Abstract
Frontotemporal lobar degeneration (FTLD), a neurodegenerative disease primarily affecting the frontal and temporal lobes, is one of the most common types of dementia. While the majority of FTLD cases are sporadic, approximately 10–40% of patients have an inherited form of FTLD. Mutations in the progranulin gene (GRN) have recently been identified as a major cause of FTLD with ubiquitin positive inclusions (FTLD-U). Because over 70 disease-linked GRN mutations cause abnormal deficiencies in the production of PGRN, a protein that plays a crucial role in embryogenesis, cell growth and survival, as well as wound repair and inflammation, researchers now aim to design therapies that would increase PGRN levels in affected individuals, thereby alleviating the symptoms associated with disease. Several compounds and genetic factors, as well as PGRN receptors, have recently been identified because of their ability to regulate PGRN levels. Strict quality control measures are needed given that extreme PGRN levels at either end of the spectrum – too low or too high – can lead to neurodegeneration or cancer, respectively. The aim of this review is to highlight what is known regarding PGRN biology; to improve understanding of the mechanisms involved in regulating PGRN levels and highlight studies that are laying the groundwork for the development of effective therapeutic modulators of PGRN.
Keywords: Progranulin, neurodegeneration, frontotemporal lobar degeneration, dementia, neurotrophic factors, sortilin
1. Introduction
New research shows that increasing levels of the protein progranulin (PGRN) may be beneficial to neurons and thereby prevent brain disorders such as frontotemporal lobar degeneration (FTLD). Mutations in the progranulin gene (GRN) are a major cause of frontotemporal lobar degeneration with ubiquitin positive inclusions (FTLD-U) (Baker et al., 2006; Cruts et al., 2006). Because these mutations have been linked to abnormal deficiencies in the production of PGRN, researchers currently aim to design therapies that would increase PGRN levels in affected individuals, thereby alleviating the symptoms associated with disease.
FTLD is the second-most common form of presenile dementia after Alzheimer’s disease (AD) accounting for 5–10% of all dementia patients (Graff-Radford and Woodruff, 2007). While the majority of FTLD cases are sporadic, approximately 10–40% of patients have a positive family history. The primary neuropathological hallmark of FTLD is degeneration of the brain’s frontal and temporal lobes as well as accumulation of proteinaceous inclusions found within neurons and glial cells. FTLD subtypes have been established depending on the proteins found in these inclusions. The main subtypes include FTLD-tau for microtubule associated protein tau (MAPT) positive inclusions and FTLD-TDP for ubiquitin and transactive response DNA-binding protein 43 (TDP-43) (Mackenzie et al., 2006; Mackenzie, 2007; Sampathu et al., 2006). Symptoms of FTLD include behavioral/personality changes and language dysfunction. In some cases, FTLD patients display abnormal motor function commonly referred to as motor neuron disease (Lomen-Hoerth et al., 2002).
While a tipping point exists at which PGRN causes disease—increases have been reported in various cancers but decreased levels cause FTLD (Bateman and Bennett, 2009) – the recent discovery of PGRN receptors, genetic regulators and possible therapeutic compounds provide renewed hope that proper management of PGRN levels will be beneficial. This review will include an examination of such novel applications along with recent advances in PGRN research.
2. Mutations causing FTLD
2.1 Non-GRN mutations
Identification of FTLD-causing genetic mutations has been challenging due to the variety of genes involved and their diverse protein functions. In 1998, the first mutations causing FTLD were discovered in MAPT, located on chromosome 17 (FTLD-tau) (Hutton et al., 1998). MAPT mutations reduce the ability of tau to interact with microtubules leading to the accumulation of pathogenic tau aggregates (Spillantini et al., 1998). In the following years, two other genes were determined to be rare causes of FTLD: valosin-containing protein (VCP) (Watts et al., 2004) associated with Golgi assembly/disassembly and regulation of the ubiquitin–proteasome system as well as charged multivesicular body protein 2B (CHMP2B) (Skibinski et al., 2005) which is involved in endosomal sorting. Most recently, chromosome 9 open reading frame 72 (C9ORF72) mutations were determined to be the major cause of chromosome 9 linked FTLD and amyotrophic lateral sclerosis (ALS) (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Despite these discoveries, questions remained as to why many families showing strong genetic linkage to chromosome 17 remained negative for MAPT mutations. The reason: located only 1.7Mb away from MAPT, were genetic variants in GRN.
2.2 GRN mutations
Genetic analysis of chromosome 17q21 led to the identification of 70 pathogenic mutations within GRN that cause FTLD-U (Figure 1) (Baker et al., 2006; Cruts et al., 2006). The majority of pathogenic variants are characterized as nonsense, frameshift and splice site mutations causing a premature stop codon, however, other mechanisms have also been observed (Baker et al., 2006; Cruts and Van Broeckhoven, 2008; Gass et al., 2006). Various mutations (missense, silent and intronic) of unknown pathogenicity also occur. Functional studies of specific missense mutations (p.R432C, pC139R, p.P248L, p.C521Y) point to possible changes in secretion and processing of PGRN, suggesting a partial loss of PGRN function (Shankaran et al., 2008; Wang et al., 2010a). Pathogenic GRN mutations typically lead to degradation of RNA through nonsense mediated decay, essentially resulting in a haploinsufficiency of the protein. Indeed, about a 75% reduction of PGRN in plasma were found in patients harboring GRN null mutations (Finch et al., 2009).
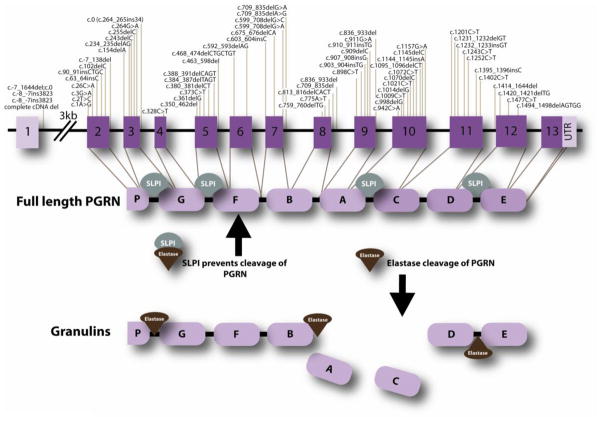
Figure 1.
Seventy pathogenic GRN mutations have been identified in over 230 families. In the schematic diagram, mutations are numbered relative to the largest GRN transcript (GenBank accession number NM_002087.2). The gene encodes for a 593 amino acid protein composed of intra-linked granulin peptides that are cleaved by proteases into individual granulins. Secretory leukocyte protease inhibitor (SLPI) protects full-length PGRN by binding to both PGRN and elastase, therefore preventing cleavage into the 6 kDa granulin repeats.
The frequency of GRN mutations is estimated to be in the range of 1 to 11.7% of the total FTLD population, and 3.4 to 25.6% of familial cases (Baker et al., 2006; Borroni et al., 2008; Bronner et al., 2007; Bruni et al., 2007; Cruts et al., 2006; Gass et al., 2006; Le Ber et al., 2007; Le Ber et al., 2008; Pickering-Brown et al., 2008). The wide rage in familial cases may be due to the patient populations, as well as strong founder effects of the higher frequencies. The mean age of patients suffering from diseases caused by pathogenic mutations in GRN is 59±7 years (Gass et al., 2006). Fifty percent of GRN mutation carriers are affected by the age of 60 years, while 90% are affected by 70 years of age, according to liability curve data (Gass et al., 2006). In addition, disease duration in GRN mutation carriers is approximately 2.4 years shorter than MAPT mutation carriers, suggesting a more rapid disease progression in patients carrying GRN mutations (Cruts and Van Broeckhoven, 2008). Still other modifying factors – genetic or environmental – may potentially impact disease progression and severity and thereby account for the variable age at onset.
In rare circumstances, GRN mutations have been reported in clinically identified AD and Parkinson’s disease (PD) patients (Brouwers et al., 2007; Kelley et al., 2010). Alternatively, PGRN is often increased in AD, ALS and PD, most likely due to microgliosis (Brouwers et al., 2007; Irwin et al., 2009; Pereson et al., 2009). Although GRN mutations are not a major cause of AD, ALS, PD, it is possible that certain genetic variants of GRN serve as risk factors.
3. Molecular biology of PGRN
GRN is composed of 13 exons, although exon 1 is non-coding (Figure 1). The PGRN protein is composed of a series of seven-and-a-half tandem repeats separated by interlinked spacer regions (Bhandari and Bateman, 1992). PGRN is a 68.5kDa protein, however, N-glycosylation of the protein at five potential sites, gives rise to secretion of a mature 88 kDa protein (Songsrirote et al., 2010). The distinctive structure allows for cleavage of PGRN into granulins, each composed of cysteine repeat motifs. Cleavage of PGRN occurs at the intra-linker spacer regions and it is achieved by several proteases; these include elastase (Zhu et al., 2002), proteinase 3 (Kessenbrock et al., 2008), matrix metalloproteinase-14 (Butler et al., 2008) and ADAM metallopeptidase with thrombosphondin type 1 motif, 7 (Bai et al., 2009). Additionally, full length PGRN is preserved from cleavage by secretory leukocyte protease inhibitor (SLPI) (Zhu et al., 2002) and high-density lipoprotein (HDL)/Apolipoprotein A-I complex (Okura et al., 2010). SLPI binds to PGRN, as well as elastase to control the levels of PGRN and granulins as depicted in Figure 1.
Another interesting point is that FTLD-U patients harbor GRN mutations that contain TDP-43 positive inclusions (FTLD-TDP) (Mackenzie et al., 2006; Sampathu et al., 2006). Currently the relationship between GRN insufficiency and TDP-43 inclusions is not completely understood; however, ongoing research is starting to unveil a link between the two and disease. Briefly, TDP-43 is involved in RNA metabolism and recently has been shown to bind GRN RNA in mice (Polymenidou et al., 2011). In disease, TDP-43 may become hyperphosphorylated, cleaved into C-terminal fragments and translocated to the cytoplasm where it forms inclusions (Neumann et al., 2006; Zhang et al., 2007). These events would induce changes in the nuclear regulation of TDP-43 RNA targets, such as GRN. Tdp-43 downregulation in mice has been shown to increase Grn RNA levels (Polymenidou et al., 2011); however, details on how Tdp-43 regulates Grn and the consequences of this increase in Grn RNA requires further investigation. Changes in PGRN expression have been shown to alter TDP-43 biology in vitro. Briefly, modeling GRN insufficiency in cell culture models by treating cells with siRNA against GRN for 72 hours leads to caspase-dependent cleavage of TDP-43 into fragments similar to the 25 and 35 kDa species observed in humans (Zhang et al., 2007). However, downregulation of PGRN in cells for shorter periods of time (48 hours) did not result in TDP-43 fragmentation (Dormann et al., 2009). Additional studies demonstrate that knock-down of PGRN in primary neuronal cultures promotes redistribution of TDP-43 into the cytosol (Guo et al., 2010). Although the relationship between PRGN and TDP-43 needs to be further evaluated, these data suggest that PGRN may be implicated not only in FTLD, but also in other neurodegenerative diseases, such as ALS, that are also characterized by TDP-43 inclusions.
4. PGRN plays pivotal role in several biological functions
Progranulin and its orthologs are highly conserved proteins found in a variety of species ranging from eubacteria to mammals. PGRN was independently identified by various groups and is known by several synonymous names including granulin-epithelin precursor (GEP) (Xu et al., 1998), proepithelin (Plowman et al., 1992), PC cell derived growth factor (PCDGF) (Zhou et al., 1993) and acrogranin (Baba et al., 1993). As its many names suggest, PGRN play roles in multiple biological functions including embryogenesis (Daniel et al., 2003; Diaz-Cueto et al., 2000), cell growth and survival (Bateman et al., 1990; Plowman et al., 1992; Xu et al., 1998), transcriptional repression (Hoque et al., 2003; Hoque et al., 2010), and wound repair and inflammation (Bateman et al., 1990; He and Bateman, 1999; Kessenbrock et al., 2008; Yin et al., 2010; Zhu et al., 2002).
4.1 Neurotrophic functions of PGRN
Recently, PGRN’s role in the central nervous system (CNS) has sparked interest. Experiments overexpressing PGRN in mouse cortical neurons have revealed that PGRN increases cell viability, suggesting PGRN can function as a neurotrophic growth factor (Ryan et al., 2009). Thorough evaluations of its neurotrophic properties revealed that when recombinant full-length PGRN and granulin E are added to cortical cultures, neurite outgrowth increases (Van Damme et al., 2008). Additionally, treatment with recombinant SLPI, which prevents cleavage, inhibits increased outgrowth produced by PGRN supplementation suggesting neurotrophic roles for granulins. In their recent publication, Tapia and colleagues determined when PGRN is knocked-down, via siRNA in rat primary hippocampal neurons, there is a decrease in neuronal arborization and length (Tapia et al., 2011). In addition to these decreases, they also observed reductions in synaptic densities that are believed to be compensated for increases in excitatory postsynaptic potentials of PGRN knockdown neurons. Such evidence demonstrates PGRN plays a crucial role in proper neuronal morphology and the connections between neurons.
4.2 Developmental functions and signaling pathways of PGRN
In terms of embryogenesis, Grn mRNA levels were measured in preimplantation mouse embryos, with the highest levels found in the blastocysts stage (Diaz-Cueto et al., 2000). Androgen treatment, or treatment with estrogen, increases PGRN levels within the neonatal rat hypothalamus, suggesting a possible regulatory role on sex differentiation (Suzuki et al., 2001; Suzuki and Nishiahara, 2002). Moreover, in the developing brain, PGRN is widely expressed in the olfactory bulbs, retinal ganglia, forebrain and spinal cord (Daniel et al., 2003). As animals mature into adults this expression is localized to certain areas of the brain, including cerebellar Purkinje cells, pyramidal neurons of the cortex, and hippocampus, as well as activated microglia (Petkau et al., 2010).
PGRN interactions outside of the cell, as well as inside, reveal its ability to regulate several other cellular functions via signaling transduction pathways. Wnt signaling is involved is a variety of physiological processes. Interestingly, it is essential for neuronal development notably functioning in axon guidance (Lucas and Salinas, 1997) and neurite outgrowth (Lyuksyutova et al., 2003). According to recent studies, PGRN is involved in Wnt signaling, suggesting disruption of this pathway due to decreased levels of PGRN may contribute to FTLD-TDP (Rosen et al., 2011). Additionally, PGRN can also activate various growth factor signaling pathways of the periphery involved in cell growth/survival including extracellular regulated kinase (ERK), phosphatydyl inositol-3 (PI3K), protein kinase B/AKT and p70S6 (He et al., 2002; Kamrava et al., 2005; Monami et al., 2006; Zanocco-Marani et al., 1999). Interestingly, some of these signaling pathways are also activated in PGRN-induced neuronal outgrowth (Gao et al., 2010). Elevated levels of PGRN in various types of cancer are known to stimulate these pathways, which aid in tumorogenesis by increasing proliferation, migration and invasiveness.
4.3 Wound repair and inflammation
Cytokines, such as tumor necrosis factor alpha (TNF-α), help initiate the inflammation process via signaling through receptors. In wound repair, PGRN, but not granulins, inhibit TNF-α induced signal transduction in neutrophils (Zhu et al., 2002). Additionally, in a murine transcutaneous wound model, PGRN was found to increase accumulation of fibroblast, endothelial cells, macrophages, neutrophils and blood vessels after application to the wounded areas (He et al., 2003). Such data illustrate the function of PGRN in the early stages of wound healing. Thanks to the recent discovery of tumor necrosis factor receptors (TNFR) as possible receptors for PGRN, researchers will be able to pinpoint the role PGRN plays in inflammation.
5. PGRN deficient animal models mimic human disease
Many researchers have begun to study the effects of PGRN loss through the generation of Grn knockout animal models (Grn−/−). Despite variations in the design, these models share many commonalities. For instance, murine Grn−/− models have increased microglial and astroglial activation with age when compared to Grn+/+ and Grn−/+ mice (Ahmed et al., 2007; Yin et al., 2010). Furthermore, Grn−/− mice have increased accumulation of ubiquitin staining that begins around 7 months. Unlike FTLD patients, the increased ubiquitin inclusions are not TDP-43 positive. Additional analyses determined that these aggregates where mainly composed of lipofuscin granules (Ahmed et al., 2007). Lipofuscin, alternatively know as “the aging pigment”, forms in response to normal aging but accelerates accumulation during mitochondrial stress and lysosomal injury, however, it has not been reported in FTLD patients. In a Grn−/− mouse generated by Petkau et al, synaptic connectivity and plasticity were both altered (Petkau et al., 2011). Microphotographs of Golgi stained hippocampal neurons from these mice revealed decreased length and arborization of dendrites similar to experiments mentioned above using primary neurons.
Mice generated by Kayasuga and colleagues exhibit a phenotype that correlates with certain behavioral symptoms in FTLD (Kayasuga et al., 2007). Characterization of aged Grn−/− mice using a resident-intruder test and Morris water maze determined that these mice display decreased social interaction and impaired learning and memory, which mimics behavior changes and advanced dementia problems observed in FTLD patients (Ghoshal et al., 2012). Another model developed by Yin et al., focused mainly on Pgrn’s involvement in inflammation (Yin et al., 2010). Here they report that loss of Pgrn in Grn−/− mice leads to an increase in the production of proinflammatory cytokines. Interestingly, these studies revealed that these mice also have behavioral abnormalities such as depression and impaired cognitive behaviors. Yin et al also report that ubiquitin positive inclusions contain TDP-43 (Yin et al., 2010), however, this finding is not observed in other models (Ahmed et al., 2007; Petkau et al., 2010).
PGRN deficiency models in zebrafish and the nematode C. elegans have been generated. Knockdown of zfPGRN-A, the ortholog of human PGRN, generated truncated motorneurons and premature branching that was rescued by overexpression of zfPGRN-A (Chitramuthu et al., 2010). Loss of PGRN in a C. elegans model caused increased apoptotic clearance of dying cells, suggesting that cells are prematurely cleared before recovery can take place (Kao et al., 2011). In other words, stressed neurons would be removed leading to increased neurodegeneration. While many commonalities exist between these animal models and FTLD patients, differences exist. Still these models will be beneficial in therapy development and elucidating the functions of PGRN.
6. Potential therapeutic targets for PGRN
6.1 Regulators of PGRN
Several compounds and genetic factors have recently been identified because of their ability to regulate PGRN levels. Strict quality control measures to ensure PGRN levels are properly regulated are crucial, given PGRN’s duality – its involvement in the progression of neurodegeneration and tumorigenesis – based on its concentrations throughout the body. Although PGRN levels are decreased in FTLD, its expression is upregulated in certain cancers and other neurodegenerative diseases.
6.1.1 Chemical Compounds
Researchers have begun to identify chemical compounds that can be used to manipulate PGRN levels. For example, Capell et al were able to pharmalogically stimulate the production of PGRN in Grn−/+ mice and patients with GRN mutations by the use of Bafilomycin A1 (BafA1) (Capell et al., 2011). BafA1, an inhibitor of vacuolar ATPase, was shown to increase PGRN levels independent of lysosomal degradation, autophagy, or endocytosis. The exact mechanism involved in BafA1 remains undetermined; however, it was suggested that translational upregulation of PGRN was initiated by increases in intracellular pH changes. Capell and colleagues also reported that other FDA-approved alkalizing compounds such as chloroquine, amiodarone and bepridil can increase PGRN levelsl. Additionally, suberoylanilide hydroxamic acid (SAHA) was found to increase GRN transcription leading to greater expression (Cenik et al., 2011). SAHA treatment led to increased GRN mRNA and protein levels in cultured Neuro-2a cells and lymphoblast cells from GRN mutational carries. While these compounds would require testing before they could be used in FTLD patients, SAHA is currently approved to treat cutaneous T-cell lymphoma.
6.1.2 Genetic modifiers of PGRN levels
MicroRNAs (miRNA) are short RNA molecules that regulate gene expression by preventing mRNA from being translated. The single nucleotide polymorphism (SNP) rs5848, located in a predicted miRNA binding site for miR-659 in the 3’ UTR of GRN, is shown to be a risk factor for FTLD-TDP (Rademakers et al., 2008). Thus, rs5848 is thought to cause a shift in the miRNA binding site when the risk T-allele of rs5848 is present, thereby decreasing GRN mRNA levels (Fenoglio et al., 2009; Rademakers et al., 2008). Nevertheless, one group has reported that they are unable to replicate these data (Rollinson et al., 2011). Additional groups have also shown that other miRNAs can regulate PGRN levels. Both miR-29b and miR-107 were determined to regulate PGRN expression (Jiao et al., 2010; Wang et al., 2010b). Increases in both miRNAs decreased PGRN levels.
The transmembrane protein 106B (TMEM106B) has been identified as a potential PGRN regulator, and thus, a possible drug target. In a collaborative study, researchers initially used a genome-wide association study that identified three SNPs in and around TMEM106B (Van Deerlin et al., 2010). These SNPs were shown to regulate TMEM106b expression, however, not much is known about its function. Independent follow up studies using GRN mutation carriers and controls report that the rare TMEM106B variants are protective, which was supported by data that these rare variants delay the age at onset of FTLD-TDP and increase plasma PGRN levels (Cruchaga et al., 2011; Finch et al., 2011; van der Zee and Van Broeckhoven, 2011).
6.2 Receptors of PGRN
Designing potential therapies to reverse or combat PGRN loss in neurodegeneration, as well as other diseases, has been difficult. Recent identification of PGRN binding receptors is a critical step in understanding how PGRN functions and the development of new therapies. Increasing PGRN levels, for example to patients at risk of FTLD-U, would reduce neuronal vulnerability to injury, inflammation or other insults that lead to increased risk of cell death and disease.
6.2.1 Sortilin
Sortilin 1 (SORT1) was identified as the first known receptor for PGRN (Hu et al., 2010). Independent of this finding, genome wide association studies also identified SORT1 as a regulator of PGRN levels (Carrasquillo et al., 2010). SORT1 belongs to a class of receptors known as vacuolar protein sorting 10 protein domain receptors (VSP10P) (Marcusson et al., 1994). This class of receptors is found in distinct areas of the CNS and peripheral nervous system suggesting roles in neuronal function. SORT1 is a sorting protein involved in endocytosis and transport to trans-golgi network (TGN) and endosomes (Petersen et al., 1997). SORT1 can form a complex with the low-affinity nerve growth factor receptor (P75NTR) and proneurotrophin to activate cell death. On the other hand, P75NTR forms complexes with tyrosine kinase receptors (Trk) to promote cell survival (Willnow et al., 2008). SORT1, however, does not associate with Trk receptors similar to P75NTR; instead, it binds to Trk receptors to enhance their anterograde transport from the soma to nerve endings (Vaegter et al., 2011). While SORT is known to interact with several proteins, its interactions with PGRN remain relatively unspecified.
Association studies have identified that several VSP10P receptors are involved in neuronal diseases such as AD (SORLA and SORSC1) and bipolar disorder (SORCS2) (Grupe et al., 2006; Rogaeva et al., 2007). In a recent genome wide association study by Carrasquillo et al, SORT1 variants were shown to regulate PGRN levels in human plasma (Carrasquillo et al., 2010). SNP rs646776 near SORT1 is associated with increased levels of SORT1. However, rs646776 genotypes correlate only with PGRN levels specific for the liver and not within the CNS. Additional evaluations of SORT1 knockdown in HeLa cells showed increased levels of PGRN (Carrasquillo et al., 2010). Furthermore, overexpression of SORT1 resulted in decreased levels of PGRN suggesting that SORT1 regulates PGRN levels.
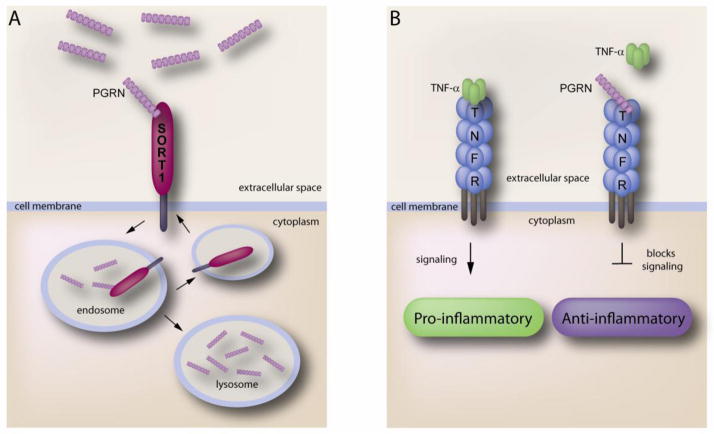
The identification of SORT1 as a major neuronal receptor for PGRN was a key development in PGRN biology. Using an alkaline phophatase tagged PGRN binding assay, Hu et al discovered that SORT1 was the only high affinity binding partner of PGRN in neurons (KD~15) (Hu et al., 2010). Additional studies revealed that when PGRN binds to SORT1 it immediately gets endocytosed and delivered to the lysomsome (Figure 2A). Uptake of PGRN via SORT1 dramatically decreases the extracellular levels of PGRN. Extensive experiments determined the C-terminal tail of PGRN (QLL) binds to the beta propeller region of SORT1 (Zheng et al., 2011). For future studies it will be important to know if SORT1 is able to act as a signaling receptor for PGRN as well as other functions. The use of this receptor for the identification of therapies will be of great use since SORT1 is known to regulate PGRN levels.
Figure 2.
A. The C-terminal end of PGRN binds to the Beta-propeller region of SORT1. Upon binding, uptake of PGRN via SORT1 traffics PGRN to the lysosome. Endocytosed SORT1 is then recycled back to the cell membrane. B. PGRN binding to TNFR acts as an antagonist of TNFα. TNFα binding to TNFR activates pro-inflammatory signaling to induce inflammation. PGRN blocks this signaling pathway when bound to TNFR therefore inhibiting inflammation.
6.2.2 TNFR
Exciting new evidence of PGRN-related therapies decreasing inflammation in arthritis may also be beneficial to patients suffering from neurodegeneration, since inflammation is often associated with neurodegenerative diseases. The discovery that PGRN binds to TNFR suggests that PGRN acts as an antagonist for TNFα (Tang et al., 2011). Tang and colleagues utilized a yeast two-hybrid system to identify tumor necrosis factor receptor 1 and 2 (TNFR1 and TNFR2) as major binding proteins of PGRN (Tang et al., 2011). Further experiments revealed that when PGRN binds to these receptors, downstream inflammatory signaling is blocked (Figure 2.b). Utilizing a collagen-induced arthritis (CIA) mouse model, they were able to show that loss of PGRN exacerbates inflammation and joint damage associated with arthritis. Interestingly, supplementation of recombinant PGRN in this model delayed symptoms of arthritis. The authors also determined that specific domains of PGRN bind to TNFRs, which they used to develop a peptide (Asttrin) that mimics PGRN binding. Experiments using Asttrin in the CIA mouse model showed a greater efficiency in preventing inflammation than PGRN, suggesting that development of specific compounds may be a key to treating these devastating diseases.
7. Conclusions
Although the specific functions of PGRN have not been fully characterized, it is becoming apparent that the expression and function of PGRN is an important determinant of successful aging. Mutations in GRN lead to reduced PGRN levels leading to FTLD-TDP, and may also increase risk of other neurodegenerative diseases. Recently identified PGRN binding receptors may aid in the development of therapeutics designed to regulate PGRN levels. By better understanding which receptors PGRN binds to, and by extension other proteins that act as PGRN antagonists, researchers may have greater success therapeutically modulating PGRN levels. Much remains unknown regarding the impact environmental insults have on the health of neurons, which also are sensitive to age-related stress accumulation. However given our increasing knowledge of PGRN function and its key regulators, researchers now aim to develop novel therapies in the hopes of improving patient care.
Highlights.
We review PGRN’s role in several biological functions
We review how PGRN knockout animal models mimic human disease
We review potential therapeutic targets for PGRN
Acknowledgments
This work was supported by Mayo Clinic Foundation (LP), National Institutes of Health/National Institute on Aging [5R01AG026251-04(LP)], National Institutes of Health/National Institute of Neurological Disorders and Stroke [R01 NS 063964-01 (LP), R01 NS077402 (LP)], Amyotrophic Lateral Sclerosis Association (LP) and Department of Defense [W81XWH-10-1-0512-1 and W81XWH-09-1-0315AL093108 (LP)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Hoff HB, 3rd, Nemoto H, Lee H, Orth J, Arai Y, Gerton GL. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev. 1993;34:233–43. doi: 10.1002/mrd.1080340302. [DOI] [PubMed] [Google Scholar]
- Bai XH, Wang DW, Kong L, Zhang Y, Luan Y, Kobayashi T, Kronenberg HM, Yu XP, Liu CJ. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29:4201–19. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun. 1990;173:1161–8. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–54. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- Bhandari V, Bateman A. Structure and chromosomal location of the human granulin gene. Biochem Biophys Res Commun. 1992;188:57–63. doi: 10.1016/0006-291x(92)92349-3. [DOI] [PubMed] [Google Scholar]
- Borroni B, Archetti S, Alberici A, Agosti C, Gennarelli M, Bigni B, Bonvicini C, Ferrari M, Bellelli G, Galimberti D, Scarpini E, Di Lorenzo D, Caimi L, Caltagirone C, Di Luca M, Padovani A. Progranulin genetic variations in frontotemporal lobar degeneration: evidence for low mutation frequency in an Italian clinical series. Neurogenetics. 2008;9:197–205. doi: 10.1007/s10048-008-0127-3. [DOI] [PubMed] [Google Scholar]
- Bronner IF, Rizzu P, Seelaar H, van Mil SE, Anar B, Azmani A, Kaat LD, Rosso S, Heutink P, van Swieten JC. Progranulin mutations in Dutch familial frontotemporal lobar degeneration. Eur J Hum Genet. 2007;15:369–74. doi: 10.1038/sj.ejhg.5201772. [DOI] [PubMed] [Google Scholar]
- Brouwers N, Nuytemans K, van der Zee J, Gijselinck I, Engelborghs S, Theuns J, Kumar-Singh S, Pickut BA, Pals P, Dermaut B, Bogaerts V, De Pooter T, Serneels S, Van den Broeck M, Cuijt I, Mattheijssens M, Peeters K, Sciot R, Martin JJ, Cras P, Santens P, Vandenberghe R, De Deyn PP, Cruts M, Van Broeckhoven C, Sleegers K. Alzheimer and Parkinson diagnoses in progranulin null mutation carriers in an extended founder family. Arch Neurol. 2007;64:1436–46. doi: 10.1001/archneur.64.10.1436. [DOI] [PubMed] [Google Scholar]
- Bruni AC, Momeni P, Bernardi L, Tomaino C, Frangipane F, Elder J, Kawarai T, Sato C, Pradella S, Wakutani Y, Anfossi M, Gallo M, Geracitano S, Costanzo A, Smirne N, Curcio SA, Mirabelli M, Puccio G, Colao R, Maletta RG, Kertesz A, St George-Hyslop P, Hardy J, Rogaeva E. Heterogeneity within a large kindred with frontotemporal dementia: a novel progranulin mutation. Neurology. 2007;69:140–7. doi: 10.1212/01.wnl.0000265220.64396.b4. [DOI] [PubMed] [Google Scholar]
- Butler GS, Dean RA, Tam EM, Overall CM. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–914. doi: 10.1128/MCB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell A, Liebscher S, Fellerer K, Brouwers N, Willem M, Lammich S, Gijselinck I, Bittner T, Carlson AM, Sasse F, Kunze B, Steinmetz H, Jansen R, Dormann D, Sleegers K, Cruts M, Herms J, Van Broeckhoven C, Haass C. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J Neurosci. 2011;31:1885–94. doi: 10.1523/JNEUROSCI.5757-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo MM, Nicholson AM, Finch N, Gibbs JR, Baker M, Rutherford NJ, Hunter TA, DeJesus-Hernandez M, Bisceglio GD, Mackenzie IR, Singleton A, Cookson MR, Crook JE, Dillman A, Hernandez D, Petersen RC, Graff-Radford NR, Younkin SG, Rademakers R. Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet. 2010;87:890–7. doi: 10.1016/j.ajhg.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenik B, Sephton CF, Dewey CM, Xian X, Wei S, Yu K, Niu W, Coppola G, Coughlin SE, Lee SE, Dries DR, Almeida S, Geschwind DH, Gao FB, Miller BL, Farese RV, Jr, Posner BA, Yu G, Herz J. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. J Biol Chem. 2011;286:16101–8. doi: 10.1074/jbc.M110.193433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitramuthu BP, Baranowski DC, Kay DG, Bateman A, Bennett HP. Progranulin modulates zebrafish motoneuron development in vivo and rescues truncation defects associated with knockdown of Survival motor neuron 1. Mol Neurodegener. 2010;5:41. doi: 10.1186/1750-1326-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Graff C, Chiang HH, Wang J, Hinrichs AL, Spiegel N, Bertelsen S, Mayo K, Norton JB, Morris JC, Goate A. Association of TMEM106B gene polymorphism with age at onset in granulin mutation carriers and plasma granulin protein levels. Arch Neurol. 2011;68:581–6. doi: 10.1001/archneurol.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Loss of progranulin function in frontotemporal lobar degeneration. Trends Genet. 2008;24:186–94. doi: 10.1016/j.tig.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Daniel R, Daniels E, He Z, Bateman A. Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Dev Dyn. 2003;227:593–9. doi: 10.1002/dvdy.10341. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Cueto L, Stein P, Jacobs A, Schultz RM, Gerton GL. Modulation of mouse preimplantation embryo development by acrogranin (epithelin/granulin precursor) Dev Biol. 2000;217:406–18. doi: 10.1006/dbio.1999.9564. [DOI] [PubMed] [Google Scholar]
- Dormann D, Capell A, Carlson AM, Shankaran SS, Rodde R, Neumann M, Kremmer E, Matsuwaki T, Yamanouchi K, Nishihara M, Haass C. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem. 2009;110:1082–94. doi: 10.1111/j.1471-4159.2009.06211.x. [DOI] [PubMed] [Google Scholar]
- Fenoglio C, Galimberti D, Cortini F, Kauwe JS, Cruchaga C, Venturelli E, Villa C, Serpente M, Scalabrini D, Mayo K, Piccio LM, Clerici F, Albani D, Mariani C, Forloni G, Bresolin N, Goate AM, Scarpini E. Rs5848 variant influences GRN mRNA levels in brain and peripheral mononuclear cells in patients with Alzheimer’s disease. J Alzheimers Dis. 2009;18:603–12. doi: 10.3233/JAD-2009-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch N, Baker M, Crook R, Swanson K, Kuntz K, Surtees R, Bisceglio G, Rovelet-Lecrux A, Boeve B, Petersen RC, Dickson DW, Younkin SG, Deramecourt V, Crook J, Graff-Radford NR, Rademakers R. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain. 2009;132:583–91. doi: 10.1093/brain/awn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch N, Carrasquillo MM, Baker M, Rutherford NJ, Coppola G, Dejesus-Hernandez M, Crook R, Hunter T, Ghidoni R, Benussi L, Crook J, Finger E, Hantanpaa KJ, Karydas AM, Sengdy P, Gonzalez J, Seeley WW, Johnson N, Beach TG, Mesulam M, Forloni G, Kertesz A, Knopman DS, Uitti R, White CL, 3rd, Caselli R, Lippa C, Bigio EH, Wszolek ZK, Binetti G, Mackenzie IR, Miller BL, Boeve BF, Younkin SG, Dickson DW, Petersen RC, Graff-Radford NR, Geschwind DH, Rademakers R. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2011;76:467–74. doi: 10.1212/WNL.0b013e31820a0e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Joselin AP, Wang L, Kar A, Ray P, Bateman A, Goate AM, Wu JY. Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3beta. Protein Cell. 2010;1:552–62. doi: 10.1007/s13238-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Pickering-Brown SM, Graff-Radford N, Uitti R, Dickson D, Wszolek Z, Gonzalez J, Beach TG, Bigio E, Johnson N, Weintraub S, Mesulam M, White CL, 3rd, Woodruff B, Caselli R, Hsiung GY, Feldman H, Knopman D, Hutton M, Rademakers R. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Ghoshal N, Dearborn JT, Wozniak DF, Cairns NJ. Core features of frontotemporal dementia recapitulated in progranulin knockout mice. Neurobiol Dis. 2012;45:395–408. doi: 10.1016/j.nbd.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford NR, Woodruff BK. Frontotemporal dementia. Semin Neurol. 2007;27:48–57. doi: 10.1055/s-2006-956755. [DOI] [PubMed] [Google Scholar]
- Grupe A, Li Y, Rowland C, Nowotny P, Hinrichs AL, Smemo S, Kauwe JS, Maxwell TJ, Cherny S, Doil L, Tacey K, van Luchene R, Myers A, Wavrant-De Vrieze F, Kaleem M, Hollingworth P, Jehu L, Foy C, Archer N, Hamilton G, Holmans P, Morris CM, Catanese J, Sninsky J, White TJ, Powell J, Hardy J, O’Donovan M, Lovestone S, Jones L, Morris JC, Thal L, Owen M, Williams J, Goate A. A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease. Am J Hum Genet. 2006;78:78–88. doi: 10.1086/498851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Tapia L, Bamji SX, Cynader MS, Jia W. Progranulin deficiency leads to enhanced cell vulnerability and TDP-43 translocation in primary neuronal cultures. Brain Res. 2010;1366:1–8. doi: 10.1016/j.brainres.2010.09.099. [DOI] [PubMed] [Google Scholar]
- He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999;59:3222–9. [PubMed] [Google Scholar]
- He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62:5590–6. [PubMed] [Google Scholar]
- He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–9. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- Hoque M, Young TM, Lee CG, Serrero G, Mathews MB, Pe’ery T. The growth factor granulin interacts with cyclin T1 and modulates P-TEFb-dependent transcription. Mol Cell Biol. 2003;23:1688–702. doi: 10.1128/MCB.23.5.1688-1702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M, Mathews MB, Pe’ery T. Progranulin (granulin/epithelin precursor) and its constituent granulin repeats repress transcription from cellular promoters. J Cell Physiol. 2010;223:224–33. doi: 10.1002/jcp.22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–67. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Irwin D, Lippa CF, Rosso A. Progranulin (PGRN) expression in ALS: an immunohistochemical study. J Neurol Sci. 2009;276:9–13. doi: 10.1016/j.jns.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Jiao J, Herl LD, Farese RV, Gao FB. MicroRNA-29b regulates the expression level of human progranulin, a secreted glycoprotein implicated in frontotemporal dementia. PLoS One. 2010;5:e10551. doi: 10.1371/journal.pone.0010551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrava M, Simpkins F, Alejandro E, Michener C, Meltzer E, Kohn EC. Lysophosphatidic acid and endothelin-induced proliferation of ovarian cancer cell lines is mitigated by neutralization of granulin-epithelin precursor (GEP), a prosurvival factor for ovarian cancer. Oncogene. 2005;24:7084–93. doi: 10.1038/sj.onc.1208857. [DOI] [PubMed] [Google Scholar]
- Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, Zhou P, de Luis A, Neukomm LJ, Cabello J, Farese RV, Jr, Kenyon C. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci U S A. 2011;108:4441–6. doi: 10.1073/pnas.1100650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res. 2007;185:110–8. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Haidar W, Boeve BF, Baker M, Shiung M, Knopman DS, Rademakers R, Hutton M, Adamson J, Kuntz KM, Dickson DW, Parisi JE, Smith GE, Petersen RC. Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch Neurol. 2010;67:171–7. doi: 10.1001/archneurol.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Frohlich L, Sixt M, Lammermann T, Pfister H, Bateman A, Belaaouaj A, Ring J, Ollert M, Fassler R, Jenne DE. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118:2438–47. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, van der Zee J, Hannequin D, Gijselinck I, Campion D, Puel M, Laquerriere A, De Pooter T, Camuzat A, Van den Broeck M, Dubois B, Sellal F, Lacomblez L, Vercelletto M, Thomas-Anterion C, Michel BF, Golfier V, Didic M, Salachas F, Duyckaerts C, Cruts M, Verpillat P, Van Broeckhoven C, Brice A. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat. 2007;28:846–55. doi: 10.1002/humu.20520. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, Hahn-Barma V, van der Zee J, Clot F, Bakchine S, Puel M, Ghanim M, Lacomblez L, Mikol J, Deramecourt V, Lejeune P, de la Sayette V, Belliard S, Vercelletto M, Meyrignac C, Van Broeckhoven C, Lambert JC, Verpillat P, Campion D, Habert MO, Dubois B, Brice A. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131:732–46. doi: 10.1093/brain/awn012. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–9. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–8. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Baker M, Pickering-Brown S, Hsiung GY, Lindholm C, Dwosh E, Gass J, Cannon A, Rademakers R, Hutton M, Feldman HH. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain. 2006;129:3081–90. doi: 10.1093/brain/awl271. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol. 2007;114:49–54. doi: 10.1007/s00401-007-0223-8. [DOI] [PubMed] [Google Scholar]
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–86. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–10. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Okura H, Yamashita S, Ohama T, Saga A, Yamamoto-Kakuta A, Hamada Y, Sougawa N, Ohyama R, Sawa Y, Matsuyama A. HDL/Apolipoprotein A-I Binds to Macrophage-Derived Progranulin and Suppresses its Conversion into Proinflammatory Granulins. J Atheroscler Thromb. 2010 doi: 10.5551/jat.3921. [DOI] [PubMed] [Google Scholar]
- Pereson S, Wils H, Kleinberger G, McGowan E, Vandewoestyne M, Van Broeck B, Joris G, Cuijt I, Deforce D, Hutton M, Van Broeckhoven C, Kumar-Singh S. Progranulin expression correlates with dense-core amyloid plaque burden in Alzheimer disease mouse models. J Pathol. 2009;219:173–81. doi: 10.1002/path.2580. [DOI] [PubMed] [Google Scholar]
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- Petkau TL, Neal SJ, Orban PC, MacDonald JL, Hill AM, Lu G, Feldman HH, Mackenzie IR, Leavitt BR. Progranulin expression in the developing and adult murine brain. J Comp Neurol. 2010;518:3931–47. doi: 10.1002/cne.22430. [DOI] [PubMed] [Google Scholar]
- Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, Gregg J, Lu G, Feldman HH, Mackenzie IR, Raymond LA, Leavitt BR. Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AM, Neary D, Snowden JS, Mann DM. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–31. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- Plowman GD, Green JM, Neubauer MG, Buckley SD, McDonald VL, Todaro GJ, Shoyab M. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem. 1992;267:13073–8. [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–68. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ, Crook RJ, Josephs KA, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Caselli RJ, Wszolek ZK, Uitti RJ, Feldman H, Hutton ML, Mackenzie IR, Graff-Radford NR, Dickson DW. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17:3631–42. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song YQ, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollinson S, Rohrer JD, van der Zee J, Sleegers K, Mead S, Engelborghs S, Collinge J, De Deyn PP, Mann DM, Van Broeckhoven C, Pickering-Brown SM. No association of PGRN 3′UTR rs5848 in frontotemporal lobar degeneration. Neurobiol Aging. 2011;32:754–5. doi: 10.1016/j.neurobiolaging.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Rosen EY, Wexler EM, Versano R, Coppola G, Gao F, Winden KD, Oldham MC, Martens LH, Zhou P, Farese RV, Jr, Geschwind DH. Functional genomic analyses identify pathways dysregulated by progranulin deficiency, implicating Wnt signaling. Neuron. 2011;71:1030–42. doi: 10.1016/j.neuron.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CL, Baranowski DC, Chitramuthu BP, Malik S, Li Z, Cao M, Minotti S, Durham HD, Kay DG, Shaw CA, Bennett HP, Bateman A. Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci. 2009;10:130. doi: 10.1186/1471-2202-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–52. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran SS, Capell A, Hruscha AT, Fellerer K, Neumann M, Schmid B, Haass C. Missense mutations in the progranulin gene linked to frontotemporal lobar degeneration with ubiquitin-immunoreactive inclusions reduce progranulin production and secretion. J Biol Chem. 2008;283:1744–53. doi: 10.1074/jbc.M705115200. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sorensen SA, Gydesen S, Fisher EM, Collinge J. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–8. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- Songsrirote K, Li Z, Ashford D, Bateman A, Thomas-Oates J. Development and application of mass spectrometric methods for the analysis of progranulin N-glycosylation. J Proteomics. 2010 doi: 10.1016/j.jprot.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Bird TD, Ghetti B. Frontotemporal dementia and Parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol. 1998;8:387–402. doi: 10.1111/j.1750-3639.1998.tb00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Yonezawa T, Fujioka H, Matuamuro M, Nishihara M. Induction of granulin precursor gene expression by estrogen treatment in neonatal rat hypothalamus. Neurosci Lett. 2001;297:199–202. doi: 10.1016/s0304-3940(00)01699-2. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nishiahara M. Granulin precursor gene: a sex steroid-inducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab. 2002;75:31–7. doi: 10.1006/mgme.2001.3274. [DOI] [PubMed] [Google Scholar]
- Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, Syed NM, Lai Y, Lin EA, Kong L, Su J, Yin F, Ding AH, Zanin-Zhorov A, Dustin ML, Tao J, Craft J, Yin Z, Feng JQ, Abramson SB, Yu XP, Liu CJ. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia L, Milnerwood A, Guo A, Mills F, Yoshida E, Vasuta C, Mackenzie IR, Raymond L, Cynader M, Jia W, Bamji SX. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J Neurosci. 2011;31:11126–32. doi: 10.1523/JNEUROSCI.6244-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B, Nyengaard JR, Tessarollo L, Lewin GR, Willnow TE, Chao MV, Nykjaer A. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci. 2011;14:54–61. doi: 10.1038/nn.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, Arnold SE, Mann DM, Pickering-Brown SM, Seelaar H, Heutink P, van Swieten JC, Murrell JR, Ghetti B, Spina S, Grafman J, Hodges J, Spillantini MG, Gilman S, Lieberman AP, Kaye JA, Woltjer RL, Bigio EH, Mesulam M, Al-Sarraj S, Troakes C, Rosenberg RN, White CL, 3rd, Ferrer I, Llado A, Neumann M, Kretzschmar HA, Hulette CM, Welsh-Bohmer KA, Miller BL, Alzualde A, de Munain AL, McKee AC, Gearing M, Levey AI, Lah JJ, Hardy J, Rohrer JD, Lashley T, Mackenzie IR, Feldman HH, Hamilton RL, Dekosky ST, van der Zee J, Kumar-Singh S, Van Broeckhoven C, Mayeux R, Vonsattel JP, Troncoso JC, Kril JJ, Kwok JB, Halliday GM, Bird TD, Ince PG, Shaw PJ, Cairns NJ, Morris JC, McLean CA, DeCarli C, Ellis WG, Freeman SH, Frosch MP, Growdon JH, Perl DP, Sano M, Bennett DA, Schneider JA, Beach TG, Reiman EM, Woodruff BK, Cummings J, Vinters HV, Miller CA, Chui HC, Alafuzoff I, Hartikainen P, Seilhean D, Galasko D, Masliah E, Cotman CW, Tunon MT, Martinez MC, Munoz DG, Carroll SL, Marson D, Riederer PF, Bogdanovic N, Schellenberg GD, Hakonarson H, Trojanowski JQ, Lee VM. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–9. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee J, Van Broeckhoven C. TMEM106B a novel risk factor for frontotemporal lobar degeneration. J Mol Neurosci. 2011;45:516–21. doi: 10.1007/s12031-011-9555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Van Damme P, Cruchaga C, Gitcho MA, Vidal JM, Seijo-Martinez M, Wang L, Wu JY, Robberecht W, Goate A. Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J Neurochem. 2010a;112:1305–15. doi: 10.1111/j.1471-4159.2009.06546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WX, Wilfred BR, Madathil SK, Tang G, Hu Y, Dimayuga J, Stromberg AJ, Huang Q, Saatman KE, Nelson PT. miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol. 2010b;177:334–45. doi: 10.2353/ajpath.2010.091202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–81. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Petersen CM, Nykjaer A. VPS10P-domain receptors - regulators of neuronal viability and function. Nat Rev Neurosci. 2008;9:899–909. doi: 10.1038/nrn2516. [DOI] [PubMed] [Google Scholar]
- Xu SQ, Tang D, Chamberlain S, Pronk G, Masiarz FR, Kaur S, Prisco M, Zanocco-Marani T, Baserga R. The granulin/epithelin precursor abrogates the requirement for the insulin-like growth factor 1 receptor for growth in vitro. J Biol Chem. 1998;273:20078–83. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, Nathan C, Ding A. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–28. S1–4. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–40. [PubMed] [Google Scholar]
- Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, Pickering-Brown S, Dickson D, Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–4. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Brady OA, Meng PS, Mao Y, Hu F. C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PLoS One. 2011;6:e21023. doi: 10.1371/journal.pone.0021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gao G, Crabb JW, Serrero G. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J Biol Chem. 1993;268:10863–9. [PubMed] [Google Scholar]
- Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–78. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]