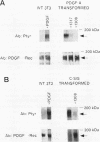
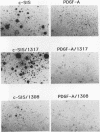
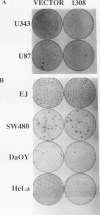
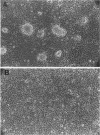
Abstract
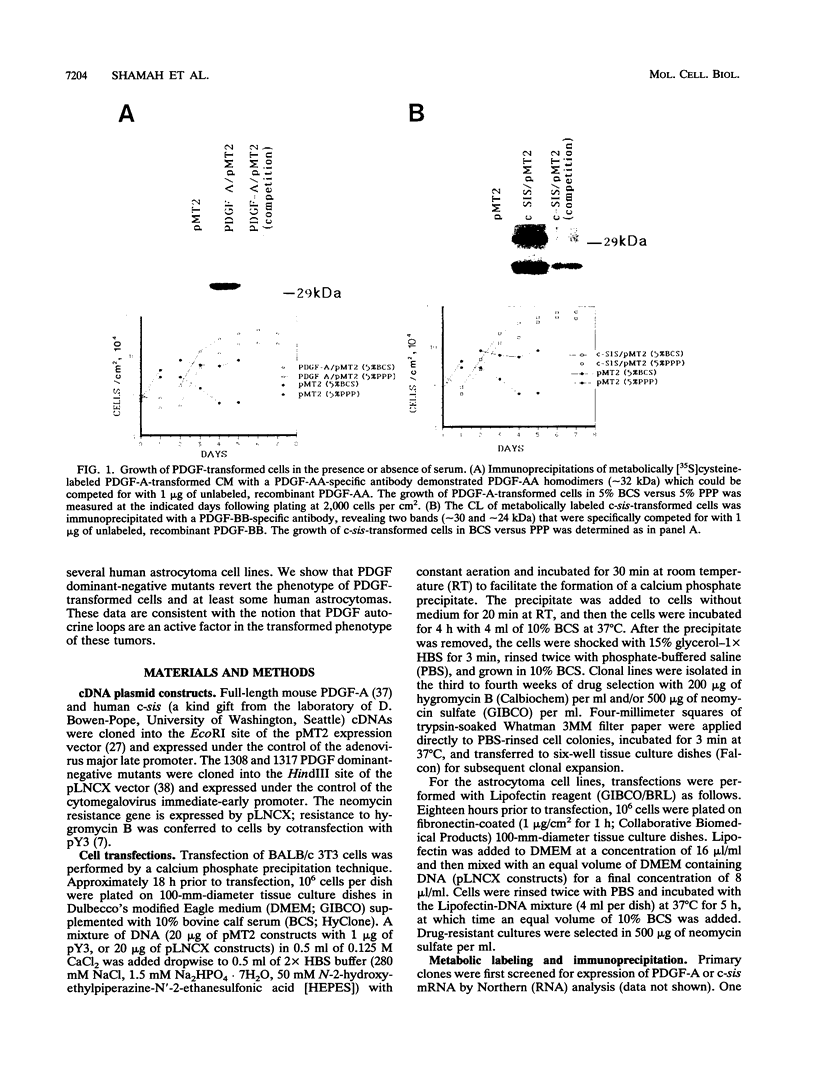
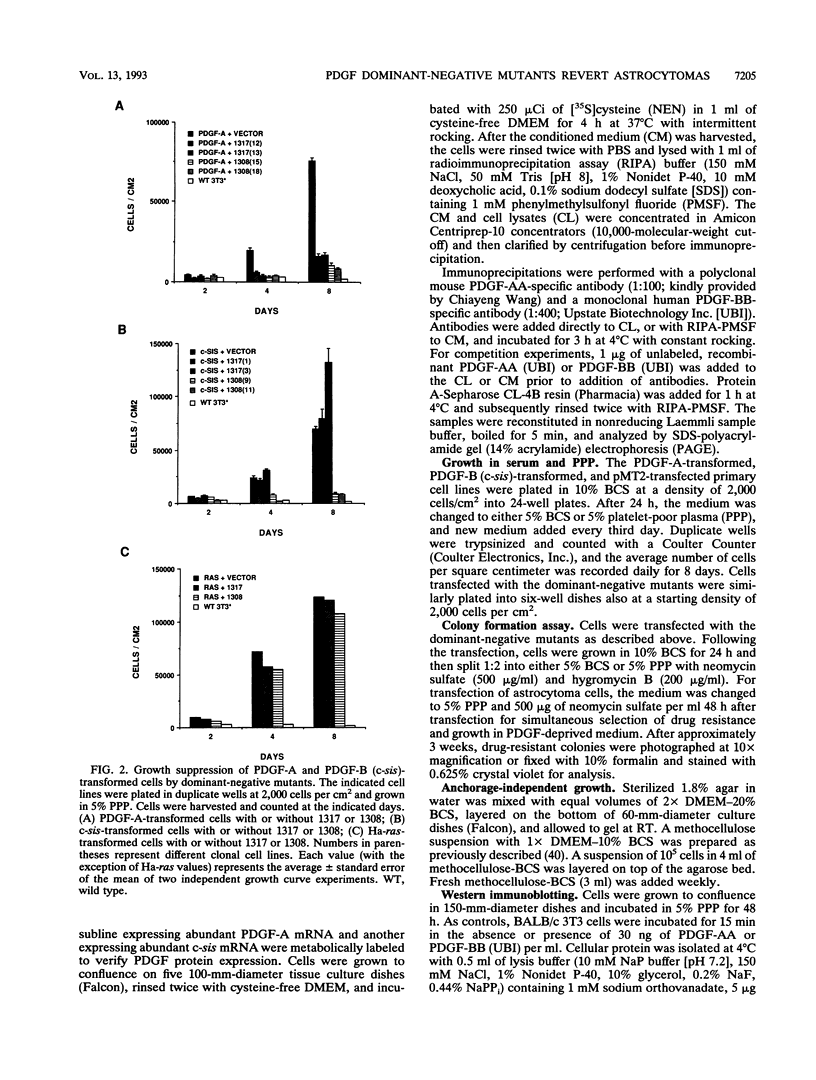
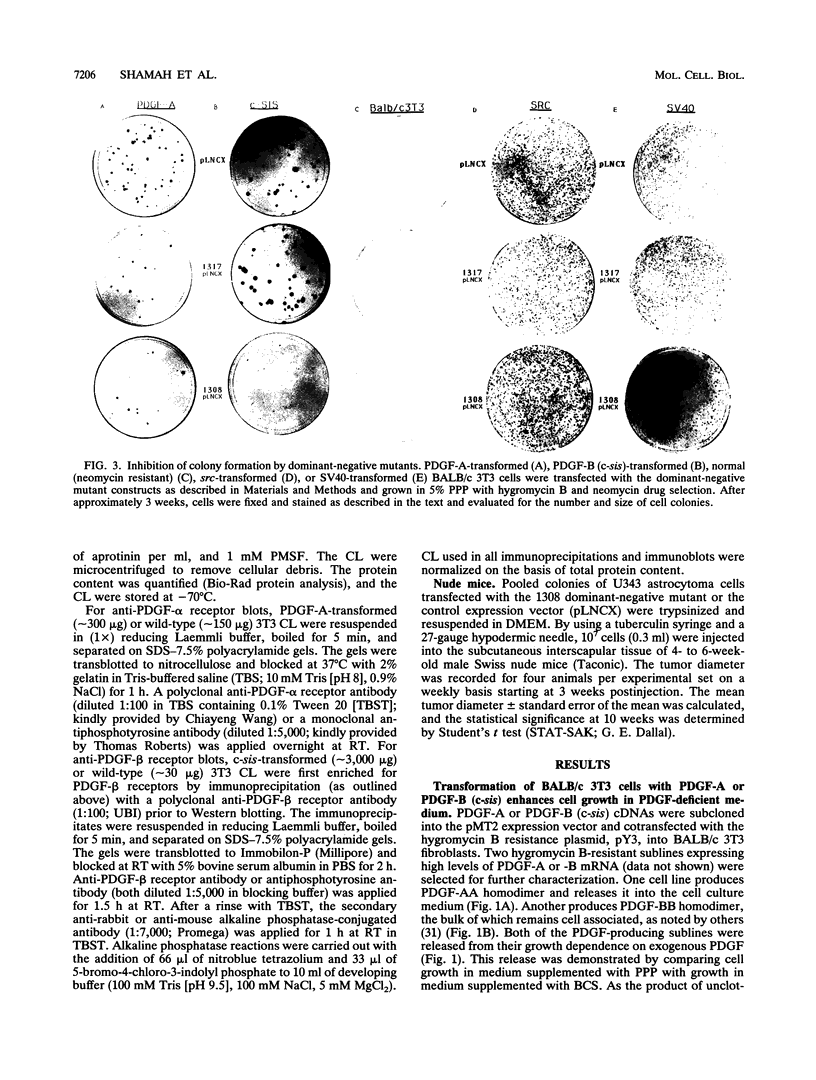
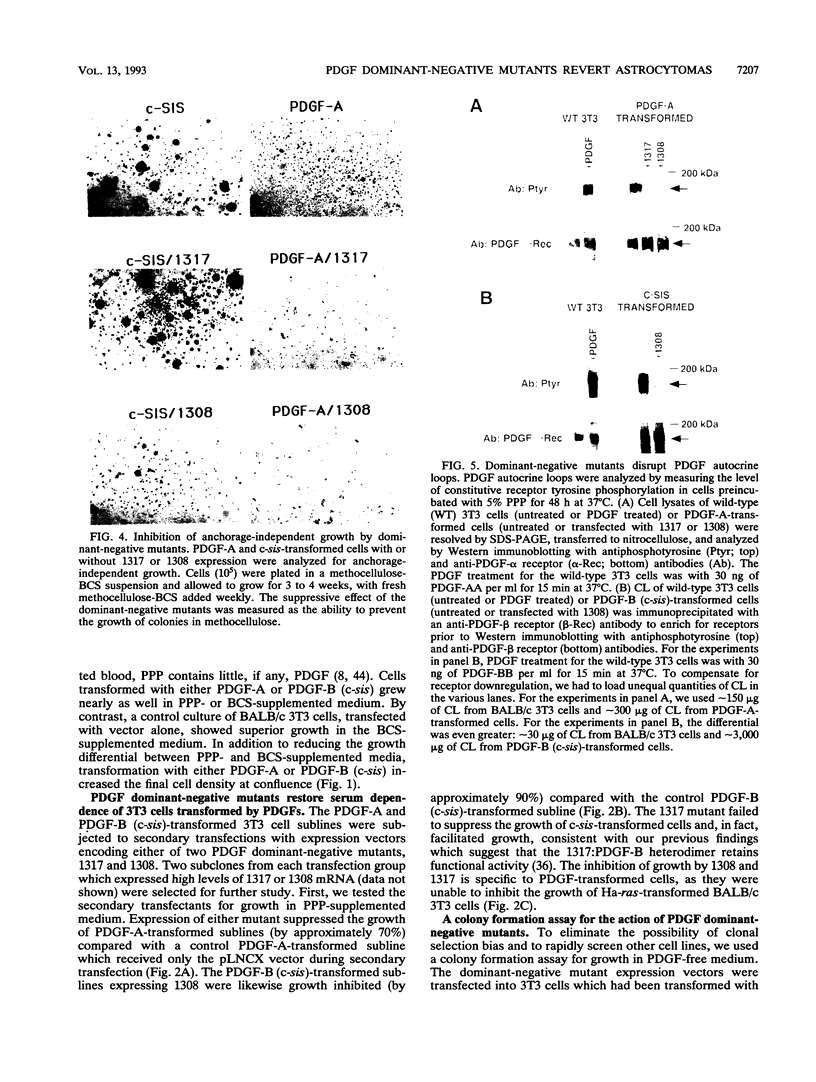
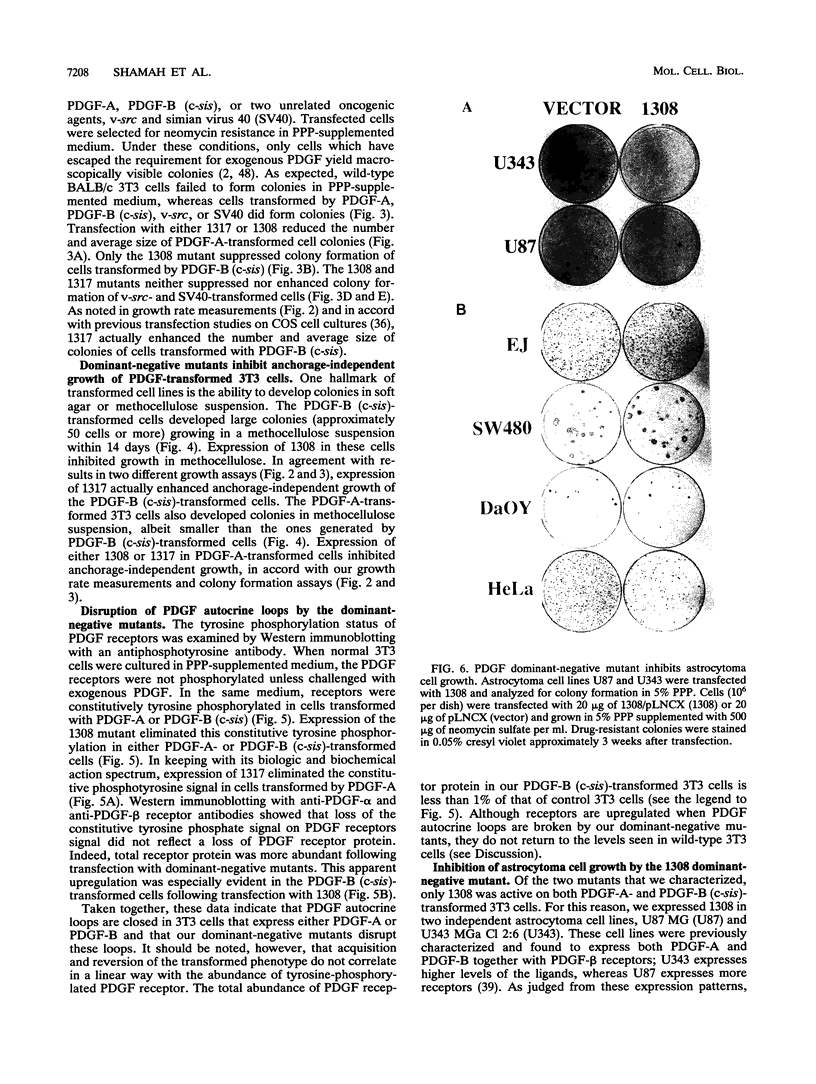
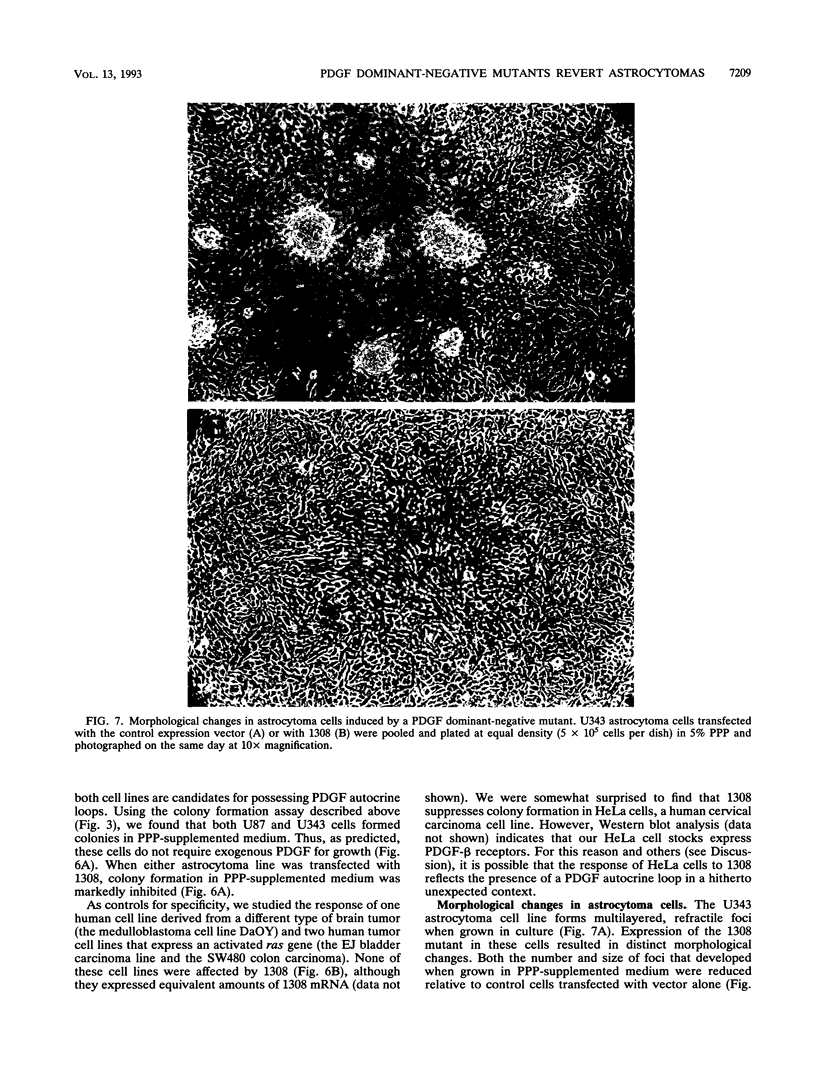
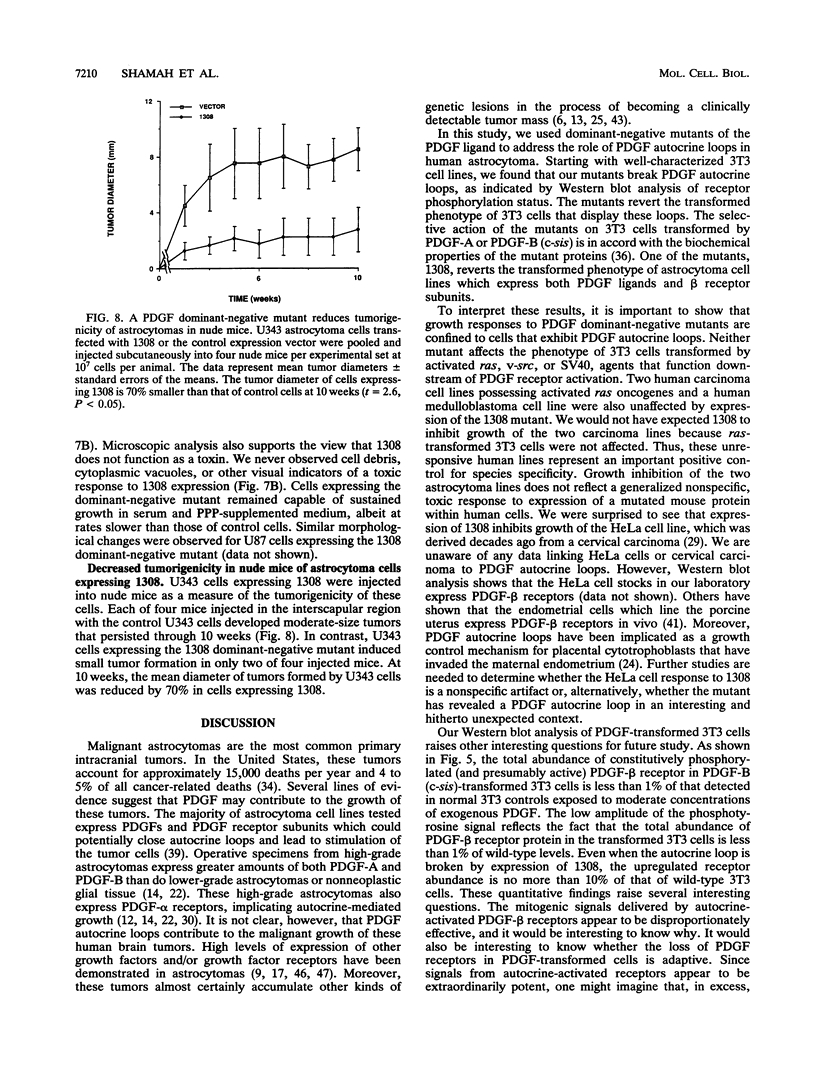
Malignant astrocytoma is the most common primary human brain tumor. Most astrocytomas express a combination of platelet-derived growth factor (PDGF) and PDGF receptor which could close an autocrine loop. It is not known whether these autocrine loops contribute to the transformed phenotype of astrocytoma cells or are incidental to that phenotype. Here we show that dominant-negative mutants of the PDGF ligand break the autocrine loop and revert the phenotype of BALB/c 3T3 cells transformed by the PDGF-A or PDGF-B (c-sis) gene. Then, we show that these mutants are selective in that they do not alter the phenotype of 3T3 cells transformed by an activated Ha-ras or v-src gene or by simian virus 40. Finally, we show that these mutants revert the transformed phenotype of two independent human astrocytoma cell lines. They have no effect on the growth of human medulloblastoma, bladder carcinoma, or colon carcinoma cell lines. These observations are consistent with the view that PDGF autocrine loops contribute to the transformed phenotype of at least some human astrocytomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Galanopoulos T., Neville-Golden J., O'Hara C. J. Malignant epithelial cells in primary human lung carcinomas coexpress in vivo platelet-derived growth factor (PDGF) and PDGF receptor mRNAs and their protein products. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3942–3946. doi: 10.1073/pnas.89.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Bejcek B. E., Hoffman R. M., Lipps D., Li D. Y., Mitchell C. A., Majerus P. W., Deuel T. F. The v-sis oncogene product but not platelet-derived growth factor (PDGF) A homodimers activate PDGF alpha and beta receptors intracellularly and initiate cellular transformation. J Biol Chem. 1992 Feb 15;267(5):3289–3293. [PubMed] [Google Scholar]
- Bejcek B. E., Li D. Y., Deuel T. F. Transformation by v-sis occurs by an internal autoactivation mechanism. Science. 1989 Sep 29;245(4925):1496–1499. doi: 10.1126/science.2551043. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Burger P. C., Mahaley M. S., Jr, Bullard D. E., Muhlbaier L. H., Bigner D. D. Specific chromosomal abnormalities in malignant human gliomas. Cancer Res. 1988 Jan 15;48(2):405–411. [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Ekstrand A. J., James C. D., Cavenee W. K., Seliger B., Pettersson R. F., Collins V. P. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991 Apr 15;51(8):2164–2172. [PubMed] [Google Scholar]
- Fleming T. P., Matsui T., Heidaran M. A., Molloy C. J., Artrip J., Aaronson S. A. Demonstration of an activated platelet-derived growth factor autocrine pathway and its role in human tumor cell proliferation in vitro. Oncogene. 1992 Jul;7(7):1355–1359. [PubMed] [Google Scholar]
- Fleming T. P., Matsui T., Molloy C. J., Robbins K. C., Aaronson S. A. Autocrine mechanism for v-sis transformation requires cell surface localization of internally activated growth factor receptors. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8063–8067. doi: 10.1073/pnas.86.20.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T. P., Saxena A., Clark W. C., Robertson J. T., Oldfield E. H., Aaronson S. A., Ali I. U. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992 Aug 15;52(16):4550–4553. [PubMed] [Google Scholar]
- Fults D., Brockmeyer D., Tullous M. W., Pedone C. A., Cawthon R. M. p53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Res. 1992 Feb 1;52(3):674–679. [PubMed] [Google Scholar]
- Hammacher A., Hellman U., Johnsson A., Ostman A., Gunnarsson K., Westermark B., Wasteson A., Heldin C. H. A major part of platelet-derived growth factor purified from human platelets is a heterodimer of one A and one B chain. J Biol Chem. 1988 Nov 5;263(31):16493–16498. [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Autocrine stimulation by the v-sis gene product requires a ligand-receptor interaction at the cell surface. J Cell Biol. 1988 Jul;107(1):287–298. doi: 10.1083/jcb.107.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsh G. R., 4th, Rosenblum M. L., Williams L. T. Oncogene-related growth factors and growth factor receptors in human malignant glioma-derived cell lines. J Neurooncol. 1989 May;7(1):47–56. doi: 10.1007/BF00149378. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Bailey M., Curtis D. A., Osborn S., Raines E., Ross R., Forstrom J. W. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990 Jan 9;29(1):166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- Heidaran M. A., Pierce J. H., Yu J. C., Lombardi D., Artrip J. E., Fleming T. P., Thomason A., Aaronson S. A. Role of alpha beta receptor heterodimer formation in beta platelet-derived growth factor (PDGF) receptor activation by PDGF-AB. J Biol Chem. 1991 Oct 25;266(30):20232–20237. [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Ernlund A., Rorsman C., Rönnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989 May 25;264(15):8905–8912. [PubMed] [Google Scholar]
- Hermanson M., Funa K., Hartman M., Claesson-Welsh L., Heldin C. H., Westermark B., Nistér M. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992 Jun 1;52(11):3213–3219. [PubMed] [Google Scholar]
- Hermansson M., Nistér M., Betsholtz C., Heldin C. H., Westermark B., Funa K. Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7748–7752. doi: 10.1073/pnas.85.20.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren L., Claesson-Welsh L., Heldin C. H., Ohlsson R. The expression of PDGF alpha- and beta-receptors in subpopulations of PDGF-producing cells implicates autocrine stimulatory loops in the control of proliferation in cytotrophoblasts that have invaded the maternal endometrium. Growth Factors. 1992;6(3):219–231. doi: 10.3109/08977199209026929. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- Kanakaraj P., Raj S., Khan S. A., Bishayee S. Ligand-induced interaction between alpha- and beta-type platelet-derived growth factor (PDGF) receptors: role of receptor heterodimers in kinase activation. Biochemistry. 1991 Feb 19;30(7):1761–1767. doi: 10.1021/bi00221a005. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Kumabe T., Sohma Y., Kayama T., Yoshimoto T., Yamamoto T. Amplification of alpha-platelet-derived growth factor receptor gene lacking an exon coding for a portion of the extracellular region in a primary brain tumor of glial origin. Oncogene. 1992 Apr;7(4):627–633. [PubMed] [Google Scholar]
- LaRochelle W. J., May-Siroff M., Robbins K. C., Aaronson S. A. A novel mechanism regulating growth factor association with the cell surface: identification of a PDGF retention domain. Genes Dev. 1991 Jul;5(7):1191–1199. doi: 10.1101/gad.5.7.1191. [DOI] [PubMed] [Google Scholar]
- Lee B. A., Donoghue D. J. Intracellular retention of membrane-anchored v-sis protein abrogates autocrine signal transduction. J Cell Biol. 1992 Sep;118(5):1057–1070. doi: 10.1083/jcb.118.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokeshwar V. B., Huang S. S., Huang J. S. Intracellular turnover, novel secretion, and mitogenically active intracellular forms of v-sis gene product in simian sarcoma virus-transformed cells. Implications for intracellular loop autocrine transformation. J Biol Chem. 1990 Jan 25;265(3):1665–1675. [PubMed] [Google Scholar]
- Mahaley M. S., Jr, Mettlin C., Natarajan N., Laws E. R., Jr, Peace B. B. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989 Dec;71(6):826–836. doi: 10.3171/jns.1989.71.6.0826. [DOI] [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercola M., Deininger P. L., Shamah S. M., Porter J., Wang C. Y., Stiles C. D. Dominant-negative mutants of a platelet-derived growth factor gene. Genes Dev. 1990 Dec;4(12B):2333–2341. doi: 10.1101/gad.4.12b.2333. [DOI] [PubMed] [Google Scholar]
- Mercola M., Wang C. Y., Kelly J., Brownlee C., Jackson-Grusby L., Stiles C., Bowen-Pope D. Selective expression of PDGF A and its receptor during early mouse embryogenesis. Dev Biol. 1990 Mar;138(1):114–122. doi: 10.1016/0012-1606(90)90181-h. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Nistér M., Claesson-Welsh L., Eriksson A., Heldin C. H., Westermark B. Differential expression of platelet-derived growth factor receptors in human malignant glioma cell lines. J Biol Chem. 1991 Sep 5;266(25):16755–16763. [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Rönnstrand L., Beckmann M. P., Faulders B., Ostman A., Ek B., Heldin C. H. Purification of the receptor for platelet-derived growth factor from porcine uterus. J Biol Chem. 1987 Mar 5;262(7):2929–2932. [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Sidransky D., Mikkelsen T., Schwechheimer K., Rosenblum M. L., Cavanee W., Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992 Feb 27;355(6363):846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Singh J. P., Chaikin M. A., Stiles C. D. Phylogenetic analysis of platelet-derived growth factor by radio-receptor assay. J Cell Biol. 1982 Nov;95(2 Pt 1):667–671. doi: 10.1083/jcb.95.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits A., Funa K., Vassbotn F. S., Beausang-Linder M., af Ekenstam F., Heldin C. H., Westermark B., Nistér M. Expression of platelet-derived growth factor and its receptors in proliferative disorders of fibroblastic origin. Am J Pathol. 1992 Mar;140(3):639–648. [PMC free article] [PubMed] [Google Scholar]
- Takahashi J. A., Mori H., Fukumoto M., Igarashi K., Jaye M., Oda Y., Kikuchi H., Hatanaka M. Gene expression of fibroblast growth factors in human gliomas and meningiomas: demonstration of cellular source of basic fibroblast growth factor mRNA and peptide in tumor tissues. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5710–5714. doi: 10.1073/pnas.87.15.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. J., Bigner S. H., Bigner D. D., Kinzler K. W., Hamilton S. R., Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Goldfarb M. Growth factor requirements of oncogene-transformed NIH 3T3 and BALB/c 3T3 cells cultured in defined media. Mol Cell Biol. 1986 Oct;6(10):3541–3544. doi: 10.1128/mcb.6.10.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]