Abstract
Pathogenicity islands (PAIs) play an important role in Shiga toxin-producing Escherichia coli (STEC) pathogenicity. The distribution of PAIs OI-122, OI-43/48, and OI-57 and a high-pathogenicity island (HPI) were determined among 98 STEC strains assigned to seropathotypes (SPTs) A to E. PCR and PCR-restriction fragment length polymorphism assays were used to identify 14 virulence genes that belonged to the four PAIs and to subtype eae and stx genes, respectively. Phylogenetic trees were constructed based on the sequences of pagC among 34 STEC strains and iha among 67 diverse pathogenic E. coli, respectively. Statistical analysis demonstrated that the prevalences of OI-122 (55.82%) and OI-57 (82.35%) were significantly greater in SPTs (i.e., SPTs A, B, and C) that are frequently associated with severe disease than in other SPTs. terC (62.5%) and ureC (62.5%) in OI-43/48 were also significantly more prevalent in SPTs A, B, and C than in SPTs D and E. In addition, OI-122, OI-57, and OI-43/48 and their associated virulence genes (except iha) were found to be primarily associated with eae-positive STEC, whereas HPI occurred independently of the eae presence. The strong association of OI-122, OI-43/48, and OI-57 with eae-positive STEC suggests in part that different pathogenic mechanisms exist between eae-positive and eae-negative STEC strains. Virulence genes in PAIs that are associated with severe diseases can be used as potential markers to aid in identifying highly virulent STEC.
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) can cause human illnesses ranging from self-limiting diarrhea to life-threatening diseases such as hemolytic-uremic syndrome (HUS), a leading cause of kidney failure in children (1). E. coli O157:H7 is the single serotype that causes most STEC outbreaks and HUS cases. Like O157, non-O157 STEC can also cause severe diseases and food-borne outbreaks (1). More than 470 non-O157 STEC serotypes have been associated with human illness (2), and public health concerns regarding non-O157 STEC are increasing (1). Estimations indicate that non-O157 STEC strains cause 112,752 illness each year in the United States, almost twice the number of O157:H7 illnesses (63,153) (3). Although some non-O157 STEC strains have been associated with disease symptoms indistinguishable from O157:H7, not all STEC serotypes can cause HUS and outbreaks, and some STEC serotypes have never been reported to be related to any human illness (4). The scientific basis for this difference, however, is poorly understood.
Increasing evidence shows that differences in virulence between pathogenic and nonpathogenic bacterial strains can be attributed in part to virulence genes located in pathogenicity islands (PAIs) (5). PAIs usually contain blocks of virulence genes and are >10 kb (6). Several PAIs have been identified and characterized in STEC. A chromosomal pathogenicity island termed the locus of enterocyte effacement (LEE) was identified in E. coli O157:H7 strain EDL933, which encodes a type III secretion system, as well as virulence genes (eae and tir) associated with the intimate attachment of bacteria to intestinal epithelial cells (4). LEE appears to confer enhanced virulence, since LEE-positive STEC are much more commonly associated with HUS and outbreaks than LEE-negative STEC (5). However, some LEE-positive STEC serotypes have never been associated with disease, and some LEE-negative STEC can cause HUS and outbreaks, indicating that virulence factors other than those in LEE may contribute to pathogenesis of STEC (5).
Pathogenicity island OI-122 is also well characterized in O157:H7 (5, 7). OI-122 is a 23-kb PAI consisting of three modules (5, 8, 9). Z4321 is located in module 1 and encodes a protein sharing 46% similarity with the phoP-activated gene C (pagC) of Salmonella enterica serovar Typhimurium (5, 9). Z4326, Z4328, and Z4329 are located in module 2. Z4326 (sen) encodes a protein that shares 38.2% similarity to Shigella flexneri enterotoxin 2 (5), whereas Z4328 and Z4329 encode proteins that have 89 and 86% similarity to non-LEE-encoded effectors NleB and NleE, respectively (9). The enterohemorrhagic E. coli factor for adherence (Efa), which is involved in epithelial cell adhesion and inhibiting the proliferation of bovine peripheral blood lymphocytes, is located in module 3 (10).
OI-43 and OI-48 are duplicate genomic islands found in EDL933 (8). OI-43/48 genes are divided into three functional groups: a seven-gene cluster ureDABCEFG that encodes urease and accessory proteins hydrolyzing urea to ammonia and carbon dioxide; telluride resistance genes terZABCDEF (11); and two putative adhesion genes, iha (iron-regulated gene A) and aidA-1 (autotransporter adhesin involved in diffuse adherence) (12).
In EDL933, OI-57 contains non-LEE-encoded effector genes nleG2-3, nleG6-2, and nleG5-2 (13, 14). NleG proteins are E3 ubiquitin ligases analogous to RING finger and U-box enzymes in eukaryotes. Although the exact functions of NleG2-3, NleG6-2, and NleG 5-2 are still unclear, similar proteins have been identified as effectors that suppress immune response from the host (15).
The high-pathogenicity island (HPI) was first detected in Yersinia pestis and other highly virulent Yersinia species and encodes a siderophore (yersiniabactin)-mediated iron-uptake system (16). HPI is required for full virulence expression in Yersinia (16) and contains two main virulence genes, fyuA and irp2. FyuA is an outer membrane protein acting as a receptor for ferric-yersiniabactin uptake and for bacteriocin pesticin, whereas Irp2 is involved in yersiniabactin synthesis (17). An orthologous and highly conserved HPI is widely distributed among different species and genera of the family Enterobacteriaceae (16).
Few studies to date have investigated PAIs other than LEE in STEC. Since PAIs are normally absent in nonpathogenic strains of the same or closely related species, they may serve as useful markers to distinguish highly virulent strains from less-virulent or harmless strains (5, 6). In addition, PAIs can be used to identify new and emerging pathogenic bacteria. We report here the distribution of OI-122, OI-43/48, OI-57, and HPI and their virulence genes in STEC and evaluate the association of the PAIs and individual virulence genes with STEC seropathotypes (SPTs) linked to severe diseases and outbreaks. In addition, the association of the four PAIs with LEE was determined.
MATERIALS AND METHODS
Bacterial strains.
A total of 98 STEC strains from humans, animals, and food were used in the present study (see Table S1 in the supplemental material). Strains were classified into SPTs A to E according to the criteria described by Karmali et al. (5). The assignment of SPTs was based on published references (5, 14, 18) and a large online database on non-O157 STEC (http://www.lugo.usc.es/ecoli/serotiposhum.htm).
stx and eae subtyping.
stx and eae subtypes were determined using PCR-restriction fragment length polymorphism (RFLP) analysis (19, 20), and stx2dact was confirmed by PCR as previously described (21). Genomic DNA was extracted using boiling method as previously described (22, 23). STEC strains S1191 (stx2e), EDL933 (stx1a and stx2a), E32511 (stx2c), EH250 (stx2b), B2F1 (stx2dact), and N15018 (stx1c) were used as positive controls for the stx subtyping; STEC strains 86-24 (gamma 1), EDL933 (gamma 1), TW06584 (kappa), E2348-69 (alpha), TW07920 (epsilon), RDEC-1 (beta), TW10366 (rho), TW03501 (iota), TW07892 (eta), and TW01387 (gamma 2/theta) were used as positive controls for the eae subtyping. E. coli K-12 was used as a negative control strain for both stx and eae subtyping.
Presence of OI-122, OI-43/48, O-57, and HPI.
PCR assays were used to determine the presence of 14 virulence genes in STEC OI-122, OI-43/48, OI-57, and HPI as described previously (5, 11, 13, 24, 25). The presence of a PAI was determined by several marker genes located in different regions of the island, including pagC, sen, nleB, efa-1, and efa-2 for OI-122; terC, ureC, iha, and aidA-1 for OI-43/48; nle2-3, nleG6-2, and nleG5-2 for OI-57; and irp2 and fyuA for HPI. PCR was performed in a 25-μl reaction mixture, containing 2 μl of DNA template, 2.5 μl of 10× PCR buffer, 2 μl of a 25-mmol liter−1 solution of MgCl2, 2 μl of a 1.25-mmol liter−1 deoxynucleoside triphosphate mixture, 0.125 μl of a 5-U μl−1 AmpliTaq Gold DNA polymerase mixture (Applied Biosystems, Branchburg, NJ), and 0.2 μl of a 50-pmol μl−1 concentration of each primer. E. coli O157:H7 EDL 933 was used as a positive control for the virulence genes of OI-122, OI-43/48, and OI-57 and E. coli O26:H11 SJ-13 for the virulence genes of HPI. E. coli K-12 was used as a negative control for all PCR assays.
Phylogenetic and sequence analysis.
iha and pagC were the only two genes that were highly prevalent in both eae-positive and eae-negative STEC strains. To determine the evolutionary relationship between the two groups of STEC, iha and pagC were selected for phylogenetic analysis studies. iha sequences from 67 E. coli and Shigella strains were obtained from GenBank. A multiple sequence alignment of iha was performed using CLUSTAL W in MEGA 5.05, and a maximum-likelihood phylogenetic tree was generated using the general time reversible model (26). A bootstrapping of 2,000 replicates was used to estimate the confidence of the branching patterns of the phylogenetic tree using iha of E. coli SMS-3-5 as the phylogenetic tree's root.
In addition, PCR was used to amplify pagC of OI-122 from 12 selected STEC strains representing different serotypes, as described by Konczy et al. (9). PCR products were sequenced by GeneWiz (Germantown, MD). Twenty-two pagC sequences representing different STEC serotypes and one Citrobacter strain were downloaded from GenBank. The pagC sequences were cropped to 446 bp prior to alignment. Phylogenetic analysis was performed using CLUSTAL W within MEGA 5.05 (26). A phylogenetic tree based on pagC sequences was constructed using maximum-likelihood methods by MEGA 5.05 with bootstrapping of 2,000 replicates using pagC of Citrobacter rodentium IC168 as the tree's root.
Statistical analysis.
A chi-square or Fisher exact test was used for data analysis using SAS9.2 (SAS Institute, Cary, NC). A P value of <0.01 was considered statistically significant.
RESULTS
Distribution of OI-122, OI-43/48, OI-57, and HPI in STEC strains.
The 98 STEC strains were classified into SPTs A to E (see Table S1 in the supplemental material). Overall, the prevalence of OI-122 and OI-57 decreased progressively from SPT A to SPT E (see Fig. S1 in the supplemental material). The prevalences of OI-122 and OI-57 were significantly greater in SPTs associated with severe diseases (SPTs A, B, and C) and outbreaks (SPTs A and B) than in other SPTs (P < 0.0001) (Table 1). Although the prevalence of OI-43/48 was greater in SPTs associated with HUS (SPTs A, B, and C) and outbreaks (SPTs A and B) than in other SPTs, the differences were not statistically significant (P = 0.1356 and 0.02, respectively). HPI was not found in SPT A (O157) but was almost evenly distributed from SPT B to SPT E (see Fig. S1 in the supplemental material).
Table 1.
Association of PAIs and virulence genes with SPTs related to outbreak (SPTs A and B), severe disease (SPTs A, B, and C), and LEE
| PAI | Gene | Prevalence (%)a |
|||||
|---|---|---|---|---|---|---|---|
| Association with SPTs related to outbreak |
Association with SPTs related to severe disease |
Association with LEE |
|||||
| In SPTs A and B (n = 34) | In SPTs C, D and E (n = 64) | In STEC SPTs A, B, and C (n = 56) | In STEC in SPTs D and E (n = 42) | In eae-positive STEC (n = 54) | In eae-negative STEC (n = 44) | ||
| OI-122 | 55.82* | 17.18* | 46.43† | 9.52† | 55.56‡ | 0‡ | |
| pagC | 70.59 | 46.88 | 69.64 † | 35.71† | 64.81‡ | 38.64‡ | |
| sen | 100.00* | 31.25* | 76.79 † | 26.45† | 100.00‡ | 2.27‡ | |
| efa-1 | 82.35* | 31.25* | 66.07† | 30.95† | 88.89‡ | 6.82‡ | |
| efa-2 | 82.35* | 31.25* | 66.07† | 26.45† | 88.89‡ | 2.27‡ | |
| nleB | 100.00* | 31.25* | 76.79† | 26.45† | 100.00‡ | 2.27‡ | |
| OI-43/48 | 32.35 | 12.50 | 26.79 | 14.28 | 37.03‡ | 2.27‡ | |
| terC | 76.47* | 35.94* | 62.50† | 33.33† | 81.48‡ | 11.36‡ | |
| ureC | 76.47* | 31.25* | 62.50† | 26.45† | 81.48‡ | 4.54‡ | |
| iha | 64.70 | 70.31 | 64.29 | 73.80 | 57.41‡ | 84.09‡ | |
| aidA-1 | 38.23 | 32.81 | 39.28 | 28.57 | 65.91‡ | 18.18‡ | |
| OI-57 | 82.35* | 21.86* | 60.71† | 19.05† | 75.93‡ | 2.27‡ | |
| nleG2-3 | 97.06* | 31.25* | 73.21† | 28.57† | 94.44‡ | 6.82‡ | |
| nleG5-2 | 82.35* | 21.88* | 60.71† | 19.04† | 85.19‡ | 2.27‡ | |
| nleG6-2 | 97.18* | 28.13* | 66.07† | 28.57† | 75.93‡ | 4.56‡ | |
| HPI | 17.65 | 25.00 | 17.86 | 28.57 | 25.92 | 18.18 | |
| fyuA | 17.65 | 23.44 | 16.07 | 28.57 | 25.92 | 18.18 | |
| irp2 | 17.65 | 23.44 | 16.07 | 28.57 | 25.92 | 18.18 | |
*, Statistically significant difference between SPTs A and B compared to SPTs C, D, and E; †, statistically significant difference between SPTs A, B, and C compared to SPTs D and E; ‡, statistically significant difference between eae-positive and eae-negative STEC. A P value of <0.01 was considered statistically significant.
sen, nleB, efa-1, efa-2, terC, ureC, nleG2-1, nleG5-2, and nleG6-2 were significantly more prevalent in SPTs A and B than in SPTs C, D, and E (Table 1). pagC, sen, nleB, efa-1, efa-2, terC, ureC, nleG2-1, nleG5-2, and nleG6-2 were statistically more prevalent in SPTs A, B, and C than in SPTs D and E (Table 1). Although aidA-1 was more prevalent in SPTs A and B than in SPTs C, D, and E, the difference was not statistically significant (P = 0.27). iha, fyuA, and irp2 were less prevalent in SPTs A, B, and C than in SPTs D and E, but the differences were not statistically significant.
Distribution of OI-122, OI-43/48, OI-57, and HPI in EHEC O157.
PAIs showed three patterns of distribution in EHEC O157 (see Table S1 in the supplemental material). In the case of β-glucuronidase (GUD)-negative O157:H7, four strains all contained marker genes for OI-122, OI-57, and OI-43/48. In GUD-positive O157: H7, none of the five strains carried efa-1 and efa-2 (located at the third module of OI-122) or aidA-1 (located at the end of OI-43/48). Sorbitol-fermenting O157:NM strains contained all of the virulence genes of OI-122 and OI-57 but were negative for all of the OI-43/48 virulence marker genes, indicating the absence of OI-43/48 in O157:NM. In addition, none of the O157:H7 and O157:NM strains were positive for HPI virulence genes.
Association of OI-122, OI-43/48, OI-57, and HPI with eae.
We compared the distribution of virulence genes of OI-122, OI-43/48, OI-57, and HPI between eae-positive and eae-negative STEC strains. All virulence genes of OI-122 and OI-57 (pagC, sen, nleB, efa-1, efa-2, nleG2-3, nleG5-2, and nleG6-2) were highly prevalent in eae-positive strains (Table 1 and see Table S1 in the supplemental material). However, these genes, with the exception of pagC, were less prevalent in eae-negative STEC (Table 1 and see Table S1 in the supplemental material). Although 38.6% of eae-negative STEC strains were positive for pagC, its prevalence was significantly higher (64.8%) in eae-positive STEC (P = 0.005). There was no apparent physical or functional relationship identified between OI-43/48 and LEE, but three OI-43/48 virulence genes (ureC, terC, and aidA-1) were mainly associated with the presence of eae (P < 0.0001). On the other hand, iha was more prevalent in eae-negative than in eae-positive STEC strains (P = 0.007). For HPI, there were no significant differences in the distribution of fyuA or irp2 between eae-positive and eae-negative STEC (P = 0.36).
Phylogenetic analysis of iha from diverse pathogenic E. coli.
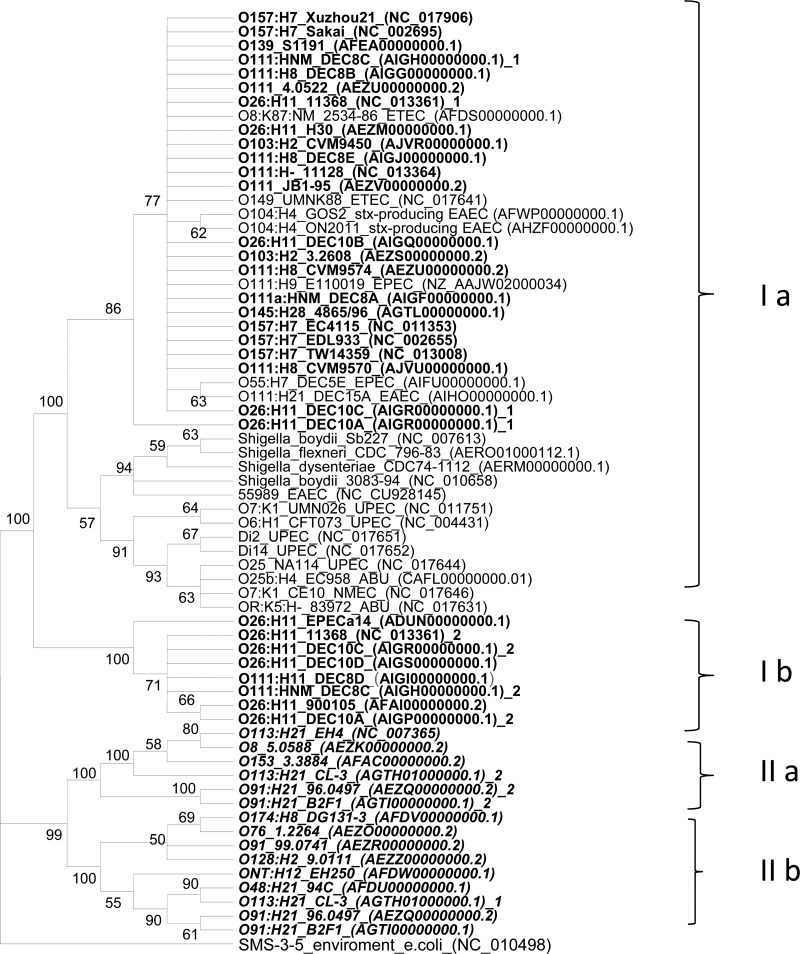
A phylogenetic tree based on iha separated eae-positive and eae-negative STEC strains into two distinct clades (Fig. 1). In clade I, two subgroups—Ia and Ib—shared at least 98.0% sequence similarity. eae-positive EHEC serotypes highly associated with outbreaks and severe diseases were located in clade I (O157:H7, O26:H11, O103:H2, O111:NM, and O145:H28). These sequences shared at least 99% similarity and clustered together with iha from other pathogenic E. coli, including enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), uropathogenic E. coli (UPEC), neonatal meningitis E. coli (NMEC), and Shiga toxin-producing EAEC O104:H4 (from a German outbreak in 2011). iha sequences from the O26:H11, O111:H11, and O111:NM strains formed subgroup Ib and shared at least 98.0% sequence similarity with subgroup Ia. Interestingly, strains DEC10A (O26:H11), DEC10C (O26:H11), 11368 (O26:H11), and DEC8C (O111:NM) carried two iha that clustered separately in Ia and Ib.
Fig 1.
Phylogenetic tree based on iha sequences from 67 E. coli and Shigella strains. iha sequences were aligned, and a tree was constructed using the maximum-likelihood method with 2,000 iterations utilizing MEGA 5.05 (26). iha sequences from eae-positive and eae-negative STEC strains segregated into two distinct clades: clade I (with subgroup Ia and Ib) and clade II (with subgroups IIa and IIb). iha sequences from eae-negative STEC are marked in boldface italic type, and eae-positive STEC strains are marked in boldface regular type. EPEC, enteropathogenic E. coli; EIEC, enteroinvasive E. coli; EAEC, enteroaggregative E. coli; stx-producing EAEC, Shiga toxin-producing EAEC; ETEC, enterotoxigenic E. coli; UPEC, uropathogenic E. coli; NMEC, neonatal meningitis E. coli; ABU, asymptomatic bacteriuria E. coli.
All 15 iha sequences from eae-negative STEC clustered together to form clade II. Multiple sequence alignments demonstrated that iha from eae-negative STEC shared only 91.1–93.6% sequence similarity with iha from clade I. iha from subgroups IIa and IIb shared only 93.8 to 94.3% sequence similarity. As in some eae-positive strains, eae-negative STEC strains CL-3 (O113:H21), 96.0497 (O91:H21), and B2F1 (O91:H21) also carried two iha genes that clustered separately in subgroups IIa and IIb.
Phylogenetic and sequence analysis of pagC.
The pagC phylogenetic tree showed four clades (Fig. 2). eae-positive E. coli STEC formed a single clade with EPEC and ETEC, whereas the eae-negative STEC strains formed two clades, along with one strain that clustered with a C. rodentium strain. We identified 15 single nucleotide polymorphisms and one indel among the 35 pagC sequences. Sequence analysis revealed that an insertion of adenine at nucleotide 388 in two O103:H25 strains, two O45:H2 strains, one O103:H2 strain, and one O103:H6 strain led to a frameshift mutation and that a premature stop codon truncated the protein at the third loop, resulting in the loss of the fourth and last loops.
Fig 2.
Phylogenetic tree based on pagC sequences from 34 pathogenic E. coli strains. pagC sequences were aligned, and a tree was constructed using the maximum-likelihood method with 2,000 iterations utilizing MEGA 5.05 (26). pagC sequences from eae-negative STEC are marked in boldface italic type, and eae-positive STEC strains were marked in boldface regular type. pagC genes sequenced in this study are marked by black frames. EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli.
DISCUSSION
In the present study, STEC PAIs OI-122 and OI-57 were found to be highly associated with seropathotypes that can cause severe disease and outbreaks, as previously demonstrated (7, 13, 14). Several OI-122 virulence factors play important roles in bacterial pathogenesis. For example, PagC can promote the survival of Salmonella within macrophages (5, 9). Efa is an adhesion protein originally described in some EHEC strains (27). The efa-1 gene is almost identical to lifA, an EPEC gene encoding lymphostatin (LifA) (28), which inhibits the proliferation of mitogen-activated lymphocytes and the synthesis of proinflammatory cytokines (28). Efa1/LifA also contributes to EPEC adherence to epithelial cells and is critical for intestinal colonization by C. rodentium (29). NleB is required for full colonization and colonic hyperplasia in mice, and a mutation of nleB abolished the lethality of C. rodentium in C3H/HeJ mice (7, 30).
Whereas OI-122 is highly related to colonization and suppression of the host immune system, the function of OI-57 is largely unknown. Wu et al. (15) determined that NleG-like proteins and U-box enzymes in eukaryotes. Although the targets of the OI-57 Nle effectors are unknown, several similar effectors are primarily involved in suppressing host immune response by degrading immune-related host proteins (15). Thus, it is possible that OI-57, similar to OI-122, would be also related to suppression of the host immune system.
In addition to the virulence genes in OI-122 and OI-57, the genes ureC and terC, located on OI-43/48, were also highly associated with seropathotypes related to severe disease and outbreaks. Urease has been confirmed as an important virulence factor in several bacterial species, such as Helicobacter pylori, Yersinia enterocolitica, Proteus mirabilis, Brucella species, and Klebsiella pneumoniae (31). Mutation of ureC has led to a reduced adherence of EHEC O157:H7 in ligated pig intestine (12). A recent study by Steyert and Kaper (32) revealed that strains with nonfunctional urease were 2-fold less likely to survive passage through the stomach and had a reduced ability to colonize the mouse intestinal tract compared to urease-positive strains. These data demonstrate that urease can help STEC strains survive in the stomach and enhance its competitiveness in colonization in calf and human intestinal tracts. The role of tellurite resistance genes (terZABCDEF) in STEC is still not well understood. Yin et al. (12) showed that mutation of the ter cluster in O157:H7 led to fewer adherence to epithelial cells and smaller bacterial clusters compared to wild-type strains. Therefore, ter genes might encode an adhesin or a gene product that promotes the function of adhesion(s). In addition, tellurite salts are strong oxidative agents, and it is possible that ter genes might offer a selective advantage in the host environment and aid STEC in general stress response (12).
Interestingly, ureC has been more frequently found in eae-positive STEC (113/132) than in eae-negative strains (4/70), although no physical linkage of ureC and eae has been identified (33). The prevalence of ureC in eae-positive STEC (45/55) was significantly higher than in eae-negative STEC (2/44) (P < 0.0001). Similarly, terC was also more prevalent in eae-positive STEC (45/55) than eae-negative strains (5/44) (P < 0.0001). Even though OI-43/48 and LEE are physically distant, our observations indicated that there might be a functional relationship between them.
The arrangement of OI-122 genes was found to be serotype dependent, and all O157:H7 strains have a complete OI-122 (5, 9). However, we found that two patterns of OI-122 existed in O157:H7. An incomplete OI-122 lacking the third module was identified in all GUD-positive O157:H7 strains. In addition, aidA-1 of OI-43/48 was absent in GUD-positive O157:H7.
Most OI-122, OI-43/48, and OI-57 virulence genes (pagC, sen, nleB, efa-1, efa-2, terC, ureC, iha, aidA-1, nleG2-3, nleG6-2, and nleG5-2) were highly prevalent in eae-positive STEC. However, they were largely absent in eae-negative STEC, with the exception of pagC and iha. Phylogenetic analysis revealed that iha genes from eae-positive STEC had high similarity (99.6%), whereas they had lower sequence similarity (91.1 to 93.6%) to iha genes from eae-negative STEC, indicating that iha from eae-positive and eae-negative STEC strains may have evolved independently or have different origins. Such a difference also existed in pagC between eae-positive and eae-negative STEC strains. Schmidt et al. (34) reported that iha was carried by a 33,014-bp PAI in STEC serotype O91:H− strains (eae negative). In addition, iha was found in plasmid pO113 of STEC serotype O113:H21 (eae negative) (35). Moreover, Shen et al. (36, 37) reported that pagC was identified within a mosaic PAI from STEC O113:H21 strain CL-3 (eae negative). Thus, the higher prevalence of iha and pagC in the eae-negative STEC strains, compared to other virulence marker genes in the present study, is likely due to the presence of the same or similar PAIs and/or plasmids, as previously described. The similar prevalence of iha genes in the seropathotypes highly associated with severe diseases and other seropathotypes indicates that iha is not related to severe clinical outcomes, but the significantly higher prevalence of pagC in the seropathotypes associated with severe diseases indicates that this gene has some association with severe clinical outcome whether a strain carries the gene in OI-122 or in some other PAIs.
The distribution of PAI virulence genes and the phylogenetic analysis of iha and pagC support the hypothesis that OI-122, OI-43/48, and OI-57 are primarily associated with eae-positive strains in STEC. However, some eae-negative STEC serotypes, for example, O113:H21 and O91:H21, are also associated with life-threatening diseases such as HUS (5). Virulence factors such as subtilase cytotoxin AB5 (subAB5) and Saa (STEC autoagglutinating adhesion) are more commonly associated with eae-negative STEC. Moreover, it has been shown that some LEE-negative STEC strains, especially O113:H21, can invade tissue culture cells (38). Whole-genome comparison between nine eae-negative and five eae-positive STEC strains revealed that eae-negative strains did not carry any LEE-encoded effectors or other phage-encoded non-LEE effectors (39). These observations indicate that some differences in pathogenesis mechanisms may exist between eae-positive and eae-negative STEC strains. Additional studies, especially genomics and proteomics, are needed to determine the difference in the pathogenicity mechanisms between eae-negative and eae-positive STEC strains.
The strong association of OI-122, OI-57, and OI-43 with eae-positive STEC offers an important basis for STEC molecular risk assessment (MRA). MRA, which uses 14 non-LEE-encoded virulence factors to distinguish high-risk from low-risk non-O157 STEC, was proposed by Coombes et al. in 2008 (13). Other researchers adopted this concept and applied it to their own studies (40–43). However, we demonstrated here that some of non-LEE-encoded effectors (nleB, nle2-3, nleG5-2, and nleG6-2) were primarily associated with eae-positive STEC strains. In addition, Mundy et al. (44) reported that nleA was present in 37 of 43 (86%) eae-positive STEC clinical strains but absent in 50 eae-negative STEC clinical strains. Konczy et al. (9) reported that nleB and nleE of OI-122 were highly correlated with LEE. Moreover, comparative genomics analysis demonstrated that all known phage-encoded non-LEE effector genes were absent in eae-negative STEC (39). Based on the MRA framework, which uses non-LEE effector genes as sole markers, all eae-negative virulence STEC strains, including HUS-associated O113:H21, O91:H21, and O104:H21, would be categorized as harmless STEC; other serotypes, for example, O103:H11 and O119:H25, which have not been reported to be associated with severe disease or outbreaks but carry non-LEE-encoded virulence effectors similar to those of O157 EHEC, would be considered outbreak- and severe disease-associated serotypes. Therefore, additional markers or methods of assessment, especially for eae-negative STEC, are needed to accurately distinguish highly pathogenic STEC from low-virulence or harmless STEC.
In summary, O-122 and OI-57 and their virulence genes were highly associated with seropathotypes that cause severe diseases and outbreaks. In addition, ureC and terC, located at OI-43/48, were also identified as markers related to high-risk seropathotypes. Virulence genes in PAIs that are associated with severe diseases can be used as markers to identify potentially highly virulent STEC. Furthermore, we demonstrated here that OI-122, OI-43/48, and OI-57 are highly associated with eae-positive STEC, which offers an important basis for STEC MRA.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported in part by the Joint Institute for Food Safety and Applied Nutrition, University of Maryland, College Park, MD.
We thank Julie Kase, FDA/CFSAN, for providing DNA from positive controls for stx and eae subtyping.
Footnotes
Published ahead of print 22 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03661-12.
REFERENCES
- 1. Bettelheim KA. 2007. The non-O157 Shiga-toxigenic (verocytotoxigenic) Escherichia coli: under-rated pathogens. Crit. Rev. Microbiol. 33: 67–87 [DOI] [PubMed] [Google Scholar]
- 2. Blanco M, Blanco JE, Mora A, Dahbi G, Alonso MP, Gonzalez EA, Bernardez MI, Blanco J. 2004. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-xi). J. Clin. Microbiol. 42: 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States: major pathogens. Emerg. Infect. Dis. 17: 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coombes BK, Gilmour MW, Goodman CD. 2011. The evolution of virulence in non-O157 Shiga toxin-producing Escherichia coli. Front. Microbiol. 2: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karmali MA, Mascarenhas M, Shen S, Ziebell K, Johnson S, Reid-Smith R, Isaac-Renton J, Clark C, Rahn K, Kaper JB. 2003. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41: 4930–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gal-Mor O, Finlay BB. 2006. Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell. Microbiol. 8: 1707–1719 [DOI] [PubMed] [Google Scholar]
- 7. Wickham ME, Lupp C, Mascarenhas M, Vazquez A, Coombes BK, Brown NF, Coburn BA, Deng W, Puente JL, Karmali MA, Finlay BB. 2006. Bacterial genetic determinants of non-O157 STEC outbreaks and hemolytic-uremic syndrome after infection. J. Infect. Dis. 194: 819–827 [DOI] [PubMed] [Google Scholar]
- 8. Perna NT, Plunkett G, III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta ET, Potamousis KD, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409: 529–533 [DOI] [PubMed] [Google Scholar]
- 9. Konczy P, Ziebell K, Mascarenhas M, Choi A, Michaud C, Kropinski AM, Whittam TS, Wickham M, Finlay B, Karmali MA. 2008. Genomic O island 122, locus for enterocyte effacement, and the evolution of virulent verocytotoxin-producing Escherichia coli. J. Bacteriol. 190: 5832–5840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abu-Median AB, van Diemen PM, Dziva F, Vlisidou I, Wallis TS, Stevens MP. 2006. Functional analysis of lymphostatin homologues in enterohaemorrhagic Escherichia coli. FEMS Microbiol. Lett. 258: 43–49 [DOI] [PubMed] [Google Scholar]
- 11. Taylor DE, Rooker M, Keelan M, Ng LK, Martin I, Perna NT, Burland NT, Blattner FR. 2002. Genomic variability of O islands encoding tellurite resistance in enterohemorrhagic Escherichia coli O157:H7 isolates. J. Bacteriol. 184: 4690–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yin X, Wheatcroft R, Chambers JR, Liu B, Zhu J, Gyles CL. 2009. Contributions of O island 48 to adherence of enterohemorrhagic Escherichia coli O157:H7 to epithelial cells in vitro and in ligated pig ileal loops. Appl. Environ. Microbiol. 75: 5779–5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coombes BK, Wickham ME, Mascarenhas M, Gruenheid S, Finlay BB, Karmali MA. 2008. Molecular analysis as an aid to assess the public health risk of non-O157 Shiga toxin-producing Escherichia coli strains. Appl. Environ. Microbiol. 74: 2153–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imamovic L, Tozzoli R, Michelacci V, Minelli F, Marziano ML, Caprioli A, Morabito S. 2010. OI-57, a genomic island of Escherichia coli O157, is present in other seropathotypes of Shiga toxin-producing E. coli associated with severe human disease. Infect. Immun. 78: 4697–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu B, Skarina T, Yee A, Jobin MC, Dileo R, Semesi A, Fares C, Lemak A, Coombes BK, Arrowsmith CH, Singer AU, Savchenko A. 2010. NleG type 3 effectors from enterohaemorrhagic Escherichia coli are U-box E3 ubiquitin ligases. PLoS Pathog. 6: e1000960 doi:10.1371/journal.ppat.1000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schubert S, Darlu P, Clermont O, Wieser A, Magistro G, Hoffmann C, Weinert K, Tenaillon O, Matic I, Denamur E. 2009. Role of intraspecies recombination in the spread of pathogenicity islands within the Escherichia coli species. PLoS Pathog. 5: e1000257 doi:10.1371/journal.ppat.1000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benedek O, Schubert S. 2007. Mobility of the Yersinia high-pathogenicity island (HPI): transfer mechanisms of pathogenicity islands (PAIs) revisited (a review). Acta Microbiol. Immunol. Hung. 54: 89–105 [DOI] [PubMed] [Google Scholar]
- 18. Toma C, Martínez Espinosa E, Song T, Miliwebsky E, Chinen I, Iyoda S, Iwanaga M, Rivas M. 2004. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 42: 4937–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beutin L, Miko A, Krause G, Pries K, Haby S, Steege K, Albrecht N. 2007. Identification of human-pathogenic strains of Shiga toxin-producing Escherichia coli from food by a combination of serotyping and molecular typing of Shiga toxin genes. Appl. Environ. Microbiol. 73: 4769–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tramuta C, Robino P, Oswald E, Nebbia P. 2008. Identification of intimin alleles in pathogenic Escherichia coli by PCR-restriction fragment length polymorphism analysis. Vet. Res. Commun. 32: 1–5 [DOI] [PubMed] [Google Scholar]
- 21. Zheng J, Cui S, Teel LD, Zhao S, Singh R, O'Brien AD, Meng J. 2008. Identification and characterization of Shiga toxin type 2 variants in Escherichia coli isolates from animals, food, and humans. Appl. Environ. Microbiol. 74: 5645–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xia X, Meng J, McDermott PF, Ayers S, Blickenstaff K, Tran TT, Abbott J, Zheng J, Zhao S. 2010. Presence and characterization of Shiga toxin-producing Escherichia coli and other potentially diarrheagenic E. coli strains in retail meats. Appl. Environ. Microbiol. 76: 1709–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ju W, Shen J, Li Y, Toro MA, Zhao S, Ayers S, Najjar MB, Meng J. 2012. Non-O157 Shiga toxin-producing Escherichia coli in retail ground beef and pork in the Washington D.C. area. Food Microbiol. 32: 371–377 [DOI] [PubMed] [Google Scholar]
- 24. Nakano M, Iida T, Ohnishi M, Kurokawa K, Takahashi A, Tsukamoto T, Yasunaga T, Hayashi T, Honda T. 2001. Association of the urease gene with enterohemorrhagic Escherichia coli strains irrespective of their serogroups. J. Clin. Microbiol. 39: 4541–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karch H, Schubert S, Zhang D, Zhang W, Schmidt H, Olschläger T, Hacker J. 1999. A genomic island, termed high-pathogenicity island, is present in certain non-O157 Shiga toxin-producing Escherichia coli clonal lineages. Infect. Immun. 67: 5994–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum-parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicholls L, Grant TH, Robins-Browne RM. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35: 275–288 [DOI] [PubMed] [Google Scholar]
- 28. Klapproth JM, Scaletsky IC, McNamara BP, Lai LC, Malstrom C, James SP, Donnenberg MS. 2000. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68: 2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klapproth JM, Sasaki M, Sherman M, Babbin B, Donnenberg MS, Fernandes PJ, Scaletsky IC, Kalman D, Nusrat A, Williams IR. 2005. Citrobacter rodentium lifA/efa1 is essential for colonic colonization and crypt cell hyperplasia in vivo. Infect. Immun. 73: 3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelly M, Hart E, Mundy R, Marches O, Wiles S, Badea L, Luck S, Tauschek M, Frankel G, Robins-Browne RM, Hartland EL. 2006. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect. Immun. 74: 2328–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steyert SR, Rasko DA, Kaper JB. 2011. Functional and phylogenetic analysis of ureD in Shiga toxin-producing Escherichia coli. J. Bacteriol. 193: 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steyert SR, Kaper JB. 2012. Contribution of urease to colonization by Shiga toxin-producing Escherichia coli. Infect. Immun. 80: 2589–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedrich AW, Lukas R, Mellmann A, Kock R, Zhang W, Mathys W, Bielaszewska M, Karch H. 2006. Urease genes in non-O157 Shiga toxin-producing Escherichia coli: mostly silent but valuable markers for pathogenicity. Clin. Microbiol. Infect. 12: 483–486 [DOI] [PubMed] [Google Scholar]
- 34. Schmidt H, Zhang WL, Hemmrich U, Jelacic S, Brunder W, Tarr PI, Dobrindt U, Hacker J, Karch H. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69: 6863–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Newton HJ, Sloan J, Bulach DM, Seemann T, Allison CC, Tauschek M, Robins-Browne RM, Paton JC, Whittam TS, Paton AW, Hartland EL. 2009. Shiga toxin-producing Escherichia coli strains negative for locus of enterocyte effacement. Emerg. Infect. Dis. 15: 372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen S, Mascarenhas M, Rahn K, Kaper JB, Karmali MA. 2004. Evidence for a hybrid genomic island in verocytotoxin-producing Escherichia coli CL3 (serotype O113:H21) containing segments of EDL933 (serotype O157:H7) O islands 122 and 48. Infect. Immun. 72: 1496–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Girardeau JP, Bertin Y, Martin C. 2009. Genomic analysis of the PAI ICL3 locus in pathogenic LEE-negative Shiga toxin-producing Escherichia coli and Citrobacter rodentium. Microbiology 155: 1016–1027 [DOI] [PubMed] [Google Scholar]
- 38. Luck SN, Badea L, Bennett-Wood V, Robins-Browne R, Hartland EL. 2006. Contribution of FliC to epithelial cell invasion by enterohemorrhagic Escherichia coli O113:H21. Infect. Immun. 74: 6999–7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steyert SR, Sahl JW, Fraser CM, Teel LD, Scheutz F, Rasko DA. 2012. Comparative genomics and stx phage characterization of LEE-negative Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bugarel M, Beutin L, Fach P. 2010. Low-density macroarray targeting non-locus of enterocyte effacement effectors (nle genes) and major virulence factors of Shiga toxin-producing Escherichia coli (STEC): a new approach for molecular risk assessment of STEC isolates. Appl. Environ. Microbiol. 76: 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bugarel M, Beutin L, Martin A, Gill A, Fach P. 2010. Micro-array for the identification of Shiga toxin-producing Escherichia coli (STEC) seropathotypes associated with hemorrhagic colitis and hemolytic uremic syndrome in humans. Int. J. Food Microbiol. 142: 318–329 [DOI] [PubMed] [Google Scholar]
- 42. Bugarel M, Martin A, Fach P, Beutin L. 2011. Virulence gene profiling of enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli strains: a basis for molecular risk assessment of typical and atypical EPEC strains. BMC Microbiol. 11: 142 doi:10.1186/1471-2180-11-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bosilevac JM, Koohmaraie M. 2011. Prevalence and characterization of non-O157 Shiga toxin-producing Escherichia coli isolates from commercial ground beef in the United States. Appl. Environ. Microbiol. 77: 2103–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mundy R, Jenkins C, Yu J, Smith H, Frankel G. 2004. Distribution of espI among clinical enterohaemorrhagic and enteropathogenic Escherichia coli isolates. J. Med. Microbiol. 53: 1145–1149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.