Abstract
Sterol import has been characterized under various conditions in three distinct fungal species, the model organism Saccharomyces cerevisiae and two human fungal pathogens Candida glabrata and Candida albicans, employing cholesterol, the sterol of higher eukaryotes, as well as its fungal equivalent, ergosterol. Import was confirmed by the detection of esterified cholesterol within the cells. Comparing the three fungal species, we observe sterol import under three different conditions. First, as previously well characterized, we observe sterol import under low oxygen levels in S. cerevisiae and C. glabrata, which is dependent on the transcription factor Upc2 and/or its orthologs or paralogs. Second, we observe sterol import under aerobic conditions exclusively in the two pathogenic fungi C. glabrata and C. albicans. Uptake emerges during post-exponential-growth phases, is independent of the characterized Upc2-pathway and is slower compared to the anaerobic uptake in S. cerevisiae and C. glabrata. Third, we observe under normoxic conditions in C. glabrata that Upc2-dependent sterol import can be induced in the presence of fetal bovine serum together with fluconazole. In summary, C. glabrata imports sterols both in aerobic and anaerobic conditions, and the limited aerobic uptake can be further stimulated by the presence of serum together with fluconazole. S. cerevisiae imports sterols only in anaerobic conditions, demonstrating aerobic sterol exclusion. Finally, C. albicans imports sterols exclusively aerobically in post-exponential-growth phases, independent of Upc2. For the first time, we provide direct evidence of sterol import into the human fungal pathogen C. albicans, which until now was believed to be incapable of active sterol import.
INTRODUCTION
Candida albicans and Candida glabrata are two medically important pathogens in humans, which cause disease predominantly in immunocompromised individuals (resulting from AIDS, transplants, and cancer treatment). C. albicans accounts for more than 50% of all Candida infections, causing oral, vaginal, and systemic disease, the last with a high morbidity rate between 30 and 50%. C. glabrata is the second or third most common species causing Candida infections (1). Azole antifungals are one of the most commonly used classes of drugs used to treat fungal infections. These drugs target the biosynthesis of ergosterol, the fungal equivalent of the sterol found in higher eukaryotes, cholesterol. Azoles inhibit 14α-lanosterol demethylase, the product of the ERG11 gene. In general, sterols are essential components of eukaryotic cell membranes, and therefore cells tightly regulate sterol levels and sterol metabolism. Alteration of sterol levels can have an effect on the susceptibility of fungal cells to antifungal drugs, and to a variety of stresses, including osmotic and oxidative pressures.
Saccharomyces cerevisiae.
Sterol metabolism in S. cerevisiae, a eukaryotic model organism, has been intensively studied (reviewed in references 2, 3, and 4). The fungal sterol ergosterol is synthesized in the endoplasmic reticulum (ER), the cellular membrane with otherwise the lowest levels of ergosterol. From there, ergosterol is transported through the Golgi bodies to the plasma membrane (PM), the membrane with the highest ergosterol content. Mitochondria also contain low levels of ergosterol, mostly in its inner membrane (5). In addition to its structural role in the membranes, a minimal level of ergosterol is required for cell cycle progression and for cell viability in S. cerevisiae, a process known as “sparking” (6, 7).
Under anaerobic conditions, or in mutants with defects in heme biosynthesis (heme biosynthesis is oxygen dependent, and its decreased levels are thought to be the primary sensor of oxygen availability [8]), S. cerevisiae requires exogenous methionine, sterols, and unsaturated fatty acids, since the synthesis of these requires both molecular oxygen and heme (9–11). Oxygen is necessary for several ergosterol biosynthesis reactions, including those performed by squalene epoxidase, sterol demethylase, and sterol desaturase. S. cerevisiae cells import exogenous sterols under anaerobic conditions, but not under aerobic conditions, a phenomenon known as “aerobic sterol exclusion” (12). Anaerobic sterol uptake in S. cerevisiae is found to be inversely proportional to the concentration of sterol already present in the cell. Cells that are saturated for free sterols stop accumulating exogenously supplied sterols (13–15). In S. cerevisiae, anaerobic sterol uptake is mediated by two ABC transporters: the more active ScAus1 and its paralog ScPdr11 (16, 17). Both proteins are localized to the plasma membrane, with portion of ScAus1 localized intracellularly (18).
The transcription factor ScUpc2 regulates expression of most of the genes in sterol biosynthesis, as well as sterol import, and up to one-third of anaerobically expressed genes. Thus, ScUpc2 is important for to adaptation to hypoxia (19). Constitutively active mutants of ScUpc2, or its paralog ScEcm22, induce sterol uptake under aerobic conditions, overcoming the aerobic sterol exclusion (20, 21).
Sterol uptake in S. cerevisiae is not limited to ergosterol; various sterols from the environment, including cholesterol, can also be imported. Import of cholesterol and other sterols results in alterations in phospholipids, fatty acid compositions, and sterol to phospholipid ratios within the cells (22). Before incorporation into the S. cerevisiae plasma membrane, ergosterol, but not cholesterol, binds tightly to the cell wall and is resistant to detergent wash (16, 23). The ScUpc2-regulated cell wall protein ScDan1 is involved in sterol uptake associated with the cell wall (17, 24). After sterol saturation of membranes, excess imported cholesterol is esterified via ScAre1p and ScAre2p (25), while excess ergosterol remains unesterified (15, 18, 26). Many other genes involved in sterol uptake have been described, including several related to mitochondrial metabolism (23).
Candida glabrata.
C. glabrata is more closely related to S. cerevisiae than to C. albicans, and the two species share some features of sterol import. C. glabrata requires the same anaerobic supplements as S. cerevisiae. However, C. glabrata differs in the conditions of sterol import. Recent studies (27, 28) revealed that C. glabrata wild-type strains import sterol under aerobic conditions, independent of the mutations in heme synthesis or the Upc2 orthologs that are required for S. cerevisiae aerobic sterol uptake. Wild-type C. glabrata responds to sterol-containing serum and bile, but not free cholesterol, whereas CgERG mutants can utilize free sterols, as well as components containing sterol. In addition, wild-type C. glabrata continuously synthesizes ergosterol, even while importing exogenous cholesterol from serum (29, 30).
The C. glabrata sterol transporter CgAus1 was identified based on homology to ScAus1, although a C. glabrata ortholog of ScPDR11 was not found. CgAus1 is transcriptionally induced in the presence of serum, a process dependent on the C. glabrata paralogs CgUPC2A and CgUPC2B (28).
Candida albicans.
Previous work suggests that C. albicans, unlike the two species mentioned above, depends solely on the synthesis of endogenous ergosterol. No mechanism of sterol uptake was identified in previous work (27, 31, 32). Unlike S. cerevisiae and C. glabrata, there is lack of response to exogenous sterols by C. albicans wild-type, CaHEM1-null mutant, or sterol auxotrophs (27). Moreover, no clear orthologs of ScAUS1 or ScPDR11 can be identified in the C. albicans genome. However, C. albicans is able to grow anaerobically without need for exogenous sterols or even unsaturated fatty acids, both of which are crucial for S. cerevisiae and C. glabrata growth, although addition of oleic acid to the medium significantly increases growth (33). Sterols on the other hand have no such effect. C. albicans can grow without ergosterol in the membranes (34). Unfortunately, sterol levels in anaerobically grown C. albicans have never been determined. There is some indirect evidence for C. albicans being able to import sterols, published more than 30 years ago, where a nystatin-resistant C. albicans strain, with no ergosterol in its membranes, increased its susceptibility after cultivation in medium with the presence of ergosterol (35).
In the present study, we compare and contrast sterol import into three fungal species: C. albicans, C. glabrata, and S. cerevisiae. We determine sterol import in various conditions using two substrates, cholesterol, the major sterol of higher eukaryotes, and ergosterol, the typical fungal sterol. Moreover, for the first time we provide direct evidence of active sterol import into the fungal pathogen C. albicans. Understanding sterol metabolism, including import, in the medically important fungi C. albicans and C. glabrata will help us to understand the behavior of these fungi in infection, especially in response to treatment with azoles and other drugs targeting sterol biosynthesis pathway. This deeper understanding could help us to design better treatment strategies for these infections.
MATERIALS AND METHODS
Strains and growth conditions.
The strains used in the present study are listed in Table 1. C. albicans, C. glabrata, and S. cerevisiae strains were propagated on YPD agar plates (1% yeast extract, 2% Bacto peptone, 2% glucose, and 2% agar) and otherwise were grown in liquid complete synthetic medium (CSM; 0.17% yeast nitrogen base without amino acids [Difco], 0.5% ammonium sulfate, 2% glucose, 0.79-g/liter CSM complete supplement mixture [Sunrise Science Products]) supplemented with 50 mg of uridine/liter. For microaerophilic growth, the GasPak EZ anaerobe gas generating pouch system with indicator (Becton Dickinson) or the MCG AnaeroPack System (Mitsubishi Gas Chemical Company, Inc.) with anaerobic indicator (Oxoid) were used according to the manufacturer's instructions.
Table 1.
Strains used in this study
| Strain | Designation | Genotype | Source or reference |
|---|---|---|---|
| C. albicans | |||
| BWP17 | TW19412 | ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 51 |
| S-7 | TW19413 | ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG UPC2/upc2::URA3 | 43 |
| D-6 | TW19414 | ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG upc2::URA3/upc2::ARG4 | 43 |
| EC-2 | TW19415 | ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG upc2::ARG4/upc2::UPC2-HIS1 | 43 |
| S. cerevisiae | |||
| W303-1a | TW19404 | MATa ade2-1 leu2-3,112 his3-1 ura3-52 trp1-100 can1-100 | Jasper Rine |
| JRY7179 | TW19405 | MATa ade2-1 leu2-3,112 his3-1 ura3-52 trp1-100 can1-100 upc2::HIS3 | 44 |
| JRY7180 | TW19406 | MATa ade2-1 leu2-3,112 his3-1 ura3-52 trp1-100 can1-100 ecm22::TRP1 | 44 |
| JRY7181 | TW19407 | MATa ade2-1 leu2-3,112 his3-1 ura3-52 trp1-100 can1-100 ecm22::TRP1 upc2::HIS3 | 44 |
| BY4742 | TW19408 | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | 52 |
| YRS1945 | TW19410 | MAT? his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 aus1::kanMX4 pdr11::kanMX4 | 23 |
| C. glabrata | |||
| KUE200 | TW19037 | trp1::Scura3 his3::ScURA3 ura3 FRT-YKU80 | 53 |
| upc2AΔ | TW19038 | trp1::Scura3 his3::ScURA3 ura3 FRT-YKU80 upc2A::HIS3 | 36 |
| upc2AΔ/UPC2A | TW19039 | trp1::Scura3 his3::ScURA3 ura3 FRT-YKU80 upc2A::HIS3-UPC2A-TRP1 | 36 |
| upc2BΔ | TW19040 | trp1::Scura3 his3::ScURA3 ura3 FRT-YKU80 upc2B::HIS3 | 36 |
| upc2BΔ/UPC2B | TW19041 | trp1::Scura3 his3::ScURA3 ura3 FRT-YKU80 upc2B::HIS3-UPC2B-TPR1 | 36 |
| upc2A/upc2B | TW20017 | trp1::Scura3 his3::ScURA3 ura3 FRT-YKU80 upc2B::HIS3 upc2A::SAT1 | This study |
| aus1 | TW20047 | trp1::Scura3 his3::ScURA3 ura3 FRT-YKU80 aus1::CgHIS3 | This study |
Construction of C. glabrata Δupc2A/Δupc2B mutant.
Since S. cerevisiae paralogs, ScUpc2 and ScEcm22, can partially complement for each other, we expected the same for C. glabrata. The CgUPC2A allele was deleted in the C. glabrata Δupc2B strain (36) in order to obtain the C. glabrata Δupc2A/Δupc2B double mutant. The dominant selectable marker SAT1 form plasmid pSFS2A (37) was amplified by PCR using Phusion polymerase (Thermo Scientific) with the oligonucleotides CgUPC2A_SAT1_F (5′-TAAGTCTTAGTTGTTTATCATCAAAACCACTGTTGGGTTTATTTTGGGTGTGATCAATGAAAATTTCGGTGATCCC-3′) and CgUPC2A_SAT1_R (5′-ACAGTATACCATAAATGTATCTTTGAAAGAACTACAAAAGAACAATAAACTCATTAGGCGTCATCCTGTGCTC-3′). The purified PCR product was transformed into C. glabrata using the standard lithium acetate transformation protocol for S. cerevisiae (38). After the transformation, cells were kept for additional 4 h in YPD in order to express the integrated SAT1 gene and then grown on YPD plus 100 μg of nourseothricin/ml. Positive transformants were verified by PCR (see Fig. S1 in the supplemental material) using the oligonucleotides SAT1_F (5′-CATCTCGGATGATGACTCTG-3′), SAT1_R (5′-CCAGTACCAGTACATCGCTG-3′), CgUPC2A_up_F (5′-AGTAATAGCACATCGTATAG-3′), and CgUPC2A_down_R (5′-TTGATTTCAAACTGACCTAG-3′).
Construction of C. glabrata Δaus1 mutant.
CgHIS3 gene deletion cassette was originally amplified by using the oligonucleotides CgAUS1_HIS3_F (5′-TAGAATAAAATTTTTTTAAACTTAACTTGTTGCCGCGCTATAGCCATATACAAGTTCTCCTAACTCCTTGTTCAACAGTG-3′) and CgAUS1_HIS3_R (5′-GTGTTAAATTTAAGAATAAAATGGAATTGTTATTTCATTAAAAGCTTGTAGGAGTCACTCTGGTAGCTGTGGGCTGTGTT-3′), both carrying 60 bases homologous to CgAUS1 upstream and downstream regions. However, the transformation in this case did not result in a strain with correct integration of the cassette. For this reason, three additional PCRs were performed in order to increase the length of the homologous region of the deletion cassette. The CgAUS1 upstream region was amplified using the oligonucleotides CgAUS1_uF (5′-CCCGTTTCACCATCCCATTC-3′) and CgAUS1_uR (5′-GGAGAACTTGTATATGGCTA-3′). CgAUS1 downstream region was amplified using oligonucleotides CgAUS1_dF (5′-GAGTGACTCCTACAAGCTTT-3′) and CgAUS1_dR (5′-TTTCTTGGTCAGAATAGGAG-3′). All three of the resulting PCR products were then used together as templates to create a single large PCR cassette that was amplified using oligonucleotides CgAUS1_uF and CgAUS1_dR. After transformation, the correct integration of the deletion cassette was verified by PCR (see Fig. S1 in the supplemental material) using oligonucleotides CgHIS3_F (5′-CGCACTTCTTGGAGAGTTTC-3′), CgHIS3_R (5′-TACACTCCAGGATCAGAGAC-3′), CgAUS1_up_F (5′-CCACTGCAGTTACTTGCATG-3′), and CgAUS1_down_R (5′-GTAATGGGTCATGTGAGTTC-3′).
Sterol uptake experiments.
For sterol uptake experiments, CSM plus uridine (CSM+Uri) was supplemented with 0.5 μg (1:4,000) of unlabeled ergosterol or cholesterol/ml (both as 2-mg/ml stocks in Tween 80-ethanol (EtOH) at 1:1 (vol/vol), together with radiolabeled [3H]ergosterol (1 mCi/ml in EtOH; specific activity, 20 Ci/mmol; American Radiolabeled Chemicals) or [14C]cholesterol (40 μCi/ml in EtOH; specific activity, 49.78 mCi/mmol; Perkin-Elmer NEN Radiochemicals) to reach an activity of 5 nCi/ml of media. Prior to medium supplementation, the radioactively labeled sterol was mixed together with an appropriate volume of the stock of the identical cold sterol in the Tween 80-EtOH mixture in order to ensure that the sterols are properly dissolved in the CSM+Uri medium. 1 ml of media in 14-ml conical tube was inoculated to optical density at 600 nm (OD600) of 0.01 with overnight cultures grown in CSM+Uri. Heat-killed control was always included (80°C for 30 min before incubation). Cultures were grown for 48 h in normoxic or microaerophilic conditions at 30°C with constant shaking at 180 rpm. After 48 h, the cultures were vortexed, and their OD600 values were determined (1:20 dilution) to determine the cell number. Samples were filtered on glass microfiber filters (Whatman, 24-mm GF/C) prewet with PBSC/PBSE (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4 [pH 7.4]) supplemented with 1.25% Tween 80 and 50 μg of cold cholesterol (PBSC) or ergosterol (PBSE)/ml. Conical tubes were washed with an additional 5 ml of PBSC/PBSE and filtered with the sample. Filters were transferred to 7-ml scintillation vials, and 5 ml of scintillation cocktail Ecoscint XR (National Diagnostics) was added. After 24 h, the radioactivity associated with the filter was measured with a liquid scintillation counter (Beckman Coulter, LS 6500 multipurpose scintillation counter) and normalized to 108 cells. Absolute numbers of sterol import cannot be compared among separate experiments due to day-to-day variation. All data obtained from one experiment are thus presented as a separate graph.
For the experiments presented in Results, we used the parental strains CaBWP17 and CgKUE200, in which all subsequent mutations were derived. We have analyzed sterol import in the sequenced wild-type strains (CaSC5314 and CgCBS138) and the auxotrophic parental strains (CaBWP17 and CaKUE200). We did not observe any statistical differences.
Drop test analysis.
Microdilution assays were performed in order to test growth phenotypes in the presence of various supplements. CSM+Uri plates containing 2% agar and additional supplements were used. Fluconazole (FLC) plates contained 256 μg of fluconazole (Sigma-Aldrich)/ml. If the plates contained cholesterol or ergosterol (20 μg/ml), these sterols were added from their stocks (2 mg/ml in Tween 80-EtOH at 1:1 [vol/vol]), in which Tween 80 provides a source of oleic acid.
In a 96-well plate, 1:10 dilution series of strains starting from an OD600 of 1.0 in sterile-distilled water (dH2O) were prepared. Dilution series of strains were then transferred from a 96-well plate to the agar plates by using a 48-Replica Plater (DAN-KAR Corp.), transferring ∼3 μl of the suspension. Agar plates were incubated at 30°C for 2 days in normoxic or microaerophilic conditions and documented by photography.
Sterol isolation and thin-layer chromatography (TLC) separation.
Parental strains of C. albicans (BWP17), C. glabrata (KUE200), and S. cerevisiae (W303-1a) were grown in CSM+Uri plus cold ergosterol-cholesterol (0.5 μg/ml; 2 mg/ml stock in Tween 80-EtOH at 1:1 [vol/vol]) and [3H]ergosterol (resulting in a specific activity of 500 nCi/ml of medium) or [14C]cholesterol (resulting in a specific activity of 5 nCi/ml of medium). Prior to medium supplementation, the radioactively labeled sterol was mixed together with the appropriate volume of the identical cold sterol in Tween 80-EtOH, in order to ensure its proper dissolution into the CSM+Uri. Then, 2 ml of medium in 14-ml conical tubes was inoculated to an OD600 of 0.01 with overnight cultures grown in CSM+Uri. Three conditions were used: 48-h cultivation in normoxic or microaerophilic conditions or 5-day cultivation in normoxic condition at 30°C, all with constant shaking of 180 rpm. At the end of cultivation, the cells were washed twice with PBS. Sterol isolation was based on previously published protocols (39). Briefly, the pellet was resuspended in 200 μl of methanol, and 200 μl of acid-washed glass beads (0.5 mm; BioSpec Products) were added. The suspension was vortexed at room temperature for 10 min. Next, 400 μl of chloroform was added, and the suspension was incubated at room temperature for additional 1 h, with a 10-s vortex every 10 min. Finally, the suspension was spun down (16,000 × g, 5 min), the supernatant was transferred into a new Eppendorf tube, and the solvent was evaporated in a heating block set at 60°C within a fume hood. The dried pellet was dissolved in 25 μl of methanol-chloroform at 1:1 (vol/vol) and spotted onto precoated TLC plates (silica gel on glass [thickness, 250 μm; particle size, 2 to 25 μm; mean pore diameter, 60 Å]; Aldrich Chemical Co., Inc.). Sterols were separated in mobile phase of petroleum ether-diethyl ether-acetic acid at 70:30:2 (vol/vol/vol), and the plates were dried out. Radioactivity was detected using phosphor screen and tritium phosphor screen (GE Healthcare) and scanned by Typhoon 9400, Variable mode imager (Amersham Biosciences). For band quantification, ImageJ software (Wayne Rasband, National Institutes of Health) was used.
Cholesteryl-BODIPY import on plates.
Solid medium CSM+Uri supplemented with 0.25 μg of cholesteryl-BODIPY 542/563 (Molecular Probes)/ml dissolved in Tween 80-EtOH at 1:1 (vol/vol) as a 1-mg/ml stock was used for characterization of sterol import on solid media. Overnight cultures in CSM+Uri were diluted to OD600 of 1.0, and 3 μl was spotted onto the plate. Two parallel plates were cultivated for 48 h at 30°C under normoxic and microaerophilic conditions. After the incubation, the fluorescence from the plates was read on a Typhoon 9400 variable-mode imager (Amersham Biosciences) with excitation and emission wavelengths of 532 and 555 nm, respectively. Images were processed with Photoshop CS5 (Adobe).
MIC.
Normoxic and microaerophilic MICs of the yeast strains for fluconazole were determined by using a protocol based on the CLSI broth microdilution protocol that determines the MIC of drug needed to inhibit 80% of cell growth. Strains were grown in a 96-well plate containing a gradient of drug by using serial 2-fold dilutions. Media used for cultivation were based on CSM+Uri plus 2% glucose and eventually supplemented with 0.5% Tween 80, 10% fetal bovine serum (FBS; Gibco), or 20 μg of cholesterol/ml (a 2-mg/ml stock in Tween 80-EtOH at 1:1 [vol/vol]). The plates were incubated for 48 h at 30°C with constant shaking at 180 rpm. Wells containing no drug served as a positive control for growth (100%), and cell growth in wells containing drug dilutions were standardized to the positive control.
RESULTS
Aerobic and anaerobic sterol uptake in three fungal species.
In the present study, we compare sterol uptake in three fungal species C. albicans, S. cerevisiae, and C. glabrata, using both radioactively labeled cholesterol and ergosterol. Cholesterol uptake and metabolism within cells is more thoroughly characterized due to its better commercial availability as a fluorescently or radiolabeled substrate; thus, it is used for most of the uptake studies. Although there are several structural differences between cholesterol and ergosterol, they both efficiently support the growth of S. cerevisiae and C. glabrata incapable of ergosterol biosynthesis (15, 27). The third species, C. albicans, has never been directly analyzed for sterol import. Moreover, most studies characterizing sterol import in S. cerevisiae use strains with ScHEM1 deleted, which is inducing cell response to hypoxia and promoting aerobic sterol import. In our studies, we use microaerophilic conditions and ScHEM1 prototrophs in order to get closer to the true in vivo hypoxic situation.
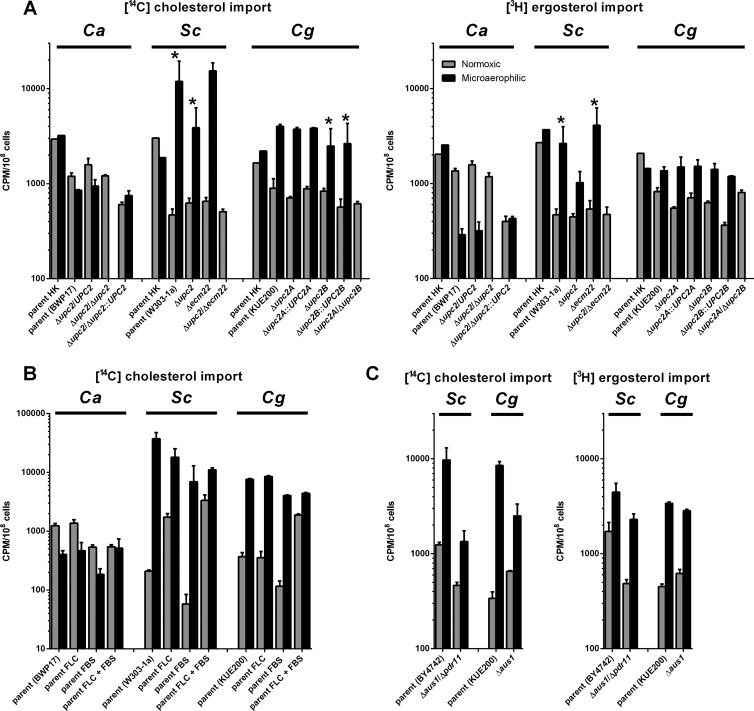
First, we analyzed cholesterol and ergosterol import into the three fungal species (Fig. 1A). For each species, we used a mutant strain deleted for UPC2 homologs, as well as the standard parental strain. It has been described previously that mutations of ScUPC2 or ScECM22 can promote aerobic sterol uptake (20, 21, 40, 41). However, the deletion of ScUPC2 and ScECM22 together leads to anaerobic inviability (our observation). The deletion of UPC2 and its paralogs has the same effect in C. albicans (42), S. cerevisiae, and C. glabrata (Fig. 1A). Upc2 is believed to be involved in the sensing of intracellular sterol levels. In C. albicans and S. cerevisiae, the deletion of UPC2 and its paralogs lead to the decrease of cellular ergosterol levels (43, 44).
Fig 1.
Sterol uptake in C. albicans, S. cerevisiae, and C. glabrata strains under various conditions. Cholesterol (A, B, and C) and ergosterol (A and C) uptake under normoxic and microaerophilic conditions into the strains of three fungal species. Sterol import was measured in parental strains and their UPC2-deficient derivatives (A), parental strains in the presence of fluconazole (FLC) and/or fetal bovine serum (FBS) (B), or in parental strains and their AUS1- and PDR11-deficient derivatives (C), comparing heat-killed (HK) cells with actively growing strains. The graphs in panel C represent separate experiment from those in panel A and thus are presented separately. In all cases strains were grown for 48 h under normoxic (gray bars) and microaerophilic condition (black bars) in CSM complete supplemented with uridine, oleic acid in the form of Tween 80, and the appropriate sterol (cholesterol or ergosterol) with its radiolabeled form. In the experiment in panel C, fluconazole (FLC; each strain's MIC80) and/or fetal bovine serum (FBS; 10%) was added as noted. Samples were normalized to cpm/108 cells. Graphs represent the average of three biological replicates with the standard errors. All differences between normoxic and microaerophilic conditions are statistically significant as determined by using the Student t test (P < 0.05), and data pairs marked with asterisks are also significant (to P < 0.1). In panel B the difference between C. albicans cholesterol uptake in the presence of FLC and FBS is not statistically significant. Heat-killed controls were only performed once due to the number of samples in the experiment. The heat killing causes significant disruption of the cell membranes, as proven by an increase in propidium iodide permeability (45).
As seen in Fig. 1A, induction of sterol import under microaerophilic conditions is observed for S. cerevisiae (parental strain W303-1a; cholesterol, 25.4-fold upregulation, P = 0.06; ergosterol, 5.7-fold upregulation, P = 0.05) and C. glabrata (parental strain KUE200; cholesterol, 4.5-fold upregulation, P < 0.01; ergosterol, 1.7-fold upregulation P < 0.01), while C. albicans displays a downregulation (parental strain BWP17; cholesterol, 1.4-fold downregulation, P = 0.01; ergosterol, 4.7-fold downregulation, P < 0.01).
In C. albicans the CaUPC2 heterozygous deletion has a similar phenotype to its parent, i.e., no induction of microaerophilic sterol uptake. However, the deletion of both CaUPC2 alleles leads to an inability to grow under microaerophilic conditions.
In S. cerevisiae, the deletion of either ScUPC2 or ScECM22 does not have a significant effect on cholesterol or ergosterol import compared to the parental strain (P > 0.05). The deletion of both ScUPC2 and ScECM22 leads to an inability to grow under microaerophilic conditions.
In C. glabrata, the deletion of either of the UPC2 paralogs, CgUPC2A or CgUPC2B, does not have any significant effect on microaerophilic cholesterol or ergosterol import. However, deletion of both UPC2 paralogs, as in S. cerevisiae and C. albicans, leads to a strain incapable of growth under microaerophilic conditions.
In all cases, heat-killed cells were associated with higher amounts of radiolabeled sterols than any of the aerobically grown strains. We suggest that this is an artifact associated with the heat killing, which disrupts cellular membranes. Heat killing makes cells permeable also to propidium iodide and thus is likely to have significant effects on membrane structure and function (45).
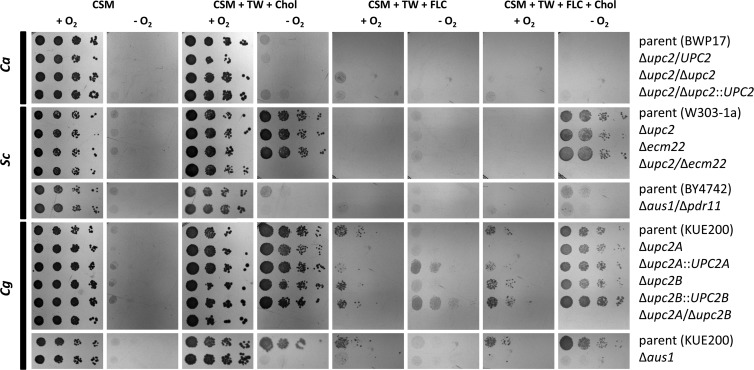
To determine the effect of exogenous sterols on strain growth, we characterized aerobic and microaerophilic growth of C. albicans, S. cerevisiae, and C. glabrata on agar media supplemented with various microaerophilic supplements and drugs affecting ergosterol biosynthesis (Fig. 2). We did not see any significant growth of any of the three fungal species under microaerophilic conditions on media that did not contain both necessary anaerobic supplements for S. cerevisiae and C. glabrata—oleic acid (in the form of Tween 80) and sterol (cholesterol or ergosterol).
Fig 2.
Normoxic and microaerophilic growth of C. albicans, S. cerevisiae, and C. glabrata strains. A dilution series (10-fold) of cells were grown for 48 h under normoxic and microaerophilic conditions at 30°C on various agar media. CSM (CSM complete supplemented with uridine), TW (Tween 80, 0.1%), Chol (cholesterol, 20 μg/ml), and FLC (fluconazole, 256 μg/ml) were used. S. cerevisiae parental strains differ for the UPC2 mutants (W303-1a) and the AUS1/PDR11 mutant (BY4742) and thus are presented separately with the appropriate mutants. CSM supplemented with Tween 80 was performed, and it was not significantly different from CSM alone (data not shown).
For C. albicans, we did not observe microaerophilic growth for the parental or the Δupc2/Δupc2 mutant on solid media with or without supplementation with either Tween 80 alone (data not shown) or with Tween 80 and either cholesterol (Fig. 2) or ergosterol (data not shown). In the previous experiment, we saw C. albicans growing under microaerophilic conditions in liquid CSM+Uri supplemented with Tween 80 and either cholesterol or ergosterol. Comparing solid and liquid media, we see significantly slower growth on the agar plates of the same composition. Observation of any growth differences on agar media might require significantly longer incubation time. The difference between liquid and agar media is probably caused by a lower rate of oxygen level decrease in liquid media compared to the surface of agar media, directly exposed to the microaerophilic atmosphere. Thus, cells may utilize the limited amounts of oxygen dissolved in liquid medium.
The S. cerevisiae parental strain and S. cerevisiae Δupc2 or Δecm22 single mutants were able to grow microaerophilically if the medium was supplemented with Tween 80 and either cholesterol (Fig. 2) or ergosterol (data not shown). This restoration of microaerophilic growth in the presence of Tween 80 and either cholesterol or ergosterol was observed even in the presence of high concentrations of fluconazole (256 μg/ml), which compromises normoxic growth of these S. cerevisiae strains. However, the S. cerevisiae Δupc2/Δecm22 double mutant is inviable under microaerophilic conditions, regardless of the plate supplements.
The C. glabrata parental strain and single mutants can grow microaerophilically if supplemented with Tween 80 and sterols. However, the susceptibility of C. glabrata to fluconazole is much lower than S. cerevisiae (MIC80 of 256 μg/ml for the C. glabrata parent versus 32 μg/ml for the S. cerevisiae parent). This high MIC allows some growth in the presence of 256 μg of fluconazole/ml. However, all of the C. glabrata strains, except C. glabrata Δupc2A/Δupc2B double mutant, display increased microaerophilic growth when sterols are present in the medium, even in the presence of high levels of fluconazole. This is proof that sterol uptake occurs in S. cerevisiae and C. glabrata under microaerophilic conditions, and they can overcome high concentrations of fluconazole, blocking de novo intracellular ergosterol biosynthesis. This is not observed for C. albicans.
Characterization of sterol import in later growth phases.
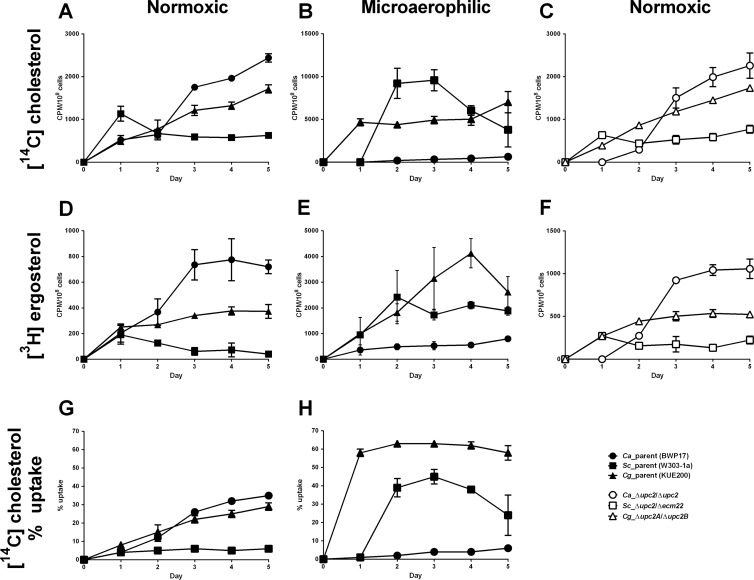
Interestingly, in some cases we saw an increase of sterol uptake with long-term cultivation under normoxic conditions. Thus, sterol import was analyzed over 5 days of growth both aerobically and microaerophilically and normalized to the cell count (Fig. 3A to F). In general, we see an increase of sterol import associated with aerobically grown C. albicans and C. glabrata cells, while for S. cerevisiae after 1 day, the amount of cell-associated sterols remain constant, or even decreases (Fig. 3A and D). This is the first direct evidence for C. albicans sterol import, which has not been previously described.
Fig 3.
Time course experiments of sterol uptake into C. albicans, S. cerevisiae, and C. glabrata. Cholesterol (A, B, and C) and ergosterol (D, E, and F) uptake by C. albicans (circles), S. cerevisiae (squares), and C. glabrata (triangles) under normoxic (A, C, D, and F) and microaerophilic (B and E) conditions. Parental strains are represented by solid symbols and UPC2-null mutants by open symbols. Strains were grown for 5 days in CSM complete supplemented with uridine, Tween 80, and a mix of cold and radioactively labeled cholesterol-ergosterol. Samples were analyzed every 24 h. The graphs represent average of three biological replicates with standard deviation. (G and H) Cholesterol uptake in three fungal species presented as a percentage of all available cholesterol taken up from the medium under normoxic (G) and microaerophilic (H) conditions. The graphs in panels G and H were created from data presented also in panels A and B.
Under microaerophilic conditions (Fig. 3B and E), S. cerevisiae and C. glabrata displayed rapid uptake of both sterols with saturation within 2 days. In some cases for these two fungi we observed a decrease in sterols associated with the cells after 4 or 5 days. We do not know the reason for this, but under these conditions sterols could be metabolized and the metabolic products secreted into the medium. In contrast to the other two fungal species, C. albicans shows minimum microaerophilic uptake of sterols, although the levels do increase slowly with time.
Because microaerophilic and normoxic growth lead to different efficiencies of carbon and nitrogen source utilization and each yeast species reaches different cell density under these conditions, we also present the percentage of cholesterol taken up from the medium (Fig. 3G and H).
The UPC2-null mutants of each of the three fungal species were also tested aerobically in order to determine whether aerobic sterol uptake in later growth phases is Upc2 dependent (Fig. 3C and F). The behavior of all UPC2 mutants was consistent with their respective parents. Due to a delay in growth, C. albicans shows a lag of 24 h before sterol import begins. Thus, sterol uptake in later growth phases is exclusive to the two fungal pathogens, C. albicans and C. glabrata, and is independent of the presence of Upc2.
Under normoxia, C. albicans and C. glabrata take cholesterol from the medium relatively slowly but with constant increases over time. These species might use sterols as a nonpreferable carbon source. On the other hand, microaerophilically S. cerevisiae and C. glabrata take up most of the available sterol in the media within 2 days. This termination of cholesterol uptake might happen when the cells run out of other nutrients in the medium and sterols are no longer needed for cell growth.
Esterification of radiolabeled sterols and uptake of fluorescently labeled sterol.
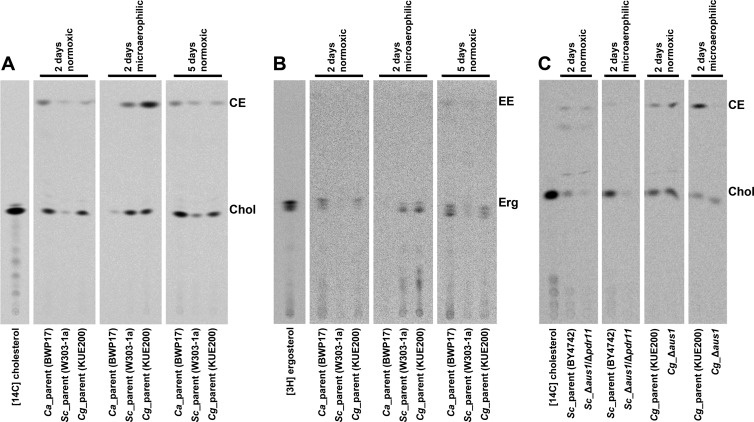
The radioactive sterols associated with the cells may have been actively taken up by the cell and incorporated to the plasma membrane, or they may just be bound to the cell wall. Recently, it was shown that the S. cerevisiae Δaus1/Δpdr11/Δhem1 mutant associates with significant amounts of dehydroergosterol. However, it remains bound to the cell wall and never gets incorporated in the plasma membrane (16). In order to exclude such effect, we grew the parental strains of C. albicans, S. cerevisiae, and C. glabrata under different conditions and in the presence of radioactively labeled cholesterol or ergosterol. Phospholipids and sterols were isolated from the cells and separated on TLC (Fig. 4). The proof of sterol membrane incorporation is their esterification inside the cells. This can be clearly demonstrated by [14C]cholesterol uptake (Fig. 4A).
Fig 4.
TLC separation of radioactively labeled cholesterol and ergosterol associated with the cells of C. albicans, S. cerevisiae, and C. glabrata. Set of parental strains of the three fungal species were compared to each other under normoxic (2 and 5 days) and microaerophilic (2 days) conditions in the presence of radioactively labeled cholesterol (A) or ergosterol (B). AUS1/PDR11 mutants were compared to their parents only in the presence of radioactively labeled cholesterol (C). Total cell lipids were extracted from the cells and separated by TLC. The samples represent the amount of cells grown in equal volume of culture medium. Chol, cholesterol; CE, cholesteryl ester; Erg, ergosterol; EE, ergosteryl ester.
Both C. albicans and C. glabrata take substantial amounts of radiolabeled cholesterol under normoxic conditions (Chol in Fig. 4A), and some of that labeled cholesterol is esterified (CE in Fig. 4A), which is strong evidence for sterol import. The amounts taken by S. cerevisiae are significantly reduced under these conditions. For all three species, the Chol and CE bands are increased after 5 days of normoxic growth.
On the other hand, under microaerophilic conditions, only S. cerevisiae and C. glabrata take up significant amounts of radiolabeled cholesterol and both of them esterify a portion of it. Very little Chol or CE is observed for C. albicans. These findings are consistent with the earlier assays characterizing each species (Fig. 1A and 3), which show that C. albicans has reduced import of sterols microaerophilically.
Ergosterol, on the other hand, is poorly esterified after uptake for any species (Fig. 4B). However, the import characteristics of the unmodified ergosterol, in terms of the amounts associated with each fungal species, are consistent with the cholesterol levels. Exogenous ergosterol, in comparison to cholesterol, is known to be poorly esterified in S. cerevisiae (18, 26). This appears to be true for all three fungal species.
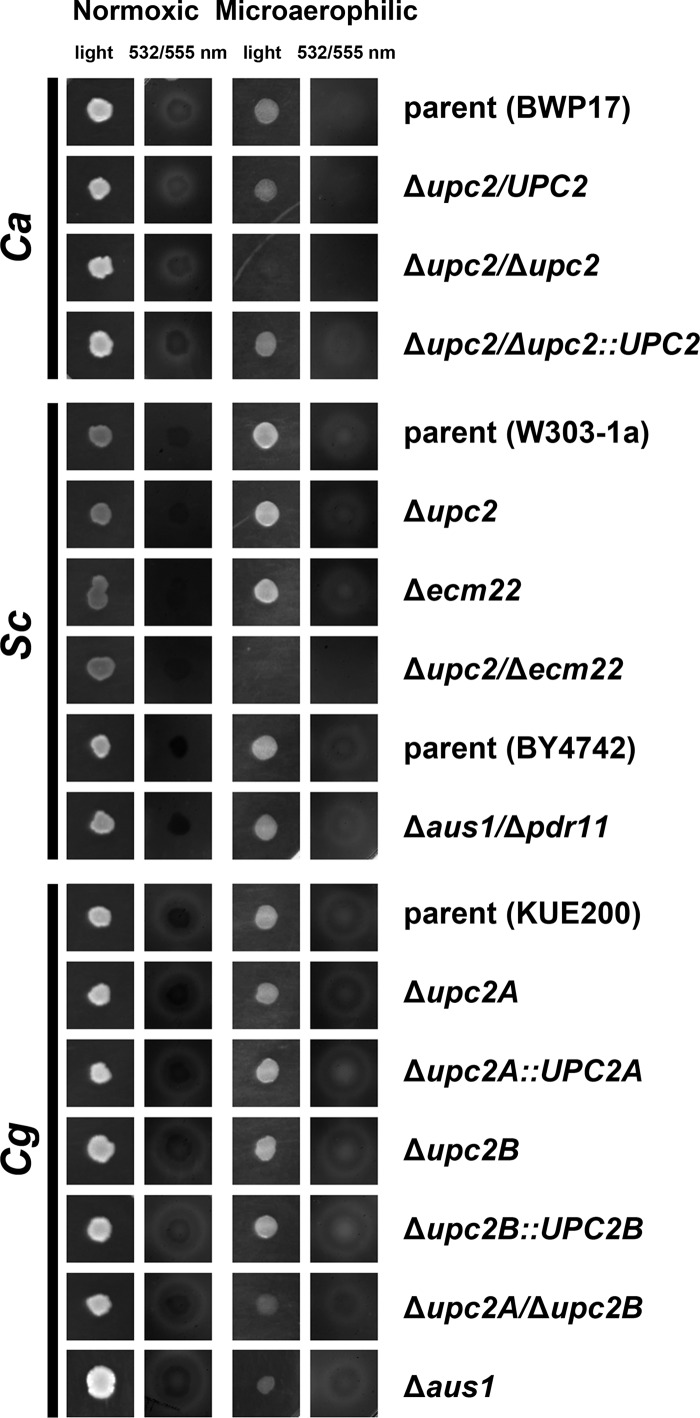
Another method to show sterol import is to monitor the uptake of fluorescently labeled sterol available in the agar media (Fig. 5). Cell cultures were spotted on the CSM complete agar plates supplemented with uridine and cholesteryl-BODIPY, which is fluorescently labeled cholesterol. The cells were grown for 48 h under normoxic and microaerophilic conditions. The uptake of the fluorescently labeled sterol was indicated by zone of clearing in close proximity of the colony. This study characterizes uptake of sterols by the cells, rather than their fate within the cell. As proposed previously (16), before incorporation to the PM the sterols seem to be bound to the cell wall first. Thus, in this assay we cannot distinguish whether the sterols were incorporated into the PM or are just bound to the cell wall.
Fig 5.
Uptake of fluorescently labeled cholesterol from agar medium. Cell cultures of C. albicans, S. cerevisiae, and C. glabrata strains spotted on CSM complete supplemented with uridine and cholesteryl-BODIPY (fluorescently labeled cholesterol) were incubated for 2 days under normoxic and microaerophilic conditions and scanned. The zone of clearance (white area) around colonies is indicative of decreased local concentration of cholesteryl-BODIPY and thus active uptake of this sterol. S. cerevisiae parental strains differ for the UPC2 mutants (W303-1a) and AUS1/PDR11 mutant (BY4742) and thus are presented both separately with the appropriate mutants.
As expected for S. cerevisiae grown in normoxia, no evidence for the sterol uptake can be seen. On the other hand, under microaerophilic conditions, the S. cerevisiae parental strain and the S. cerevisiae Δupc2 and Δecm22 single mutants are capable of sterol uptake from the medium. The anaerobically nonviable S. cerevisiae Δupc2/Δecm22 double mutant displays no uptake.
In C. glabrata, we see sterol uptake in all strains studied, both in aerobic and microaerophilic conditions. This was quite surprising for the C. glabrata Δupc2A/Δupc2B double mutant under microaerophilic conditions, where the cells grow very poorly. C. glabrata appears to be the most tolerant species to the low oxygen levels, and the double mutant could probably undergo a limited number of divisions on the agar medium before all of the available oxygen was removed by the anaerobic pouch. The same strain under microaerophilic conditions displayed no measurable growth in liquid medium.
C. albicans displays sterol uptake under normoxic conditions and reduced levels under microaerophilic conditions, which is in agreement with previous experiments (Fig. 1A and 3). As expected, the C. albicans Δupc2/Δupc2 mutant displays no microaerophilic growth, and thus no uptake can be observed, while under normoxic conditions it does not differ from the parental strain, since it is capable of sterol import in later growth stages (Fig. 3C and F).
Effect of fluconazole and fetal bovine serum on sterol import.
In S. cerevisiae, the depletion of ergosterol in the fungal membranes is thought to activate the Upc2-dependent pathway and induce de novo sterol synthesis under normoxic conditions or sterol uptake under hypoxia. Drugs such as azoles block ergosterol biosynthesis and activate Upc2 (46). For this reason, we focused on fluconazole and its possible role in sterol uptake in the three fungal species. Fetal bovine serum (FBS) and bile have also been described as substrates that may induce sterol uptake in C. glabrata (28). Thus, we analyzed these compounds and their relationship to sterol uptake in the three fungal species.
We tested the parental strains of all three species and used unsupplemented medium, medium supplemented with fluconazole (an MIC80 for BWP17 of 1 μg/ml, an MIC80 for W303-1a of 32 μg/ml, and an MIC80 for KUE200 of 512 μg/ml), 10% FBS, or both (10% FBS and fluconazole [FLC] MIC80s) (Fig. 1B). In several cases, there is a small decrease in sterol import in media containing FBS (aerobically and microaerophilically grown C. albicans, and aerobically grown S. cerevisiae and C. glabrata). This is probably caused by the presence of additional cholesterol in the FBS and thus a dilution of the specific activity of [14C]cholesterol.
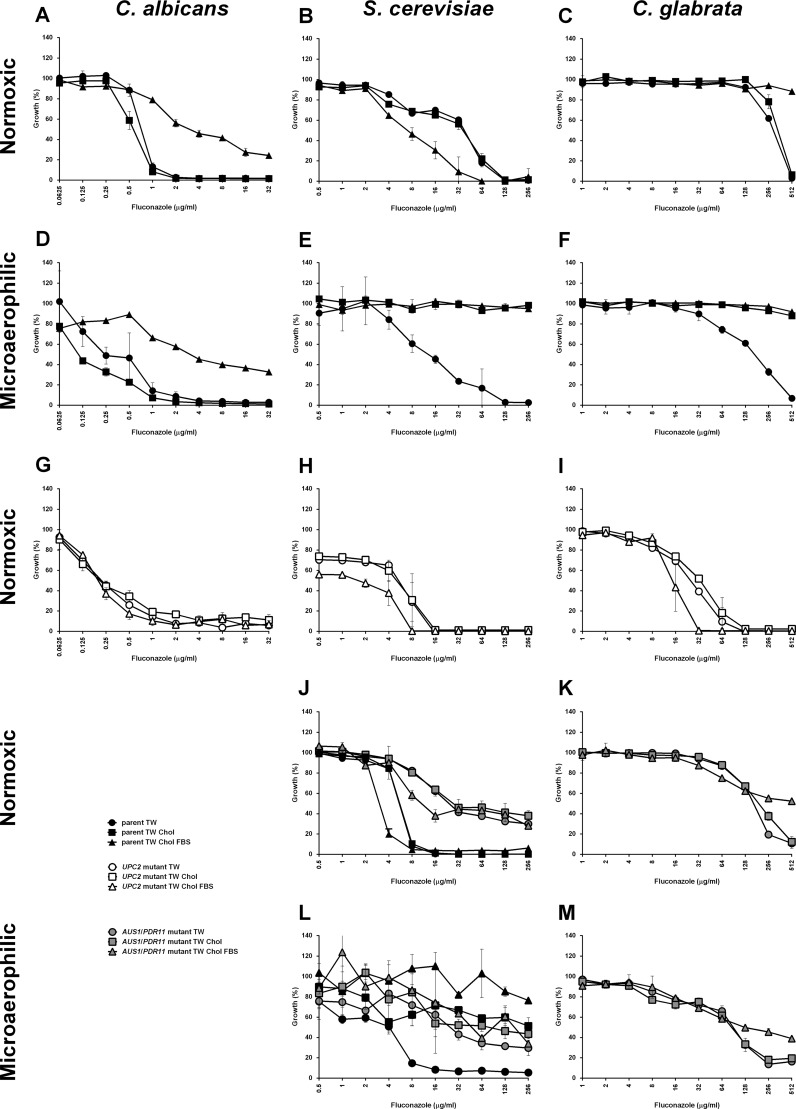
In C. albicans, there is little effect on sterol uptake by serum or fluconazole. S. cerevisiae responds to the presence of fluconazole in normoxic condition by increased sterol uptake in the absence or presence of serum. However, serum itself has no stimulating effect on S. cerevisiae (compared to medium without supplements, FLC increases cholesterol uptake 8.3-fold, P < 0.01, FBS decreases it 3.6-fold, P < 0.01 and for FLC plus FBS increase 16.0-fold, P = 0.01). In the case of FLC-stimulated sterol uptake in S. cerevisiae, this increase does not have any effect on the strain's MIC (Fig. 6B). When S. cerevisiae is grown anaerobically, it imports sterols from all of the media.
Fig 6.
MICs determined for C. albicans, S. cerevisiae, and C. glabrata. MICs determined under normoxic (A, B, C, G, H, I, J, and K) and microaerophilic (D, E, F, L, and M) conditions for C. albicans (A, D, and G), S. cerevisiae (B, E, H, J, and L), and C. glabrata (C, F, I, K, and M). Parental strains (CaBWP17, ScW303-1a/ScBY4742, and CgKUE200) are represented by black solid symbols, UPC2-null mutants (CaΔupc2/Δupc2, ScΔupc2/Δecm22, and CgΔupc2A/Δupc2B) by open symbols and AUS1/PDR11 mutants (ScΔaus1/Δpdr11 and CgΔaus1) by gray solid symbols. Circles represent medium supplemented with Tween 80, squares represent medium supplemented with Tween 80-cholesterol, and triangles represent medium supplemented with Tween 80-cholesterol-FBS. S. cerevisiae parental strains differ for the UPC2 mutants (W303-1a; graphs B, E, and H) and AUS1/PDR11 mutant (BY4742; graphs J and L) and thus are presented both in separate sections of the figure. Parental strain BY4742 is presented together with its ScΔaus1/Δpdr11 derivative (J and L). Fluconazole concentrations did not exceed 256 μg/ml, with the exception of 2-fold increase for C. glabrata, in order to prevent reaching of critical micelle concentration for this compound. Graphs represent the average of three biological replicates with standard errors.
C. glabrata does not respond under normoxic conditions to the presence of serum or fluconazole if supplemented separately (compared to unsupplemented medium, no statistically significant change with FLC alone, P > 0.05, FBS does downregulate the uptake 3.2-fold, P < 0.01). Aerobically, however, the presence of serum and fluconazole together induces sterol uptake (5.1-fold, P < 0.01). Anaerobically, C. glabrata behaves similarly to S. cerevisiae and takes up sterols regardless of other media supplements.
To complement these uptake studies, we determined the MICs of all three species under normoxic and hypoxic conditions in the presence of Tween 80, Tween 80-cholesterol, and Tween 80-cholesterol-FBS (Fig. 6), using both the parental strains and strains deleted for UPC2 (and its paralogs). For the C. albicans parental strain, there is no significant effect of sterol supplementation on the MIC under normoxic or hypoxic conditions. However, supplementation with FBS does increase the strain's resistance under both conditions (Fig. 6A and D). This is surprising, since we do not see any significant increase in sterol uptake in the presence of serum (Fig. 1B). This does not seem to be an effect of the nutrient components of the medium that would allow better growth but is likely related to the presence of CaUPC2, since this growth advantage is lost in the C. albicans Δupc2 mutant (Fig. 6G).
Under normoxic conditions S. cerevisiae does not increase resistance in the presence of serum, as seen for C. albicans, but rather serum reduces resistance, both for the parental strain and S. cerevisiae Δupc2/Δecm22 mutant (Fig. 6B and H). Under microaerophilic conditions, the S. cerevisiae wild-type becomes more resistant to fluconazole in the presence of cholesterol (Fig. 6E), with no additional effect due to supplementation with serum.
C. glabrata behaves as described previously (28). In normoxic conditions the parental strain is able to overcome ergosterol biosynthesis block caused by fluconazole only in the presence of serum, while the supplementation with cholesterol does not provide any advantage (Fig. 6C). This effect, similar to the effect seen with C. albicans, is eliminated in the C. glabrata Δupc2A/Δupc2B mutant, showing that the effect is Upc2 related (Fig. 6I). However, in C. glabrata wild-type, we can link the rescue of growth to the active sterol uptake. Under microaerophilic conditions C. glabrata takes up cholesterol regardless of the presence of serum (Fig. 6F). Surprisingly, in the MIC assays, S. cerevisiae and C. glabrata are able to grow under microaerophilic conditions in medium containing no cholesterol. It is likely that the environment is not completely without oxygen and low oxygen levels allows these strains to synthesize small amounts of their own ergosterol. The other required anaerobic supplement, oleic acid, is present as Tween 80, which has no effect on the microaerophilic MICs compared to the normoxic values.
Characterization of S. cerevisiae Δaus1/Δpdr11 and C. glabrata Δaus1 strains.
Two S. cerevisiae ABC transporter paralogs, ScAus1 and ScPdr11, were characterized previously (16–18, 23) and described as responsible for sterol import into fungal cells. Unfortunately, most of the work with these proteins was done in strain backgrounds deleted for ScHEM1, a gene whose deletion turns on a hypoxic response under normoxic conditions and thus not representing true hypoxia. We characterized S. cerevisiae Δaus1/Δpdr11 mutant under normoxic and microaerophilic conditions. In addition, we characterized the C. glabrata Δaus1 mutant, deleted for the only known homolog of the ScAus1/ScPdr11 ABC sterol transporters. No homolog has been identified in C. albicans.
We determined the cholesterol/ergosterol import into the S. cerevisiae Δaus1/Δpdr11 and C. glabrata Δaus1 deletion strains (Fig. 1C). In S. cerevisiae, we observed a decrease in cholesterol and ergosterol uptake in the S. cerevisiae Δaus1/Δpdr11 mutant under both normoxic and microaerophilic conditions. In C. glabrata, deletion of CgAUS1 under microaerophilic conditions exhibited a decrease in cholesterol uptake, but not ergosterol uptake (compared to the parent in hypoxia, for cholesterol, a 3.4-fold decrease [P < 0.01], and for ergosterol, a 1.2-fold decrease [P = 0.02]). In normoxia, the amount of cholesterol or ergosterol taken up from the medium by C. glabrata Δaus1 mutant actually slightly increased (1.9-fold for cholesterol [P < 0.01] and 1.4-fold for ergosterol [P = 0.01]).
We also checked esterification of the transported cholesterol (Fig. 4C), an indication of its incorporation into the PM. Consistent with the uptake studies, the amount of cholesterol taken up by the S. cerevisiae Δaus1/Δpdr11 mutant decreased, both under normoxic and microaerophilic conditions. Under normoxic conditions, a fraction of the cholesterol imported by the S. cerevisiae Δaus1/Δpdr11 mutant was still esterified (28%). In addition, there are two additional bands on the TLC, which are probably minor forms of chemically modified cholesterol, e.g., acetylated cholesterol. Under microaerophilic conditions, there was no evidence for cholesterol esterification for the S. cerevisiae Δaus1/Δpdr11 mutant, although the amount of uptake is very low.
In C. glabrata, normoxic cholesterol uptake and esterification were clearly evident. The C. glabrata Δaus1 mutant displayed no decrease in cholesterol uptake under normoxic conditions, and esterified this sterol extensively (30% compared to 22% in the parent), while under microaerophilic conditions, there is no evidence for esterified cholesterol (compared to 68% in parent), and the unmodified cholesterol levels are comparable to those for the parent.
Using drop tests (Fig. 2), we could see a decrease in microaerophilic growth for both S. cerevisiae Δaus1/Δpdr11 and C. glabrata Δaus1 mutants. However, S. cerevisiae parental strain BY4742 (S288C derived) displays worse microaerophilic growth in the presence of sterols than the other parent, W303-1a, background for the ScUPC2 and ScECM22 deletions. The strain S288C grows worse under microaerophilic conditions, because it has a Ty1 insertion in the carboxy terminus of ScHAP1 gene, a transcription factor important for the regulation of genes dependent on heme/oxygen levels (47).
MICs of S. cerevisiae Δaus1/Δpdr11 and C. glabrata Δaus1 mutants in presence or absence of cholesterol and serum were also checked (Fig. 6J, K, L, and M). Surprisingly, the deletion of ScAUS1 and ScPDR11 in S. cerevisiae led to tolerance of high fluconazole concentrations under normoxic and microaerophilic conditions, regardless of the supplements. However, this strain does not grow on agar media supplemented with sterols (Fig. 2). This is again most likely related to low oxygen levels available in the liquid medium. Deletion of CgAUS1 in C. glabrata led to more predictable results. Under microaerophilic condition, there is no positive effect of supplemented cholesterol on growth, and also the effect of FBS supplementation seems to be somewhat reduced, although it still provides little growth advantage.
Last, when sterol import of the fluorescently labeled cholesterol was investigated using these strains (S. cerevisiae Δaus1/Δpdr11 and C. glabrata Δaus1), there was no change from their parents under normoxic or microaerophilic conditions (Fig. 5). Thus, it seems that the collecting of the fluorescently labeled sterol from agar medium in the colony proximity is not affected by the presence of AUS1/PDR11 in these two species.
DISCUSSION
In this study, we have characterized sterol import in three fungal species: C. albicans, S. cerevisiae, and C. glabrata. We have used radioactively labeled versions of the fungal sterol, ergosterol, as well as the major sterol in higher eukaryotes, cholesterol. In general, for all three fungal species, we have not seen any clear difference in import of these two sterols. However, we observed their different fates within the cells. In S. cerevisiae, cholesterol gets extensively esterified, while ergosterol remains mostly intact (18, 26). We have confirmed this observation in S. cerevisiae, and expanded this analysis to two additional fungi, C. albicans and C. glabrata. In addition, esterification of cholesterol proves that these sterols are incorporated into the membranes, and not bound to the cell wall unmodified, as reported previously for some cases (16). Overall, growth and sterol uptake under all tested conditions are summarized in Table 2.
Table 2.
Sterol uptake overview
| Conditiona | Sterol uptake |
||
|---|---|---|---|
| S. cerevisiae | C. albicans | C. glabrata | |
| Aerobic exclusion | ± | – | – |
| Anaerobic exclusion | – | + | – |
| Tween 80 supports microaerophilic growth | ± | – | ± |
| Tween 80 and sterols support microaerophilic growth | + | – | + |
| Sterol supplement overcomes FLC inhibition microaerophilically | + | – | + |
| FBS stimulates sterol uptake | – | – | – |
| FLC stimulates sterol uptake | + | – | – |
| FBS plus FLC stimulates sterol uptake | + | – | + |
| Anaerobic growth of UPC2 mutant | – | – | – |
| Upc2 involved in serum-stimulated sterol uptake in the presence of FLC | – | – | + |
| Upc2 involved in normoxic post-exponential-phase sterol uptake | – | – | – |
| Aus1/Pdr11 required for aerobic sterol uptake* | + | NHb | – |
| Aus1/Pdr11 required for microaerophilic sterol uptake* | + | NH | + |
FBS, fetal bovine serum; FLC, fluconazole. *, In S. cerevisiae both ScAus1 and ScPdr11 paralogs are required, while in C. glabrata, only one ortholog, CgAus1, is present.
NH, no homolog.
S. cerevisiae is the best-characterized species in terms of sterol import of these three fungi. S. cerevisiae takes up sterols exclusively under hypoxic conditions and not under normoxia. This is known as “aerobic sterol exclusion” (12). The uptake of sterols under hypoxia is the only option due to the inability of S. cerevisiae to synthesize ergosterol in the absence of molecular oxygen and the inability to grow without sterols present in its membranes. However, we do see limited amounts of aerobically imported sterols during the initial 24 h of growth, with evidence of esterification for cholesterol. When we tested sterol import in later growth stages in normoxia, we did not seen any increase in sterol uptake over time, which is in agreement with previous studies (15). Interestingly, we saw a slight stimulation of sterol import in S. cerevisiae in the presence of fluconazole. This is in agreement with the finding that treatment of S. cerevisiae with lovastatin also increases cholesterol import and blocks its esterification (13). However, the sterol import we observed was insufficient to substitute for endogenous ergosterol biosynthesis or alter the strain MIC in the presence or absence of sterols.
The second organism, C. glabrata, is often discussed in the literature as an organism with low fluconazole susceptibility due to active sterol import. However, its low susceptibility is demonstrated even in media without sterols available. Under hypoxia, C. glabrata behaves similar to S. cerevisiae and takes up exogenous sterols, because it has the same need for hypoxic supplements. On the other hand, C. glabrata is capable of slow sterol import under normoxic conditions, mostly in the postexponential phases of growth. When grown in the presence of fluconazole and/or exogenous sterols in liquid medium, no increase in sterol uptake can be detected, as described previously (28). Moreover, although the sterol transporter CgAUS1 was described to be transcriptionally induced by the presence of serum in the liquid medium within a matter of hours (28), we did not observe any alteration in sterol import. Only when serum was present together with high concentrations of fluconazole was sterol import induced. For the induction of aerobic sterol uptake, C. glabrata appears to require both a block in ergosterol biosynthesis and the presence of serum. We have some indications that this induction of sterol uptake is not limited to serum or bile but could also be promoted in media containing peptone, such as YEPD (data not shown). The nature of the inducing compound has not yet been identified.
In C. albicans grown under normoxic conditions, there is a clear increase in sterol uptake over time, with evidence of esterification. Therefore, it is successfully incorporated in the membranes and reaches the ER. When grown under microaerophilic conditions, little sterol uptake is seen. We suggest that this is “anaerobic sterol exclusion,” the opposite of aerobic sterol exclusion as seen in S. cerevisiae. The growth of C. albicans in the presence of high fluconazole levels is increased when the liquid media is supplemented with FBS and is dependent on Upc2. Despite this increase in growth, there is no evidence for increased sterol uptake. Interestingly, the growth of C. albicans on agar media is severely reduced under microaerophilic conditions, regardless of the medium supplements, suggesting significant differences between growth in liquid and on solid supports. This is probably caused by the rate of oxygen level decrease in air versus liquid. We have also checked sterol import in C. albicans hypha-stage-locked mutants Canrg1 and Catup1. We do not see any alterations in sterol import for these mutants (data not shown).
For all three species, the UPC2 genes are linked to sterol uptake. In S. cerevisiae ScUPC2 transcription is induced by treatment with statins or azoles and by growth in microaerophilic conditions (40, 46, 48). Constitutively active mutants in S. cerevisiae can promote sterol uptake (20, 21, 40, 41). In C. albicans, the deletion of both UPC2 alleles results in reduced normoxic cholesterol uptake in RPMI medium (43), although we have not observed this for CSM in the present study. Moreover, in C. glabrata both the presence of serum and ergosterol depletion caused by lovastatin induce CgUPC2B expression (36). Deletion of UPC2 and its paralogs results in anaerobic inviability in all three organisms. However, it has not been determined that the lack of anaerobic growth is solely due to impaired sterol import. In support of this, C. albicans wild-type does not appear to depend on sterol import under microaerophilic conditions, and AUS1/PDR11 mutants of S. cerevisiae and C. glabrata seem to be microaerophilically viable.
By characterizing S. cerevisiae Δaus1 Δpdr11 and C. glabrata Δaus1 mutants, we confirmed the importance of these ABC transporters for sterol uptake. In S. cerevisiae the deletion of the AUS1 and PDR11 genes negatively affected sterol uptake under both normoxic and microaerophilic conditions. However, in C. glabrata, the deletion of AUS1 only had an effect on the uptake of cholesterol under microaerophilic conditions. The deletion of AUS1 and PDR11 in S. cerevisiae has also an interesting effect; it improves growth under high fluconazole concentrations. We can only speculate on this. The partial intracellular localization of ScAus1 (18) may have an effect on membrane composition and sterol distribution within cells. Overall, this phenomenon remains to be properly characterized. The deletion of AUS1 and PDR11, both in S. cerevisiae and C. glabrata, does not negatively affect their microaerophilic growth in medium supplemented with Tween 80 and sterols. It is possible that the uptake of unsaturated fatty acids (Tween 80) and low oxygen levels allow the synthesis of some ergosterol, sufficient for their growth. However, the oxygen levels may not be enough to allow for growth of the UPC2 mutants. We can speculate that at least in C. glabrata there is an alternative method of normoxic sterol import. This might be also the case in C. albicans, which is also capable of normoxic sterol import, although it has no ScAUS1/ScPDR11 homolog.
Compared to S. cerevisiae (20), constitutively active mutation of Upc2 in C. albicans increases the strains MIC (49), but it does not lead to an increase of sterol import (our unpublished observation). Moreover, the deletion of HEM1 in C. albicans does not promote sterol uptake as in S. cerevisiae and C. glabrata (27, 50). Sterol uptake in S. cerevisiae and C. glabrata under these situations is most likely linked to the Aus1/Pdr11 proteins, and the absence of the ortholog in C. albicans may be responsible for its missing sterol uptake under these conditions. On the other hand, C. albicans and C. glabrata can take up significant amounts of sterol under normoxia, even in the absence of Aus1/Pdr11 orthologs. Passive diffusion is not likely, since it is not observed in S. cerevisiae (Fig. 3). These organisms, unlike S. cerevisiae, accumulate increasing amounts of sterols as the growth of their culture progresses. The amount of sterols taken up after 5 days of cultivation is significant, ca. 30%, while in case of S. cerevisiae it is 6%. This suggests that sterols may be imported by facilitated diffusion through an as yet unidentified transporter. There is evidence that both organisms, C. albicans and C. glabrata, “collect” sterols from agar medium in their proximity under normoxic conditions, whereas S. cerevisiae does not. Overall, this area of transport remains yet to be explored.
It is still unclear how C. albicans grows under microaerophilic or anaerobic conditions. Anaerobic growth has been partially characterized (33) and the authors speculated that the early oxygen-dependent step in ergosterol biosynthesis, squalene epoxidation, might use oxygen from water. However, the significantly decreased susceptibility of anaerobically grown C. albicans to both azoles and polyenes in their work indicate decreased levels of synthesis or complete absence of ergosterol in membranes. Unfortunately, the ergosterol levels of anaerobically grown C. albicans have never been determined. In our microaerophilic system, we could see a decrease in ergosterol levels by >50% (data not shown). However, the growth under true anaerobic conditions still could lead to total absence of ergosterol or alteration of its synthesis.
This study brings new understanding of the response of two pathogenic fungi, C. albicans and C. glabrata, to treatment with drugs affecting sterol biosynthesis, the azoles. Although clinically this class of drugs works well against C. albicans, their effect against C. glabrata is limited. The explanation would be that while C. albicans relies mostly on internal ergosterol biosynthesis, which can be negatively affected by azole treatment, C. glabrata, besides its high azole resistance, can overcome the block in ergosterol biosynthesis by active transport of exogenous sterols. Although azoles work against C. glabrata in vitro, their importance in vivo seems to be limited, since human body provides conditions favoring the stimulation of sterol import from the fungal environment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Koichi Tanabe for the kind providing of C. glabrata strains, Jasper Rine for the S. cerevisiae UPC2 and ECM22 mutants, and Roger Schneiter for the S. cerevisiae aus1/pdr11 mutant.
This research was funded by National Institutes of Health NIDCR grants R01 DE11367, R01 DE14161, and RO1 DE017078, as well as Unrestricted Research Funds from the School of Biological Sciences, University of Missouri at Kansas City.
Footnotes
Published ahead of print 8 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00345-12.
REFERENCES
- 1. Fidel PL, Jr, Vazquez JA, Sobel JD. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacquier N, Schneiter R. 2012. Mechanisms of sterol uptake and transport in yeast. J. Steroid Biochem. Mol. Biol. 129:70–78 [DOI] [PubMed] [Google Scholar]
- 3. Raychaudhuri S, Prinz WA. 2006. Uptake and trafficking of exogenous sterols in Saccharomyces cerevisiae. Biochem. Soc. Trans. 34:359–362 [DOI] [PubMed] [Google Scholar]
- 4. Schneiter R. 2007. Intracellular sterol transport in eukaryotes, a connection to mitochondrial function? Biochimie 89:255–259 [DOI] [PubMed] [Google Scholar]
- 5. Schneiter R, Brugger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G, Paltauf F, Wieland FT, Kohlwein SD. 1999. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 146:741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahl C, Biemann HP, Dahl J. 1987. A protein kinase antigenically related to pp60v-src possibly involved in yeast cell cycle control: positive in vivo regulation by sterol. Proc. Natl. Acad. Sci. U. S. A. 84:4012–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez RJ, Parks LW. 1983. Structural and physiological features of sterols necessary to satisfy bulk membrane and sparking requirements in yeast sterol auxotrophs. Arch. Biochem. Biophys. 225:861–871 [DOI] [PubMed] [Google Scholar]
- 8. Zhang L, Hach A. 1999. Molecular mechanism of heme signaling in yeast: the transcriptional activator HapI serves as the key mediator. Cell. Mol. Life Sci. 56:415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andreasen AA, Stier TJ. 1953. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell Physiol. 41:23–36 [DOI] [PubMed] [Google Scholar]
- 10. Andreasen AA, Stier TJ. 1954. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J. Cell Physiol. 43:271–281 [DOI] [PubMed] [Google Scholar]
- 11. Lewis TA, Taylor FR, Parks LW. 1985. Involvement of heme biosynthesis in control of sterol uptake by Saccharomyces cerevisiae. J. Bacteriol. 163:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenz RT, Parks LW. 1987. Regulation of ergosterol biosynthesis and sterol uptake in a sterol-auxotrophic yeast. J. Bacteriol. 169:3707–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorenz RT, Parks LW. 1990. Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 34:1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lorenz RT, Parks LW. 1991. Physiological effects of fenpropimorph on wild-type Saccharomyces cerevisiae and fenpropimorph-resistant mutants. Antimicrob. Agents Chemother. 35:1532–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenz RT, Rodriguez RJ, Lewis TA, Parks LW. 1986. Characteristics of sterol uptake in Saccharomyces cerevisiae. J. Bacteriol. 167:981–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kohut P, Wustner D, Hronska L, Kuchler K, Hapala I, Valachovic M. 2010. The role of ABC proteins Aus1p and Pdr11p in the uptake of external sterols in yeast: dehydroergosterol fluorescence study. Biochem. Biophys. Res. Commun. 404:233–238 [DOI] [PubMed] [Google Scholar]
- 17. Wilcox LJ, Balderes DA, Wharton B, Tinkelenberg AH, Rao G, Sturley SL. 2002. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J. Biol. Chem. 277:32466–32472 [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Prinz WA. 2004. ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J. Biol. Chem. 279:45226–45234 [DOI] [PubMed] [Google Scholar]
- 19. Kwast KE, Lai LC, Menda N, James DT, III, Aref S, Burke PV. 2002. Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: functional roles of Rox1 and other factors in mediating the anoxic response. J. Bacteriol. 184:250–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crowley JH, Leak FW, Jr, Shianna KV, Tove S, Parks LW. 1998. A mutation in a purported regulatory gene affects control of sterol uptake in Saccharomyces cerevisiae. J. Bacteriol. 180:4177–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis TL, Keesler GA, Fenner GP, Parks LW. 1988. Pleiotropic mutations in Saccharomyces cerevisiae affecting sterol uptake and metabolism. Yeast 4:93–106 [DOI] [PubMed] [Google Scholar]
- 22. Bottema CD, Rodriguez RJ, Parks LW. 1985. Influence of sterol structure on yeast plasma membrane properties. Biochim. Biophys. Acta 813:313–320 [DOI] [PubMed] [Google Scholar]
- 23. Reiner S, Micolod D, Zellnig G, Schneiter R. 2006. A genomewide screen reveals a role of mitochondria in anaerobic uptake of sterols in yeast. Mol. Biol. Cell 17:90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alimardani P, Regnacq M, Moreau-Vauzelle C, Ferreira T, Rossignol T, Blondin B, Berges T. 2004. SUT1-promoted sterol uptake involves the ABC transporter Aus1 and the mannoprotein Dan1 whose synergistic action is sufficient for this process. Biochem. J. 381:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zweytick D, Leitner E, Kohlwein SD, Yu C, Rothblatt J, Daum G. 2000. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 267:1075–1082 [DOI] [PubMed] [Google Scholar]
- 26. Valachovic M, Hronska L, Hapala I. 2001. Anaerobiosis induces complex changes in sterol esterification pattern in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Lett. 197:41–45 [DOI] [PubMed] [Google Scholar]
- 27. Bard M, Sturm AM, Pierson CA, Brown S, Rogers KM, Nabinger S, Eckstein J, Barbuch R, Lees ND, Howell SA, Hazen KC. 2005. Sterol uptake in Candida glabrata: rescue of sterol auxotrophic strains. Diagn. Microbiol. Infect. Dis. 52:285–293 [DOI] [PubMed] [Google Scholar]
- 28. Nakayama H, Tanabe K, Bard M, Hodgson W, Wu S, Takemori D, Aoyama T, Kumaraswami NS, Metzler L, Takano Y, Chibana H, Niimi M. 2007. The Candida glabrata putative sterol transporter gene CgAUS1 protects cells against azoles in the presence of serum. J. Antimicrob. Chemother. 60:1264–1272 [DOI] [PubMed] [Google Scholar]
- 29. Nakayama H, Izuta M, Nakayama N, Arisawa M, Aoki Y. 2000. Depletion of the squalene synthase (ERG9) gene does not impair growth of Candida glabrata in mice. Antimicrob. Agents Chemother. 44:2411–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakayama H, Nakayama N, Arisawa M, Aoki Y. 2001. In vitro and in vivo effects of 14α-demethylase (ERG11) depletion in Candida glabrata. Antimicrob. Agents Chemother. 45:3037–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurtz MB, Marrinan J. 1989. Isolation of hem3 mutants from Candida albicans by sequential gene disruption. Mol. Gen. Genet. 217:47–52 [DOI] [PubMed] [Google Scholar]
- 32. Tsai HF, Bard M, Izumikawa K, Krol AA, Sturm AM, Culbertson NT, Pierson CA, Bennett JE. 2004. Candida glabrata erg1 mutant with increased sensitivity to azoles and to low oxygen tension. Antimicrob. Agents Chemother. 48:2483–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dumitru R, Hornby JM, Nickerson KW. 2004. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 48:2350–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bard M, Lees ND, Barbuch RJ, Sanglard D. 1987. Characterization of a cytochrome P450 deficient mutant of Candida albicans. Biochem. Biophys. Res. Commun. 147:794–800 [DOI] [PubMed] [Google Scholar]
- 35. Mas J, Pina E. 1980. Disappearance of nystatin resistance in Candida mediated by ergosterol. J. Gen. Microbiol. 117:249–252 [DOI] [PubMed] [Google Scholar]
- 36. Nagi M, Nakayama H, Tanabe K, Bard M, Aoyama T, Okano M, Higashi S, Ueno K, Chibana H, Niimi M, Yamagoe S, Umeyama T, Kajiwara S, Ohno H, Miyazaki Y. 2011. Transcription factors CgUPC2A and CgUPC2B regulate ergosterol biosynthetic genes in Candida glabrata. Genes Cells 16:80–89 [DOI] [PubMed] [Google Scholar]
- 37. Reuss O, Vik A, Kolter R, Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 38. Gietz RD, Woods RA. 2006. Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol. Biol. 313:107–120 [DOI] [PubMed] [Google Scholar]
- 39. Schneiter R, Daum G. 2006. Analysis of yeast lipids. Methods Mol. Biol. 313:75–84 [DOI] [PubMed] [Google Scholar]
- 40. Davies BS, Wang HS, Rine J. 2005. Dual activators of the sterol biosynthetic pathway of Saccharomyces cerevisiae: similar activation/regulatory domains but different response mechanisms. Mol. Cell. Biol. 25:7375–7385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shianna KV, Dotson WD, Tove S, Parks LW. 2001. Identification of a UPC2 homolog in Saccharomyces cerevisiae and its involvement in aerobic sterol uptake. J. Bacteriol. 183:830–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. MacPherson S, Akache B, Weber S, De Deken X, Raymond M, Turcotte B. 2005. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 49:1745–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silver PM, Oliver BG, White TC. 2004. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 3:1391–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vik A, Rine J. 2001. Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:6395–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mansfield BE, Oltean HN, Oliver BG, Hoot SJ, Leyde SE, Hedstrom L, White TC. 2010. Azole drugs are imported by facilitated diffusion in Candida albicans and other pathogenic fungi. PLoS Pathog. 6:e1001126 doi:10.1371/journal.ppat.1001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davies BS, Rine J. 2006. A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics 174:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaisne M, Becam AM, Verdiere J, Herbert CJ. 1999. A ‘natural’ mutation in Saccharomyces cerevisiae strains derived from S288c affects the complex regulatory gene HAP1 (CYP1). Curr. Genet. 36:195–200 [DOI] [PubMed] [Google Scholar]
- 48. Abramova NE, Cohen BD, Sertil O, Kapoor R, Davies KJ, Lowry CV. 2001. Regulatory mechanisms controlling expression of the DAN/TIR mannoprotein genes during anaerobic remodeling of the cell wall in Saccharomyces cerevisiae. Genetics 157:1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoot SJ, Smith AR, Brown RP, White TC. 2011. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 55:940–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gollub EG, Liu KP, Dayan J, Adlersberg M, Sprinson DB. 1977. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J. Biol. Chem. 252:2846–2854 [PubMed] [Google Scholar]
- 51. Wilson RB, Davis D, Mitchell AP. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115–132 [DOI] [PubMed] [Google Scholar]
- 53. Ueno K, Uno J, Nakayama H, Sasamoto K, Mikami Y, Chibana H. 2007. Development of a highly efficient gene targeting system induced by transient repression of YKU80 expression in Candida glabrata. Eukaryot. Cell 6:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.