Abstract
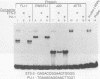
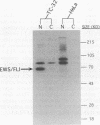
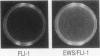
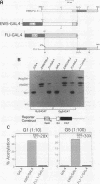
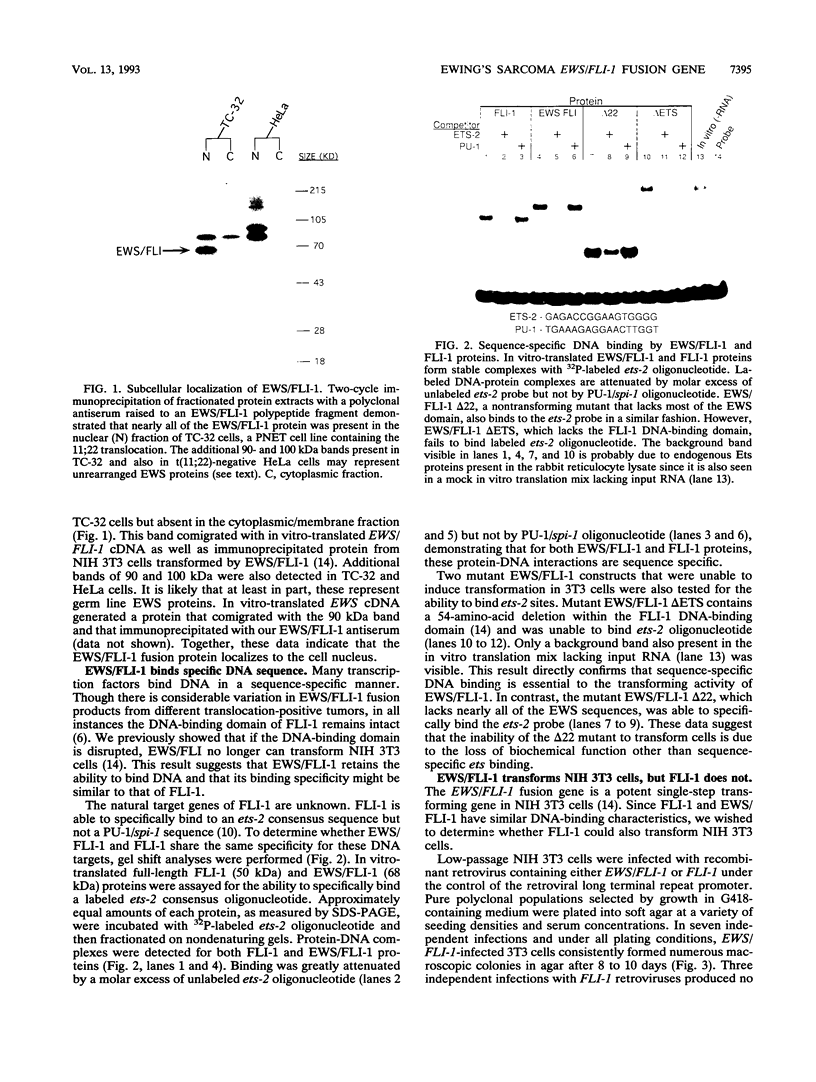
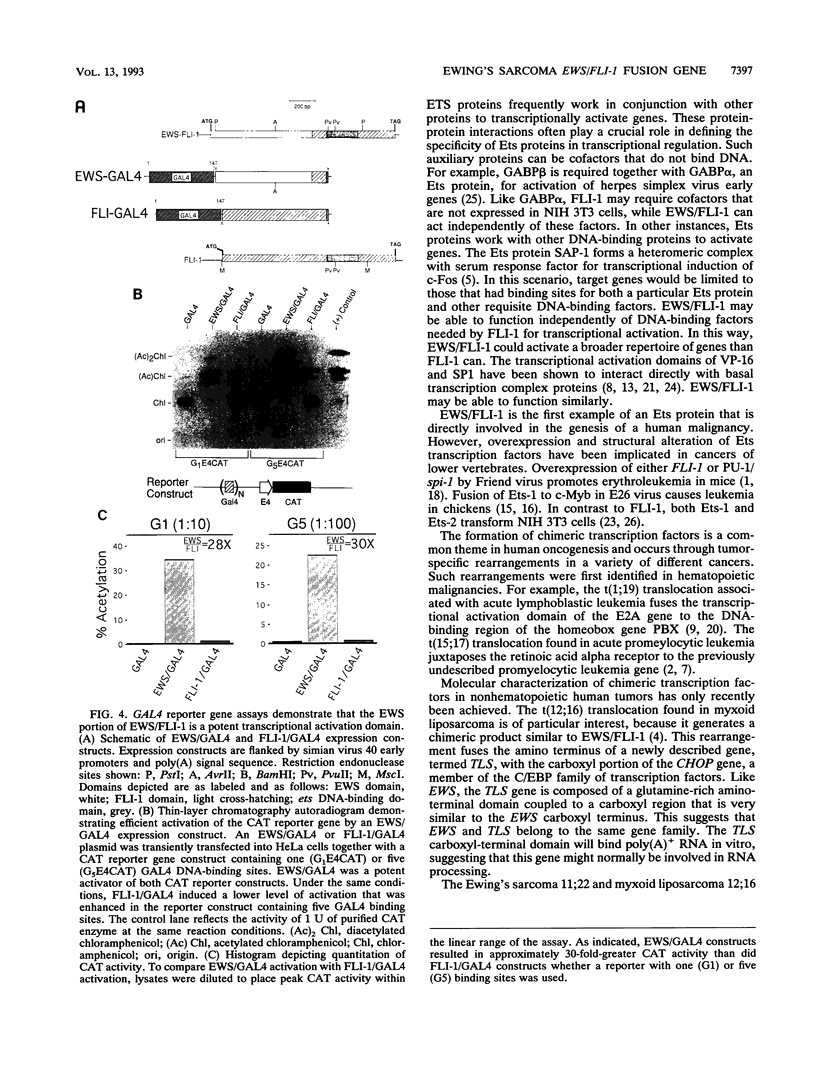
EWS/FLI-1 is a chimeric protein formed by a tumor-specific 11;22 translocation found in both Ewing's sarcoma and primitive neuroectodermal tumor of childhood. EWS/FLI-1 has been shown to be a potent transforming gene, suggesting that it plays an important role in the genesis of these human tumors. We now demonstrate that EWS/FLI-1 has the characteristics of an aberrant transcription factor. Subcellular fractionation experiments localized the EWS/FLI-1 protein to the nucleus of primitive neuroectodermal tumor cells. EWS/FLI-1 specifically bound in vitro an ets-2 consensus sequence similarly to normal FLI-1. When coupled to a GAL4 DNA-binding domain, the amino-terminal EWS/FLI-1 region was a much more potent transcriptional activator than the corresponding amino-terminal domain of FLI-1. Finally, EWS/FLI-1 efficiently transformed NIH 3T3 cells, but FLI-1 did not. These data suggest that EWS/FLI-1, functioning as a transcription factor, leads to a phenotype dramatically different from that of cells expressing FLI-1. EWS/FLI-1 could disrupt normal growth and differentiation either by more efficiently activating FLI-1 target genes or by inappropriately modulating genes normally not responsive to FLI-1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-David Y., Giddens E. B., Letwin K., Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991 Jun;5(6):908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Borrow J., Goddard A. D., Sheer D., Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990 Sep 28;249(4976):1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- Chumakov A. M., Chen D. L., Chumakova E. A., Koeffler H. P. Localization of the c-ets-2 transactivation domain. J Virol. 1993 Apr;67(4):2421–2425. doi: 10.1128/jvi.67.4.2421-2425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat A., Aman P., Mandahl N., Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993 Jun 17;363(6430):640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992 Sep 10;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Hoey T., Weinzierl R. O., Gill G., Chen J. L., Dynlacht B. D., Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993 Jan 29;72(2):247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., Maki R. A., Papayannopoulou T., Moore J., Hromas R. Characterization of the ets oncogene family member, fli-1. J Biol Chem. 1993 Mar 15;268(8):5769–5773. [PubMed] [Google Scholar]
- Lewin B. Oncogenic conversion by regulatory changes in transcription factors. Cell. 1991 Jan 25;64(2):303–312. doi: 10.1016/0092-8674(91)90640-k. [DOI] [PubMed] [Google Scholar]
- Lim F., Kraut N., Framptom J., Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992 Feb;11(2):643–652. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991 Mar 8;64(5):971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- May W. A., Gishizky M. L., Lessnick S. L., Lunsford L. B., Lewis B. C., Delattre O., Zucman J., Thomas G., Denny C. T. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5752–5756. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz T., Graf T. Fusion of the nuclear oncoproteins v-Myb and v-Ets is required for the leukemogenicity of E26 virus. Cell. 1991 Jul 12;66(1):95–105. doi: 10.1016/0092-8674(91)90142-l. [DOI] [PubMed] [Google Scholar]
- Metz T., Graf T. v-myb and v-ets transform chicken erythroid cells and cooperate both in trans and in cis to induce distinct differentiation phenotypes. Genes Dev. 1991 Mar;5(3):369–380. doi: 10.1101/gad.5.3.369. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Tavitian A., Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988 Jan 21;331(6153):277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Muller A. J., Young J. C., Pendergast A. M., Pondel M., Landau N. R., Littman D. R., Witte O. N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991 Apr;11(4):1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Roberts S. G., Ha I., Maldonado E., Reinberg D., Green M. R. Interaction between an acidic activator and transcription factor TFIIB is required for transcriptional activation. Nature. 1993 Jun 24;363(6431):741–744. doi: 10.1038/363741a0. [DOI] [PubMed] [Google Scholar]
- Seneca S., Punyammalee B., Bailly M., Ghysdael J., Crabeel M. Ets1, when fused to the GAL4 DNA binding domain, efficiently enhances galactose promotor dependent gene expression in yeast. Oncogene. 1991 Mar;6(3):357–360. [PubMed] [Google Scholar]
- Seth A., Watson D. K., Blair D. G., Papas T. S. c-ets-2 protooncogene has mitogenic and oncogenic activity. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7833–7837. doi: 10.1073/pnas.86.20.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Brown T. A., McKnight S. L. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science. 1991 Aug 16;253(5021):762–768. doi: 10.1126/science.1876833. [DOI] [PubMed] [Google Scholar]
- Topol L. Z., Tatosyan A. G., Ascione R., Thompson D. M., Blair D. G., Kola I., Seth A. C-ets-1 protooncogene expression alters the growth properties of immortalized rat fibroblasts. Cancer Lett. 1992 Oct 30;67(1):71–78. doi: 10.1016/0304-3835(92)90010-s. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Hahn S. L., Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993 Jan 15;211(1-2):7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Kerckaert J. P., Wasylyk B. A novel modulator domain of Ets transcription factors. Genes Dev. 1992 Jun;6(6):965–974. doi: 10.1101/gad.6.6.965. [DOI] [PubMed] [Google Scholar]
- Watanabe S. M., Rosenberg N. E., Witte O. N. A membrane-associated, carbohydrate-modified form of the v-abl protein that cannot be phosphorylated in vivo or in vitro. J Virol. 1984 Sep;51(3):620–627. doi: 10.1128/jvi.51.3.620-627.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., Smyth F. E., Thompson D. M., Cheng J. Q., Testa J. R., Papas T. S., Seth A. The ERGB/Fli-1 gene: isolation and characterization of a new member of the family of human ETS transcription factors. Cell Growth Differ. 1992 Oct;3(10):705–713. [PubMed] [Google Scholar]
- Whang-Peng J., Triche T. J., Knutsen T., Miser J., Kao-Shan S., Tsai S., Israel M. A. Cytogenetic characterization of selected small round cell tumors of childhood. Cancer Genet Cytogenet. 1986 Apr 1;21(3):185–208. doi: 10.1016/0165-4608(86)90001-4. [DOI] [PubMed] [Google Scholar]
- Wu Y. L., Tam S. P., Davies P. L. A modified CAT expression vector with convenient cloning sites. Nucleic Acids Res. 1990 Apr 11;18(7):1919–1919. doi: 10.1093/nar/18.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lemarchandel V., Romeo P. H., Ben-David Y., Greer P., Bernstein A. The Fli-1 proto-oncogene, involved in erythroleukemia and Ewing's sarcoma, encodes a transcriptional activator with DNA-binding specificities distinct from other Ets family members. Oncogene. 1993 Jun;8(6):1621–1630. [PubMed] [Google Scholar]
- de Thé H., Chomienne C., Lanotte M., Degos L., Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990 Oct 11;347(6293):558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]