Abstract
There has been extensive research regarding T cell recognition of Epstein-Barr virus-transformed cells; however, less is known regarding the recognition of B cells immortalized by gamma-2 herpesviruses. Here we show that B cells immortalized by murine gammaherpesvirus 68 (MHV-68, γHV-68) can be controlled by either CD4 or CD8 T cells in vivo. We present evidence for the direct recognition of infected B cells by CD4 and CD8 T cells. These data will help in the development of immunotherapeutic approaches combating gamma-2 herpesvirus-related disease.
TEXT
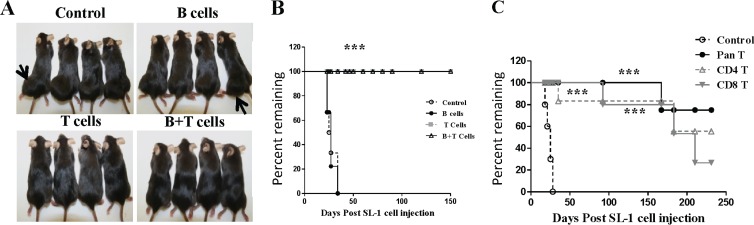
Tcell immunity is critical to control virus replication and latently infected cells in most herpesvirus infections. Using the murine gammaherpesvirus 68 (MHV-68, γHV-68) mouse model of gamma-2 herpesvirus infection, we now have a good understanding of the immunological factors necessary to control virus replication (1–9). However, we have a much poorer understanding of T cell recognition of latently infected B cells. This has been due largely to our inability to immortalize B cells by MHV-68 infection in vitro. However, our recently published work describes a methodology by which immature B cells can be immortalized by MHV-68 (10). These cells grow as tumors in immunodeficient mice but not immunocompetent mice, indicating immunological control of the outgrowth of MHV-68-infected B cells (10). To determine the key cell types responsible for this protection, we used an adoptive transfer system. We transferred purified T lymphocytes, B lymphocytes, or both T and B lymphocytes from naïve mice into RAG2−/− mice. MHV-68-immortalized SL1 cells were then injected into the mice, and tumor growth was monitored. In mice receiving no T cells or only B cells, the tumor grew to a size necessitating euthanasia by approximately 30 to 40 days postinjection (Fig. 1A). However, mice receiving either T cells alone or T and B cells survived for >150 days postinjection with no tumor growth (Fig. 1B). These data indicated that T cells were the predominant cells responsible for mediating protection against the outgrowth of MHV-68-immortalized tumor cells. Importantly, we did not observe virus reactivation from SL1 cells after injection in vivo, as no viral spread to the host was detectable, measured by PCR for the viral genome in the spleens of wild-type or RAG2−/− mutant mice injected with SL1 cells (data not shown).
Fig 1.
T cells control the growth of tumors induced by MHV-68-transformed SL1 cells. (A) RAG2−/− mice were injected subcutaneously with 5 × 106 SL1 cells and then received a phosphate-buffered saline (PBS) control, B cells, T cells, or T and B cells by i.p. injection. Cells (107) of the appropriate populations were administered 7 days prior to SL1 cells. Both B cells and T cells were purified by negative selection by magnetic separation. Mice were euthanized when tumors reached 3 cm in diameter, in accordance with the Emory University animal welfare policy. (B) Endpoint curves of mice with SL1 tumors receiving B cells, T cells, B and T cells, or a PBS control (n = 10; log-rank test; ***, P < 0.001). (C) RAG2−/− mutant mice received SL1 cells and T cells (Pan T), CD4 T cells, CD8 T cells, or a PBS control. Cells were isolated by negative selection by magnetic cell separation. The control group received PBS buffer only (n = 10; log-rank test; ***, P < 0.001).
Next, we adoptively transferred purified CD8 or CD4 T cells into RAG2−/− mutant mice, injected them with SL1 cells, and then monitored disease progression. As a control, other mice received either the intact T cell population (Pan T) or no T cells (Control). We observed rapid tumor growth in the control group; however, mice were protected from tumor growth if they received either CD4 or CD8 T cells (Fig. 1C). Consistent with these findings, SL1 cells injected into T cell-deficient nude mice grew tumors (10/10 mice), whereas no tumors were seen in C57BL/6 mice, major histocompatibility complex class II-deficient mice, or CD8-deficient mice (0/10 mice in all cases) (data not shown). This indicated that both CD8 and CD4 T cells could independently protect against outgrowth of MHV-68-immortalized B cells.
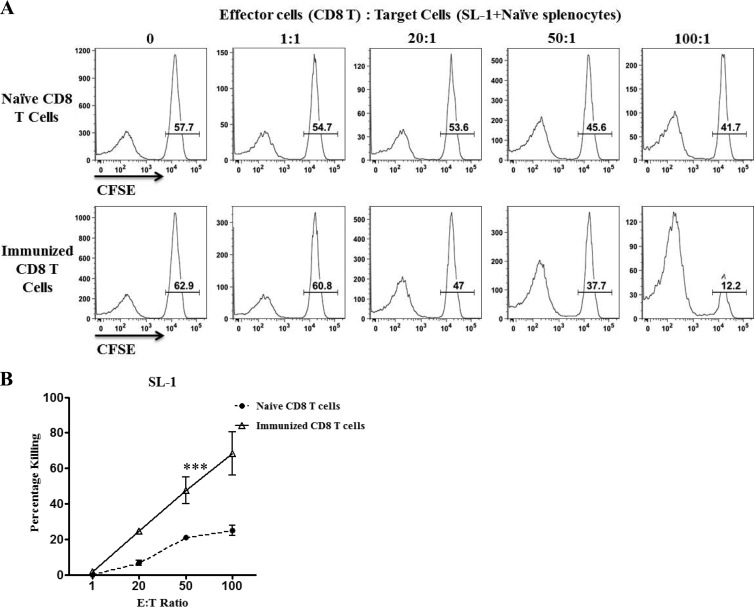
T cell-mediated protection from tumor growth can be mediated either by direct T cell recognition of tumor cells or through T cells acting indirectly by stimulating other immune defenses. We therefore tested whether SL1 cells could be recognized directly by CD4 or CD8 T cells. To generate an expanded population of T cells recognizing SL1 cells, we immunized naïve C57BL/6 mice with SL1 cells by intraperitoneal (i.p.) injection. Mice were boosted twice under the same protocol at 3-week intervals, and then 4 to 5 days after the last injection, spleen cells were harvested. Purified CD8 T cells were used in an in vitro cytotoxicity assay. Graded numbers of CD8 T cells were incubated with target cells consisting of a 1:1 ratio of SL1 cells and B cells isolated from naïve spleens. Differing levels of carboxyfluorescein succinimidyl ester (CFSE) labeling distinguished the two target populations. Significantly more killing of SL1 cells was observed than in cultures where naïve CD8 T cells were used as effector cells (Fig. 2A and B).
Fig 2.
CD8 T cells from SL1-immunized mice displayed cytolytic activity. For immunization, mice were injected i.p. with 107 SL1 cells three times at 3-week intervals, and then spleen cells were prepared 4 to 5 days after the last immunization. (A) Representative histogram plots showing the selective killing of SL1 cells by CD8 T cells from SL1-immunized mice. CD8 T cells isolated from either naïve mice or mice immunized with SL1 cells were used as effector cells. SL1 cells were labeled with 0.5 μM CFSE (gated), and naïve splenic B cells were labeled with 0.025 μM CFSE. Both cell populations were also labeled with PKH26 to distinguish effector from target cells. SL1 and B cells were mixed at a 1:1 ratio and then incubated for 16 h with effector CD8 T cells at the effector-to-target (E:T) ratios indicated. Histograms are gated on PHK26+ target cells. The values are proportions of SL1 target cells, and representative histograms are shown. (B) Percent specific lysis was calculated by using the formula [1 − %CFSEhi (+ effectors)/%CFSEhi (no effectors)] × 100. ***, P < 0.001. Representative data from three experiments are shown. Bars represent standard errors of the means.
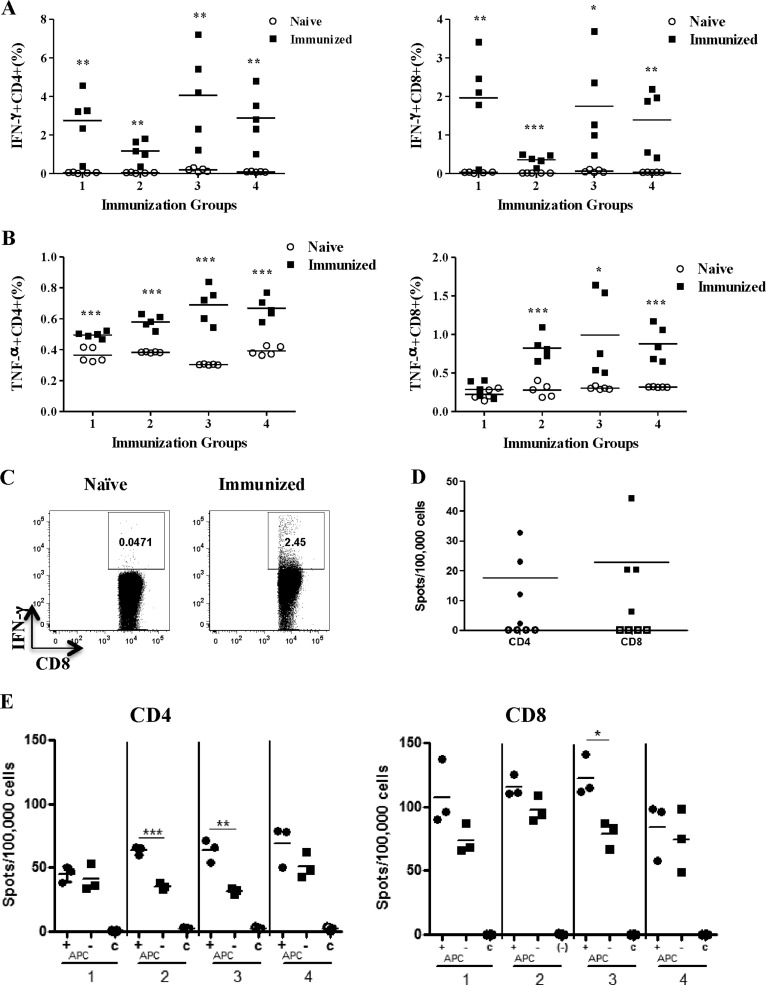
We also wished to determine whether CD8 or CD4 T cells produced effector cytokines after recognition of SL1 cells. Therefore, we briefly cocultured SL1 cells and spleen cells from either SL1-immunized or naive mice and then performed intracellular cytokine staining. Both CD4 and CD8 T cells from immunized mice produced significantly more gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) than naïve T cells did when stimulated with SL1 cells (Fig. 3A to C). To address whether SL1 cells presented viral antigens to T cells, as opposed to tumor-related antigens, we tested whether SL1 cells were recognized by T cells from MHV-68-infected mice that had not been exposed to SL1 cells. Using the highly sensitive IFN-γ enzyme-linked immunospot (ELISPOT) assay, we showed that both CD8 and CD4 T cells from infected mice mounted significant responses to SL1 cells, demonstrating the recognition of viral antigens (Fig. 3D).
Fig 3.
Cytokine production by CD4 and CD8 T cells from SL1-immunized mice and direct recognition of SL1 cells by CD4 and CD8 T cells. (A) Spleen cells from SL1-immunized mice were incubated with SL1 cells for 5 h, and then intracellular staining for IFN-γ was performed. The data show the percentages of IFN-γ intracellular cytokine production in CD4 T cells (left panel) and CD8 T cells (right panel). Each immunization group represents a different experiment, and each point represents a value from an individual mouse. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Intracellular staining for TNF-α by the same protocol as described for panel A. (C) Representative fluorescence-activated cell sorter plots of intracellular IFN-γ staining. Dot plots were gated on CD8 T cells among live lymphocytes. Plots show staining from naïve mice and mice immunized with SL1 cells. (D) Recognition of SL1 cells by CD8 or CD4 T cells from MHV-68-infected mice at day 14 postinfection. Purified CD4 or CD8 T cells were incubated with SL1 cells and antigen-presenting cells, and IFN-γ-secreting cells were detected with an ELISPOT assay. Purified CD4 or CD8 T cells (1 × 105) were incubated with 2 × 104 SL1 cells in the presence or absence of 5 × 105 naïve spleen cells, as a source of antigen-presenting cells, for 48 h before the assay was developed. Open symbols, negative control with no SL1 cells. Each point is the value from an individual mouse. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (E) Direct recognition of SL1 cells by CD4 and CD8 T cells from SL1-immunized mice. CD4 or CD8 T cells were purified by magnetic separation and then incubated with SL1 cells in the presence or absence of naïve spleen cells, acting as antigen-presenting cells (APC). C, negative-control group consisting of T cells plus antigen-presenting cells only. Values represent individual mice, and points represent sample replicates. *, P < 0.05; ***, P < 0.001.
While these data showed recognition of SL1 cells by both CD4 and CD8 T cells, it remained possible that antigen recognition occurred indirectly via other antigen-presenting cell types. To address direct versus indirect presentation, we used the IFN-γ ELISPOT assay. Purified CD8 or CD4 T cells from SL1-immunized mice were cultured with SL1 cells either alone or with the addition of naïve spleen cells as a source of antigen-presenting cells. Significantly higher frequencies of CD4 and CD8 T cells produced IFN-γ than in the negative control in cultures lacking additional antigen-presenting cells (Fig. 3E). This indicated direct antigen presentation mediated by SL1 cells. The addition of antigen-presenting cells in some cases elevated the response, indicating that processing and presentation of antigens from SL1 cells can also occur by other spleen cell types.
In this report, we show that prevention of tumor formation by MHV-68-immortalized B cells in vivo is mediated by both CD8 and CD4 T cells. This is consistent with the fact that a concerted CD8 and CD4 cell response is necessary to control MHV-68 infection (1–3, 6–9, 11). CD8 T cells play a dominant role in this process, through cytotoxicity and IFN-γ production. CD4 T cells contribute significantly, through several mechanisms, including IFN-γ production (12) and providing help for the CD8 T cell response (13, 14). Our previous work showed that CD4, but not CD8, T cells were critical for the regression of S11 B lymphoma cells latently infected with MHV-68 in BALB/c mice (15). The present study was performed with the C57BL/6 mouse strain, which may partly explain the different findings, given the demonstrated strain dependence of MHV-68-specific T cell responses (5, 8). The two cell lines were also generated in different ways, SL1 cell through direct infection of fetal liver-derived B cells, whereas the S11 cell line was cultured from a lymphoma that developed in a long-term-infected mouse. Our present findings are consistent with data from the Epstein-Barr virus literature showing that either human CD8 or CD4 T cells can prevent the growth of lymphoblastoid cell lines in SCID mice (16, 17). Our data suggest that the same may be true regarding the control of B cells infected with Kaposi's sarcoma herpesvirus, a close relative of MHV-68, which is associated with B cell tumors in the case of body cavity lymphoma. These studies present the possibility of determining key effector mechanisms responsible for suppressing the outgrowth of B cells latently infected with gamma-2 herpesviruses.
Footnotes
Published ahead of print 20 March 2013
REFERENCES
- 1. Evans AG, Moser JM, Krug LT, Pozharskaya V, Mora AL, Speck SH. 2008. A gammaherpesvirus-secreted activator of Vβ4+ CD8+ T cells regulates chronic infection and immunopathology. J. Exp. Med. 205:669–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freeman ML, Burkum CE, Lanzer KG, Jensen MK, Ahmed M, Yager EJ, Flaño E, Winslow GM, Woodland DL, Blackman MA. 2011. Cutting edge: activation of virus-specific CD4 T cells throughout γ-herpesvirus latency. J. Immunol. 187:6180–6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuse S, Obar JJ, Bellfy S, Leung EK, Zhang W, Usherwood EJ. 2006. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J. Virol. 80:9159–9170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim I-J, Flaño E, Woodland DL, Blackman MA. 2002. Antibody-mediated control of persistent γ-herpesvirus infection. J. Immunol. 168:3958–3964 [DOI] [PubMed] [Google Scholar]
- 5. Lee KS, Groshong SD, Cool CD, Kleinschmidt-DeMasters BK, van Dyk LF. 2009. Murine gammaherpesvirus 68 infection of IFNγ unresponsive mice: a small animal model for gammaherpesvirus-associated B cell lymphoproliferative disease. Cancer Res. 69:5481–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sparks-Thissen RL, Braaten DC, Kreher S, Speck SH, Virgin HW. 2004. An optimized CD4 T-cell response can control productive and latent gammaherpesvirus infection. J. Virol. 78:6827–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tibbetts SA, van Dyk LF, Speck SH, Virgin HW. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125–7132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai C-Y, Hu Z, Zhang W, Usherwood EJ. 2011. Strain-dependent requirement for IFN-γ for respiratory control and immunotherapy in murine gammaherpesvirus infection. Viral Immunol. 24:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weck KE, Dal Canto AJ, Gould JD, O'Guin AK, Roth KA, Saffitz JE, Speck SH, Virgin HW. 1997. Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat. Med. 3:1346–1353 [DOI] [PubMed] [Google Scholar]
- 10. Liang X, Paden CR, Morales FM, Powers RP, Jacob J, Speck SH. 2011. Murine gamma-herpesvirus immortalization of fetal liver-derived B cells requires both the viral cyclin D homolog and latency-associated nuclear antigen. PLoS Pathog. 7:e1002220 doi:10.1371/journal.ppat.1002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. 2006. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J. Virol. 80:8303–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christensen JP, Cardin RD, Branum KC, Doherty PC. 1999. CD4+ T cell-mediated control of a γ-herpesvirus in B cell-deficient mice is mediated by IFN-γ. Proc. Natl. Acad. Sci. U. S. A. 96:5135–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardin RD, Brooks JW, Sarawar SR, Doherty PC. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Molloy MJ, Zhang W, Usherwood EJ. 2011. Suppressive CD8+ T cells arise in the absence of CD4 help and compromise control of persistent virus. J. Immunol. 186:6218–6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robertson KA, Usherwood EJ, Nash AA. 2001. Regression of a murine gammaherpesvirus 68-positive b-cell lymphoma mediated by CD4 T lymphocytes. J. Virol. 75:3480–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lacerda JF, Ladanyi M, Louie DC, Fernandez JM, Papadopoulos EB, O'Reilly RJ. 1996. Human Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes home preferentially to and induce selective regressions of autologous EBV-induced B cell lymphoproliferations in xenografted C.B-17 scid/scid mice. J. Exp. Med. 183:1215–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merlo A, Turrini R, Bobisse S, Zamarchi R, Alaggio R, Dolcetti R, Mautner J, Zanovello P, Amadori A, Rosato A. 2010. Virus-specific cytotoxic CD4+ T cells for the treatment of EBV-related tumors. J. Immunol. 184:5895–5902 [DOI] [PubMed] [Google Scholar]