Abstract
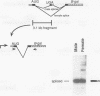
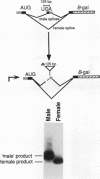
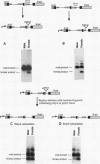
The on/off state of the binary switch gene Sex-lethal (Sxl), which controls somatic sexual development in Drosophila melanogaster, is regulated at the level of alternative splicing. In males, in which the gene is off, the default splicing machinery produces nonfunctional mRNAs; in females, in which the gene is on, the autoregulatory activity of the Sxl proteins directs the splicing machinery to produce functional mRNAs. We have used germ line transformation to analyze the mechanism of default and regulated splicing. Our results demonstrate that a blockage mechanism is employed in Sxl autoregulation. However, in contrast to transformer, in which Sxl appears to function by preventing the interaction of splicing factors with the default 3' splice site, a different strategy is used in autoregulation. (i) Multiple cis-acting elements, both upstream and downstream of the male exon, are required. (ii) These cis-acting elements are distant from the splice sites they regulate, suggesting that the Sxl protein cannot function in autoregulation by directly competing with splicing factors for interaction with the regulated splice sites. (iii) The 5' splice site of the male exon appears to be dominant in regulation while the 3' splice site plays a subordinate role.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht E. B., Salz H. K. The Drosophila sex determination gene snf is utilized for the establishment of the female-specific splicing pattern of Sex-lethal. Genetics. 1993 Jul;134(3):801–807. doi: 10.1093/genetics/134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S. Sex in flies: the splice of life. Nature. 1989 Aug 17;340(6234):521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Horabin J. I., Schedl P., Cline T. W. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Maine E. M., Schedl P., Cline T. W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988 Dec 23;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Bopp D., Bell L. R., Cline T. W., Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991 Mar;5(3):403–415. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Baker B. S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989 Mar 24;56(6):997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Cline T. W. Autoregulatory functioning of a Drosophila gene product that establish es and maintains the sexually determined state. Genetics. 1984 Jun;107(2):231–277. doi: 10.1093/genetics/107.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin C., Bank A. Reversibility of IVS 2 missplicing in a mutant human beta-globin gene. J Biol Chem. 1985 Dec 25;260(30):16332–16337. [PubMed] [Google Scholar]
- Dominski Z., Kole R. Cooperation of pre-mRNA sequence elements in splice site selection. Mol Cell Biol. 1992 May;12(5):2108–2114. doi: 10.1128/mcb.12.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes P. A., Cooke N. E., Liebhaber S. A. A difference in the splicing patterns of the closely related normal and variant human growth hormone gene transcripts is determined by a minimal sequence divergence between two potential splice-acceptor sites. J Biol Chem. 1990 Nov 15;265(32):19863–19870. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen J. P. Dosage Compensation in Drosophila: Evidence That daughterless and Sex-lethal Control X Chromosome Activity at the Blastoderm Stage of Embryogenesis. Genetics. 1987 Nov;117(3):477–485. doi: 10.1093/genetics/117.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguel V., Liao X. L., Rymond B. C., Rosbash M. U1 snRNP can influence 3'-splice site selection as well as 5'-splice site selection. Genes Dev. 1991 Aug;5(8):1430–1438. doi: 10.1101/gad.5.8.1430. [DOI] [PubMed] [Google Scholar]
- Grabowski P. J., Nasim F. U., Kuo H. C., Burch R. Combinatorial splicing of exon pairs by two-site binding of U1 small nuclear ribonucleoprotein particle. Mol Cell Biol. 1991 Dec;11(12):5919–5928. doi: 10.1128/mcb.11.12.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granadino B., Campuzano S., Sánchez L. The Drosophila melanogaster fl(2)d gene is needed for the female-specific splicing of Sex-lethal RNA. EMBO J. 1990 Aug;9(8):2597–2602. doi: 10.1002/j.1460-2075.1990.tb07441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley M. L., Maniatis T. Sex-specific splicing and polyadenylation of dsx pre-mRNA requires a sequence that binds specifically to tra-2 protein in vitro. Cell. 1991 May 17;65(4):579–586. doi: 10.1016/0092-8674(91)90090-l. [DOI] [PubMed] [Google Scholar]
- Hodges D., Bernstein S. I. Suboptimal 5' and 3' splice sites regulate alternative splicing of Drosophila melanogaster myosin heavy chain transcripts in vitro. Mech Dev. 1992 May;37(3):127–140. doi: 10.1016/0925-4773(92)90075-u. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Drosophila sex determination: a cascade of regulated splicing. Cell. 1989 Mar 24;56(6):905–906. doi: 10.1016/0092-8674(89)90619-3. [DOI] [PubMed] [Google Scholar]
- Hoffman B. E., Grabowski P. J. U1 snRNP targets an essential splicing factor, U2AF65, to the 3' splice site by a network of interactions spanning the exon. Genes Dev. 1992 Dec;6(12B):2554–2568. doi: 10.1101/gad.6.12b.2554. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Schedl P. Regulated splicing of the Drosophila sex-lethal male exon involves a blockage mechanism. Mol Cell Biol. 1993 Mar;13(3):1408–1414. doi: 10.1128/mcb.13.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima K., Inoue K., Higuchi I., Sakamoto H., Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science. 1991 May 10;252(5007):833–836. doi: 10.1126/science.1902987. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hoshijima K., Sakamoto H., Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature. 1990 Mar 29;344(6265):461–463. doi: 10.1038/344461a0. [DOI] [PubMed] [Google Scholar]
- Jamison S. F., Crow A., Garcia-Blanco M. A. The spliceosome assembly pathway in mammalian extracts. Mol Cell Biol. 1992 Oct;12(10):4279–4287. doi: 10.1128/mcb.12.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes L. N., Cline T. W., Schedl P. The primary sex determination signal of Drosophila acts at the level of transcription. Cell. 1992 Mar 6;68(5):933–943. doi: 10.1016/0092-8674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Kreivi J. P., Zerivitz K., Akusjärvi G. Sequences involved in the control of adenovirus L1 alternative RNA splicing. Nucleic Acids Res. 1991 May 11;19(9):2379–2386. doi: 10.1093/nar/19.9.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Mardon H. J., Sebastio G., Baralle F. E. A role for exon sequences in alternative splicing of the human fibronectin gene. Nucleic Acids Res. 1987 Oct 12;15(19):7725–7733. doi: 10.1093/nar/15.19.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen M. P., Smith C. W., Patton J. G., Nadal-Ginard B. Alpha-tropomyosin mutually exclusive exon selection: competition between branchpoint/polypyrimidine tracts determines default exon choice. Genes Dev. 1991 Apr;5(4):642–655. doi: 10.1101/gad.5.4.642. [DOI] [PubMed] [Google Scholar]
- Nasim F. H., Spears P. A., Hoffmann H. M., Kuo H. C., Grabowski P. J. A Sequential splicing mechanism promotes selection of an optimal exon by repositioning a downstream 5' splice site in preprotachykinin pre-mRNA. Genes Dev. 1990 Jul;4(7):1172–1184. doi: 10.1101/gad.4.7.1172. [DOI] [PubMed] [Google Scholar]
- Oliver B., Perrimon N., Mahowald A. P. Genetic evidence that the sans fille locus is involved in Drosophila sex determination. Genetics. 1988 Sep;120(1):159–171. doi: 10.1093/genetics/120.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. The regulated production of mu m and mu s mRNA is dependent on the relative efficiencies of mu s poly(A) site usage and the c mu 4-to-M1 splice. Mol Cell Biol. 1989 Feb;9(2):726–738. doi: 10.1128/mcb.9.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V. Vectors for P-mediated transformation in Drosophila. Biotechnology. 1988;10:437–456. doi: 10.1016/b978-0-409-90042-2.50028-3. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986 Aug 29;46(5):681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner L. C., Baker B. S. Regulation of doublesex pre-mRNA processing occurs by 3'-splice site activation. Genes Dev. 1991 Nov;5(11):2071–2085. doi: 10.1101/gad.5.11.2071. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Inoue K., Higuchi I., Ono Y., Shimura Y. Control of Drosophila Sex-lethal pre-mRNA splicing by its own female-specific product. Nucleic Acids Res. 1992 Nov 11;20(21):5533–5540. doi: 10.1093/nar/20.21.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M. E., Schedl P., Cline T. W. The complex set of late transcripts from the Drosophila sex determination gene sex-lethal encodes multiple related polypeptides. Mol Cell Biol. 1991 Jul;11(7):3584–3602. doi: 10.1128/mcb.11.7.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraphin B., Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell. 1989 Oct 20;59(2):349–358. doi: 10.1016/0092-8674(89)90296-1. [DOI] [PubMed] [Google Scholar]
- Sosnowski B. A., Belote J. M., McKeown M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell. 1989 Aug 11;58(3):449–459. doi: 10.1016/0092-8674(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky M. Sex determination in Drosophila: the X-chromosomal gene liz is required for Sxl activity. EMBO J. 1988 Dec 1;7(12):3889–3898. doi: 10.1002/j.1460-2075.1988.tb03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A., Kimmel B. E., Rubin G. M. rough, a Drosophila homeobox gene required in photoreceptors R2 and R5 for inductive interactions in the developing eye. Cell. 1988 Dec 2;55(5):771–784. doi: 10.1016/0092-8674(88)90133-x. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Valcárcel J., Singh R., Zamore P. D., Green M. R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature. 1993 Mar 11;362(6416):171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- Watakabe A., Tanaka K., Shimura Y. The role of exon sequences in splice site selection. Genes Dev. 1993 Mar;7(3):407–418. doi: 10.1101/gad.7.3.407. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]