Abstract
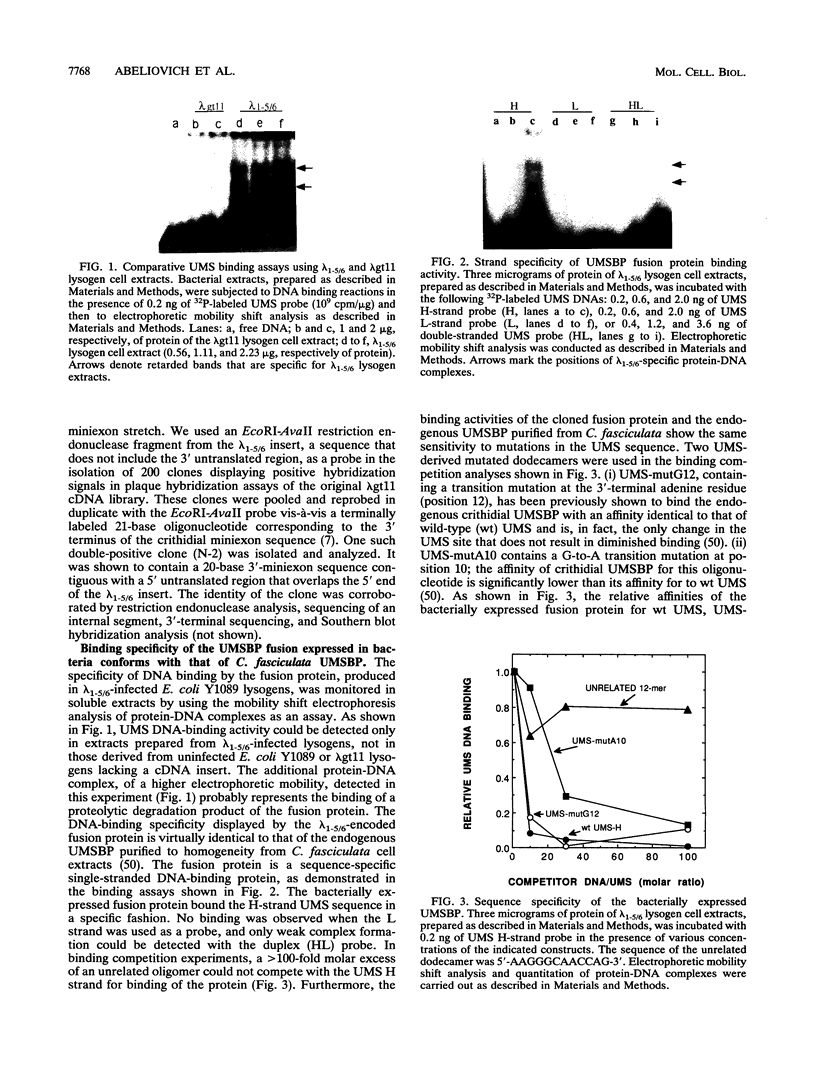
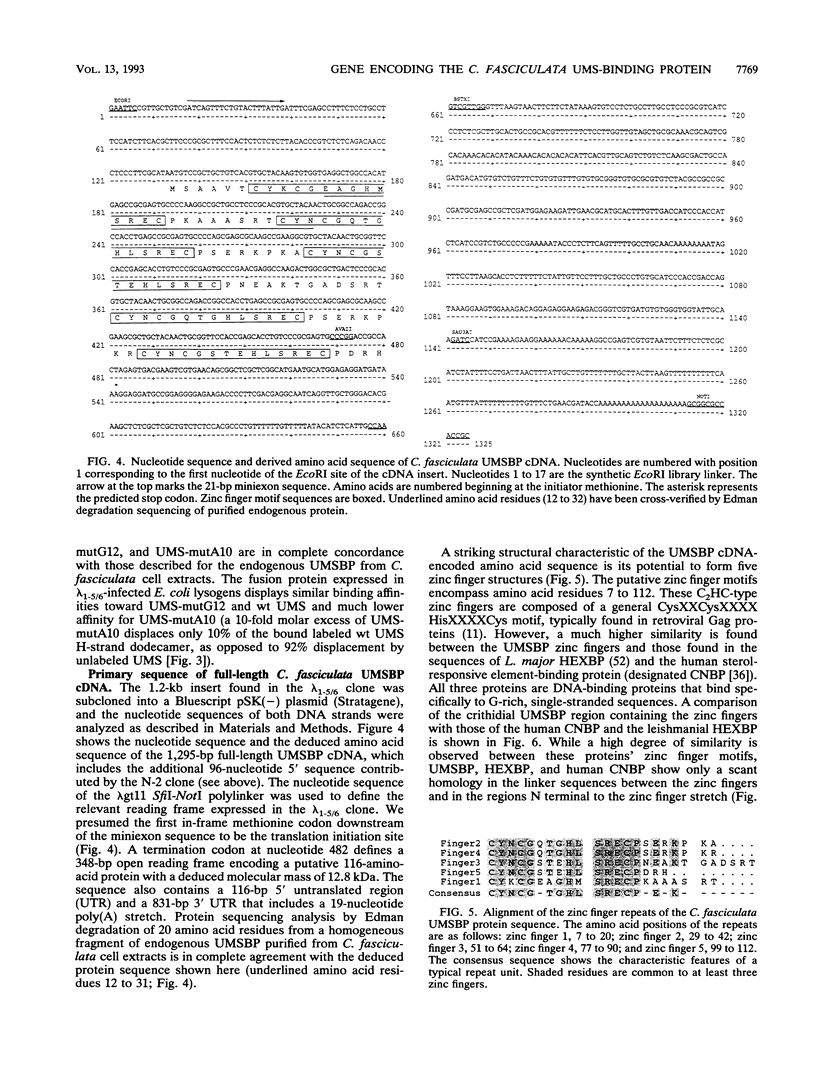
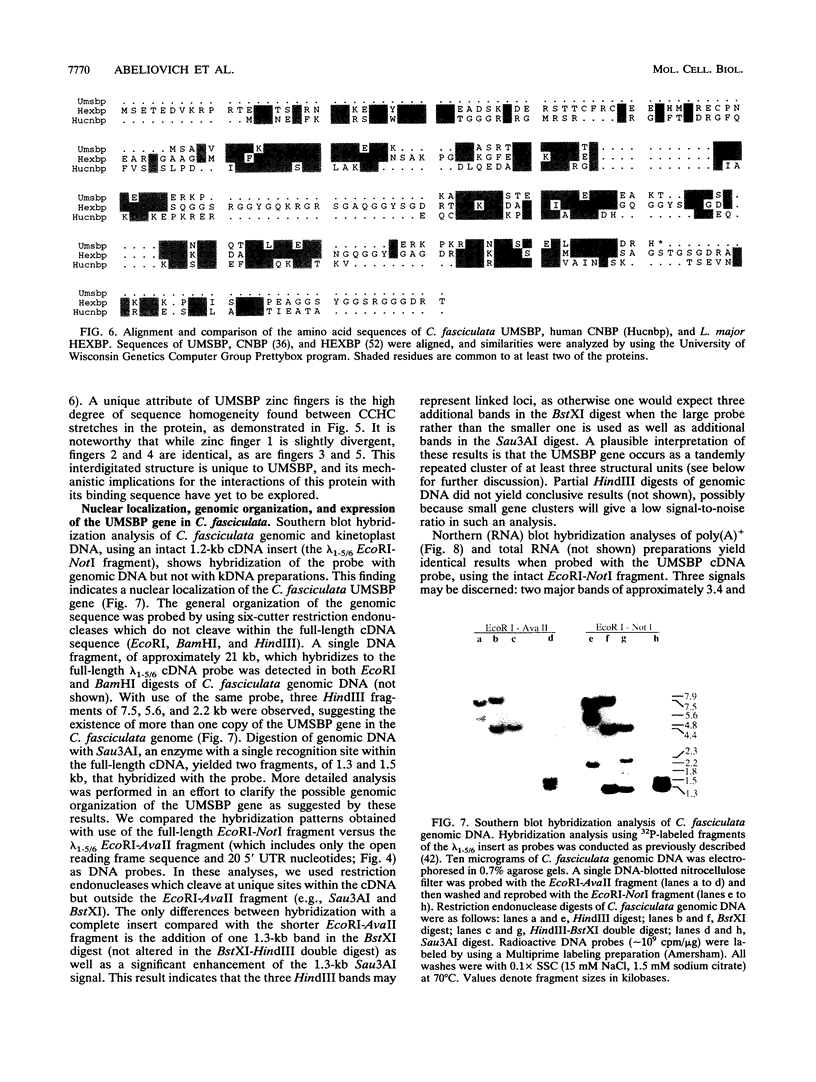
Replication of the kinetoplast DNA minicircle light strand initiates at a highly conserved 12-nucleotide sequence, termed the universal minicircle sequence. A Crithidia fasciculata single-stranded DNA-binding protein interacts specifically with the guanine-rich heavy strand of this origin-associated sequence (Y. Tzfati, H. Abeliovich, I. Kapeller, and J. Shlomai, Proc. Natl. Acad. Sci. USA 89:6891-6895, 1992). Using the universal minicircle sequence heavy-strand probe to screen a C. fasciculata cDNA expression library, we have isolated two overlapping cDNA clones encoding the trypanosomatid universal minicircle sequence-binding protein. The complete cDNA sequence defines an open reading frame encoding a 116-amino-acid polypeptide chain consisting of five repetitions of a CCHC zinc finger motif. A significant similarity is found between this universal minicircle sequence-binding protein and two other single-stranded DNA-binding proteins identified in humans and in Leishmania major. All three proteins bind specifically to single-stranded guanine-rich DNA ligands. Partial amino acid sequence of the endogenous protein, purified to homogeneity from C. fasciculata, was identical to that deduced from the cDNA nucleotide sequence. DNA-binding characteristics of the cDNA-encoded fusion protein expressed in bacteria were identical to those of the endogenous C. fasciculata protein. Hybridization analyses reveal that the gene encoding the minicircle origin-binding protein is nuclear and may occur in the C. fasciculata chromosome as a cluster of several structural genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., McCammon M. T., Montgomery D. L., Douglas M. G. Sequences required for delivery and localization of the ADP/ATP translocator to the mitochondrial inner membrane. Mol Cell Biol. 1986 Feb;6(2):626–634. doi: 10.1128/mcb.6.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Bergemann A. D., Johnson E. M. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Mol Cell Biol. 1992 Mar;12(3):1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeyer L., Ray D. S. Replication of kinetoplast DNA in isolated kinetoplasts from Crithidia fasciculata. Identification of minicircle DNA replication intermediates. J Biol Chem. 1986 Feb 15;261(5):2362–2368. [PubMed] [Google Scholar]
- Birkenmeyer L., Sugisaki H., Ray D. S. Structural characterization of site-specific discontinuities associated with replication origins of minicircle DNA from Crithidia fasciculata. J Biol Chem. 1987 Feb 15;262(5):2384–2392. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Van Doren K., Hirsh D., Steitz J. A. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988 Oct 6;335(6190):559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- Collick A., Dunn M. G., Jeffreys A. J. Minisatellite binding protein Msbp-1 is a sequence-specific single-stranded DNA-binding protein. Nucleic Acids Res. 1991 Dec 11;19(23):6399–6404. doi: 10.1093/nar/19.23.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove W. B., Skeen M. J. The cell cycle in Crithidia fasciculata. Temporal relationships between synthesis of deoxyribonucleic acid in the nucleus and in the kinetoplast. J Protozool. 1970 May;17(2):172–177. doi: 10.1111/j.1550-7408.1970.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Covey S. N. Amino acid sequence homology in gag region of reverse transcribing elements and the coat protein gene of cauliflower mosaic virus. Nucleic Acids Res. 1986 Jan 24;14(2):623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann W., Kröger B., Goller M., Horak I. A recombination hotspot in the LTR of a mouse retrotransposon identified in an in vitro system. Cell. 1989 Jun 16;57(6):937–946. doi: 10.1016/0092-8674(89)90332-2. [DOI] [PubMed] [Google Scholar]
- Englund P. T. Free minicircles of kinetoplast DNA in Crithidia fasciculata. J Biol Chem. 1979 Jun 10;254(11):4895–4900. [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Erdile L. F., Heyer W. D., Kolodner R., Kelly T. J. Characterization of a cDNA encoding the 70-kDa single-stranded DNA-binding subunit of human replication protein A and the role of the protein in DNA replication. J Biol Chem. 1991 Jun 25;266(18):12090–12098. [PubMed] [Google Scholar]
- Ferguson M., Torri A. F., Ward D. C., Englund P. T. In situ hybridization to the Crithidia fasciculata kinetoplast reveals two antipodal sites involved in kinetoplast DNA replication. Cell. 1992 Aug 21;70(4):621–629. doi: 10.1016/0092-8674(92)90431-b. [DOI] [PubMed] [Google Scholar]
- Gibson W. C., Swinkels B. W., Borst P. Post-transcriptional control of the differential expression of phosphoglycerate kinase genes in Trypanosoma brucei. J Mol Biol. 1988 May 20;201(2):315–325. doi: 10.1016/0022-2836(88)90140-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Lerner T. J., Huecas M., Sosa-Pineda B., Nogueira N., Lizardi P. M. Apparent generation of a segmented mRNA from two separate tandem gene families in Trypanosoma cruzi. Nucleic Acids Res. 1985 Aug 26;13(16):5789–5804. doi: 10.1093/nar/13.16.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Sigma factors from E. coli, B. subtilis, phage SP01, and phage T4 are homologous proteins. Nucleic Acids Res. 1986 Aug 26;14(16):6745–6763. doi: 10.1093/nar/14.16.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A., Patrick R. M., Walsh K. Nucleic acid specificity of a vertebrate telomere-binding protein: evidence for G-G base pair recognition at the core-binding site. Genes Dev. 1992 May;6(5):815–824. doi: 10.1101/gad.6.5.815. [DOI] [PubMed] [Google Scholar]
- Hofmann J. F., Gasser S. M. Identification and purification of a protein that binds the yeast ARS consensus sequence. Cell. 1991 Mar 8;64(5):951–960. doi: 10.1016/0092-8674(91)90319-t. [DOI] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Englund P. T. Intermediates in the replication of kinetoplast DNA minicircles. J Biol Chem. 1985 Mar 25;260(6):3844–3851. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Fein B. I., Englund P. T. Gapped Minicircles. A novel replication intermediate of kinetoplast DNA. J Biol Chem. 1984 Dec 25;259(24):15532–15539. [PubMed] [Google Scholar]
- Krowczynska A. M., Rudders R. A., Krontiris T. G. The human minisatellite consensus at breakpoints of oncogene translocations. Nucleic Acids Res. 1990 Mar 11;18(5):1121–1127. doi: 10.1093/nar/18.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyers M. L., Keating K. M., Roberts W. J., Williams K. R., Chase J. W., Horwitz M. S. Purification and functional characterization of adenovirus ts111A DNA-binding protein. Fluorescence studies of protein-nucleic acid binding. J Biol Chem. 1990 Apr 5;265(10):5875–5882. [PubMed] [Google Scholar]
- Muhich M. L., Hughes D. E., Simpson A. M., Simpson L. The monogenetic kinetoplastid protozoan, Crithidia fasciculata, contains a transcriptionally active, multicopy mini-exon sequence. Nucleic Acids Res. 1987 Apr 10;15(7):3141–3153. doi: 10.1093/nar/15.7.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Tanase S., Morino Y., Mori M. Presequence binding factor-dependent and -independent import of proteins into mitochondria. J Biol Chem. 1992 Jul 5;267(19):13119–13122. [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ntambi J. M., Shapiro T. A., Ryan K. A., Englund P. T. Ribonucleotides associated with a gap in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1986 Sep 5;261(25):11890–11895. [PubMed] [Google Scholar]
- Pasion S. G., Hines J. C., Aebersold R., Ray D. S. Molecular cloning and expression of the gene encoding the kinetoplast-associated type II DNA topoisomerase of Crithidia fasciculata. Mol Biochem Parasitol. 1992 Jan;50(1):57–67. doi: 10.1016/0166-6851(92)90244-e. [DOI] [PubMed] [Google Scholar]
- Pestov D. G., Gladkaya L. A., Maslov D. A., Kolesnikov A. A. Characterization of kinetoplast minicircle DNA in the lower trypanosomatid Crithidia oncopelti. Mol Biochem Parasitol. 1990 Jun;41(1):135–145. doi: 10.1016/0166-6851(90)90104-t. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Taylor A. K., Andalibi A., Svenson K. L., Lusis A. J. Identification of a zinc finger protein that binds to the sterol regulatory element. Science. 1989 Aug 11;245(4918):640–643. doi: 10.1126/science.2562787. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Kinetoplast DNA minicircles: high-copy-number mitochondrial plasmids. Plasmid. 1987 May;17(3):177–190. doi: 10.1016/0147-619x(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Englund P. T. Replication of kinetoplast DNA in Trypanosoma equiperdum. Minicircle H strand fragments which map at specific locations. J Biol Chem. 1989 Jan 15;264(2):823–830. [PubMed] [Google Scholar]
- Ryan K. A., Englund P. T. Synthesis and processing of kinetoplast DNA minicircles in Trypanosoma equiperdum. Mol Cell Biol. 1989 Aug;9(8):3212–3217. doi: 10.1128/mcb.9.8.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Griffith J. D., Englund P. T. A knotted free minicircle in kinetoplast DNA. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5844–5848. doi: 10.1073/pnas.85.16.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier J. M., Benard J., da Silva J., Riou G. Occurrence of a kinetoplast DNA-protein complex in Trypanosoma cruzi. Biochem Biophys Res Commun. 1981 Aug 14;101(3):988–994. doi: 10.1016/0006-291x(81)91846-5. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sheline C., Melendy T., Ray D. S. Replication of DNA minicircles in kinetoplasts isolated from Crithidia fasciculata: structure of nascent minicircles. Mol Cell Biol. 1989 Jan;9(1):169–176. doi: 10.1128/mcb.9.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline C., Ray D. S. Specific discontinuities in Leishmania tarentolae minicircles map within universally conserved sequence blocks. Mol Biochem Parasitol. 1989 Dec;37(2):151–157. doi: 10.1016/0166-6851(89)90147-3. [DOI] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Torri A. F., Englund P. T. Purification of a mitochondrial DNA polymerase from Crithidia fasciculata. J Biol Chem. 1992 Mar 5;267(7):4786–4792. [PubMed] [Google Scholar]
- Tzfati Y., Abeliovich H., Kapeller I., Shlomai J. A single-stranded DNA-binding protein from Crithidia fasciculata recognizes the nucleotide sequence at the origin of replication of kinetoplast DNA minicircles. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6891–6895. doi: 10.1073/pnas.89.15.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Webb J. R., McMaster W. R. Molecular cloning and expression of a Leishmania major gene encoding a single-stranded DNA-binding protein containing nine "CCHC" zinc finger motifs. J Biol Chem. 1993 Jul 5;268(19):13994–14002. [PubMed] [Google Scholar]