Abstract
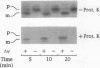
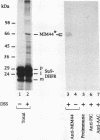
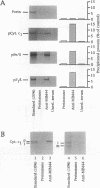
The essential yeast gene MPI1 encodes a mitochondrial membrane protein that is possibly involved in protein import into the organelle (A. C. Maarse, J. Blom, L. A. Grivell, and M. Meijer, EMBO J. 11:3619-3628, 1992). For this report, we determined the submitochondrial location of the MPI1 gene product and investigated whether it plays a direct role in the translocation of preproteins. By fractionation of mitochondria, the mature protein of 44 kDa was localized to the mitochondrial inner membrane and therefore termed MIM44. Import of the precursor of MIM44 required a membrane potential across the inner membrane and involved proteolytic processing of the precursor. A preprotein in transit across the mitochondrial membranes was cross-linked to MIM44, whereas preproteins arrested on the mitochondrial surface or fully imported proteins were not cross-linked. When preproteins were arrested at two distinct stages of translocation across the inner membrane, only preproteins at an early stage of translocation could be cross-linked to MIM44. Moreover, solubilized MIM44 was found to interact with in vitro-synthesized preproteins. We conclude that MIM44 is a component of the mitochondrial inner membrane import machinery and interacts with preproteins in an early step of translocation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Miake-Lye R. Unscrambling the puzzle of biological machines: the importance of the details. Cell. 1992 Feb 7;68(3):415–420. doi: 10.1016/0092-8674(92)90179-g. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schaniel A., Vestweber D., Schatz G. A yeast mitochondrial outer membrane protein essential for protein import and cell viability. Nature. 1990 Dec 13;348(6302):605–609. doi: 10.1038/348605a0. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Becker K., Guiard B., Rassow J., Söllner T., Pfanner N. Targeting of a chemically pure preprotein to mitochondria does not require the addition of a cytosolic signal recognition factor. J Biol Chem. 1992 Mar 15;267(8):5637–5643. [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Shilling J., Werner-Washburne M., Holmes S., Kosic-Smithers J., Nicolet C. M. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989 Jul;9(7):3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Gasser S. M., Schatz G. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13075–13080. [PubMed] [Google Scholar]
- Dingwall C., Laskey R. The nuclear membrane. Science. 1992 Nov 6;258(5084):942–947. doi: 10.1126/science.1439805. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Kurzchalia T. V., Wiedmann M., Rapoport T. A. Probing the molecular environment of translocating polypeptide chains by cross-linking. Methods Cell Biol. 1991;34:241–262. doi: 10.1016/s0091-679x(08)61684-2. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Ostermann J., Guiard B., Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987 Dec 24;51(6):1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Horst M., Jenö P., Kronidou N. G., Bolliger L., Oppliger W., Scherer P., Manning-Krieg U., Jascur T., Schatz G. Protein import into yeast mitochondria: the inner membrane import site protein ISP45 is the MPI1 gene product. EMBO J. 1993 Aug;12(8):3035–3041. doi: 10.1002/j.1460-2075.1993.tb05972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. E., Yaffe M. P. Import of proteins into yeast mitochondria: the nuclear MAS2 gene encodes a component of the processing protease that is homologous to the MAS1-encoded subunit. EMBO J. 1988 Dec 1;7(12):3863–3871. doi: 10.1002/j.1460-2075.1988.tb03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Pfaller R., Söllner T., Griffiths G., Horstmann H., Pfanner N., Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990 Dec 13;348(6302):610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- Maarse A. C., Blom J., Grivell L. A., Meijer M. MPI1, an essential gene encoding a mitochondrial membrane protein, is possibly involved in protein import into yeast mitochondria. EMBO J. 1992 Oct;11(10):3619–3628. doi: 10.1002/j.1460-2075.1992.tb05446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M., Dietmeier K., Söllner T., Segui B., Steger H. F., Neupert W., Pfanner N. Identification of the mitochondrial receptor complex in Saccharomyces cerevisiae. FEBS Lett. 1992 Oct 5;310(3):265–268. doi: 10.1016/0014-5793(92)81345-m. [DOI] [PubMed] [Google Scholar]
- Nunnari J., Walter P. Protein targeting to and translocation across the membrane of the endoplasmic reticulum. Curr Opin Cell Biol. 1992 Aug;4(4):573–580. doi: 10.1016/0955-0674(92)90074-m. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Müller H. K., Harmey M. A., Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987 Nov;6(11):3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of F1-ATPase subunit beta into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986 Dec 15;209(2):152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 1985 Nov;4(11):2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Rassow J., van der Klei I. J., Neupert W. A dynamic model of the mitochondrial protein import machinery. Cell. 1992 Mar 20;68(6):999–1002. doi: 10.1016/0092-8674(92)90069-o. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Söllner T., Neupert W. Mitochondrial import receptors for precursor proteins. Trends Biochem Sci. 1991 Feb;16(2):63–67. doi: 10.1016/0968-0004(91)90026-r. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Hartl F. U., Cheng M. Y., Ostermann J., Horwich A., Neupert W. The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. EMBO J. 1988 Nov;7(11):3493–3500. doi: 10.1002/j.1460-2075.1988.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon L., Moll T., Vestweber D., Marshallsay B., Schatz G. Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J Cell Biol. 1989 Dec;109(6 Pt 1):2603–2616. doi: 10.1083/jcb.109.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Rassow J., Guiard B., Wienhues U., Herzog V., Hartl F. U., Neupert W. Translocation arrest by reversible folding of a precursor protein imported into mitochondria. A means to quantitate translocation contact sites. J Cell Biol. 1989 Oct;109(4 Pt 1):1421–1428. doi: 10.1083/jcb.109.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Pfanner N. Mitochondrial preproteins en route from the outer membrane to the inner membrane are exposed to the intermembrane space. FEBS Lett. 1991 Nov 18;293(1-2):85–88. doi: 10.1016/0014-5793(91)81157-4. [DOI] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Schekman R. Polypeptide translocation across the endoplasmic reticulum membrane. J Biol Chem. 1992 Jul 15;267(20):13791–13794. [PubMed] [Google Scholar]
- Scherer P. E., Manning-Krieg U. C., Jenö P., Schatz G., Horst M. Identification of a 45-kDa protein at the protein import site of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11930–11934. doi: 10.1073/pnas.89.24.11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Schneider H., Söllner T., Dietmeier K., Eckerskorn C., Lottspeich F., Trülzsch B., Neupert W., Pfanner N. Targeting of the master receptor MOM19 to mitochondria. Science. 1991 Dec 13;254(5038):1659–1662. doi: 10.1126/science.1661031. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Brunner J., Baker A., Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989 Sep 21;341(6239):205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. A chimeric mitochondrial precursor protein with internal disulfide bridges blocks import of authentic precursors into mitochondria and allows quantitation of import sites. J Cell Biol. 1988 Dec;107(6 Pt 1):2037–2043. doi: 10.1083/jcb.107.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- Wienhues U., Becker K., Schleyer M., Guiard B., Tropschug M., Horwich A. L., Pfanner N., Neupert W. Protein folding causes an arrest of preprotein translocation into mitochondria in vivo. J Cell Biol. 1991 Dec;115(6):1601–1609. doi: 10.1083/jcb.115.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte C., Jensen R. E., Yaffe M. P., Schatz G. MAS1, a gene essential for yeast mitochondrial assembly, encodes a subunit of the mitochondrial processing protease. EMBO J. 1988 May;7(5):1439–1447. doi: 10.1002/j.1460-2075.1988.tb02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]