72. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch H, Suthanthiran M. The natural history, risk factors and outcomes of polyomavirus BK-associated nephropathy after renal tranpsplantation. Nature Clinical Practice Nephrology. 2006;2(5):240. doi: 10.1038/ncpneph0179. [DOI] [PubMed] [Google Scholar]
- 13.Moens U, Subramaniam N, Johansen B, Johansen T, Traavik T. A steroid hormone response unit in the late leader of the noncoding control region of the human polyomavirus BK confers enhanced host cell permissivity. J Virol. 1994;68(4):2398. doi: 10.1128/jvi.68.4.2398-2408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding R, Medeiros M, Dadhania D, et al. Noninvasive diagnosis of BK virus nephritis by measurement of messenger RNA for BK virus VP1 in urine. Transplantation. 2002;74(7):987. doi: 10.1097/00007890-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Hartono C, Ding R, et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344(13):947. doi: 10.1056/NEJM200103293441301. [DOI] [PubMed] [Google Scholar]
- 16.Ding R, Li B, Muthukumar T, et al. CD103 mRNA levels in urinary cells predict acute rejection of renal allografts. Transplantation. 2003;75(8):1307. doi: 10.1097/01.TP.0000064210.92444.B5. [DOI] [PubMed] [Google Scholar]
- 17.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353(22):2342. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 18.Dugan AS, Eash S, Atwood WJ. Update on BK virus entry and intracellular trafficking. Transpl Infect Dis. 2006;8(2):62. doi: 10.1111/j.1399-3062.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 19.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5(3):582. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 20.Drachenberg CB, Hirsch HH, Papadimitriou JC, et al. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation. 2007;84(3):323. doi: 10.1097/01.tp.0000269706.59977.a5. [DOI] [PubMed] [Google Scholar]
- 21.Viscount HB, Eid AJ, Espy MJ, et al. Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy. Transplantation. 2007;84(3):340. doi: 10.1097/01.tp.0000275205.41078.51. [DOI] [PubMed] [Google Scholar]
- 22.Basse G, Mengelle C, Kamar N, et al. Prospective evaluation of BK virus DNAemia in renal transplant patients and their transplant outcome. Transplant Proc. 2007;39(1):84. doi: 10.1016/j.transproceed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Pavlakis M, Mandelbrot D, Karp S, Khwaja K, Johnson S. “BK Virus Screening and Outcomes in a Steroid Withdrawal (SW) and Steroid Continuation (SB) in Kidney Transplantation.” Abstract # SA-FC033, 2005 Renal Week of American Society of Nephrology. J Am Soc Nephrology. 16(Suppl 2):89A–200. [Google Scholar]
- 24.Lipshutz GS, Mahanty H, Feng S, et al. BKV in simultaneous pancreas-kidney transplant recipients: a leading cause of renal graft loss in first 2 years post-transplant. Am J Transplant. 2005;5(2):366. doi: 10.1111/j.1600-6143.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 25.Gupta G, Shapiro R, Thai N, Randhawa PS, Vats A. Low incidence of BK virus nephropathy after simultaneous kidney pancreas transplantation. Transplantation. 2006;82(3):382. doi: 10.1097/01.tp.0000228899.05501.a7. [DOI] [PubMed] [Google Scholar]
- 26.Cosio FG, Amer H, Grande JP, Larson TS, Stegall MD, Griffin MD. Comparison of low versus high tacrolimus levels in kidney transplantation: assessment of efficacy by protocol biopsies. Transplantation. 2007;83(4):411. doi: 10.1097/01.tp.0000251807.72246.7d. [DOI] [PubMed] [Google Scholar]
- 27.Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 28.Hadley GA, Bartlett ST, Via CS, Rostapshova EA, Moainie S. The epithelial cell-specific integrin, CD103 (alpha E integrin), defines a novel subset of alloreactive CD8+ CTL. J Immunol. 1997;159(8):3748. [PubMed] [Google Scholar]
- 29.Robertson H, Wong WK, Talbot D, Burt AD, Kirby JA. Tubulitis after renal transplantation: demonstration of an association between CD103+ T cells, transforming growth factor beta1 expression and rejection grade. Transplantation. 2001;71(2):306. doi: 10.1097/00007890-200101270-00024. [DOI] [PubMed] [Google Scholar]
- 30.Sharma VK, Bologa RM, Xu GP, et al. Intragraft TGF-beta 1 mRNA: a correlate of interstitial fibrosis and chronic allograft nephropathy. Kidney Int. 1996;49(5):1297. doi: 10.1038/ki.1996.185. [DOI] [PubMed] [Google Scholar]
- 31.Mas VR, Maluf DG, Archer KJ, et al. Study of mRNA growth factors in urinary cells of kidney transplant recipients as predictors of chronic allograft nephropathy. Transplantation. 2005;80(12):1686. doi: 10.1097/01.tp.0000185472.79948.db. [DOI] [PubMed] [Google Scholar]
- 32.Thomas A, Dropulic LK, Rahman MH, Geetha D. Ureteral stents: a novel risk factor for polyomavirus nephropathy. Transplantation. 2007;84(3):433. doi: 10.1097/01.tp.0000269616.21698.10. [DOI] [PubMed] [Google Scholar]