Abstract
Cardiac mitochondria and the sarcolemmal (sarc)KATP channels contribute to cardioprotective signaling of anesthetic-induced preconditioning (APC). Changes in mitochondrial bioenergetics influence the sarcKATP channel function, but whether this channel has impacts on mitochondria is uncertain. We used the mouse model with deleted pore-forming Kir6.2 subunit of sarcKATP channel (Kir6.2 KO) to investigate whether the functional sarcKATP channels are necessary for isoflurane activation of mitochondrial protective mechanisms. Ventricular cardiomyocytes were isolated from C57Bl6 wild type (WT) and Kir6.2 KO mouse hearts. Flavoprotein autofluorescence, mitochondrial ROS production and mitochondrial membrane potential were monitored by laser-scanning confocal microscopy in intact cardiomyocytes. Cell survival was assessed using H2O2-induced stress. Isoflurane (0.5 mM) increased flavoprotein fluorescence to 180±14% and 190±15% and ROS production to 118±2% and 124±6% of baseline in WT and Kir6.2 KO myocytes, respectively. TMRE fluorescence decreased to 84±6% in WT and to 86±4% in Kir6.2 KO myocytes. This effect was abolished by 5HD. Pretreatment with isoflurane decreased the stress-induced cell death from 31±1% to 21±1% in WT and from 44±2% to 35±2% in Kir6.2 KO myocytes. In conclusion, Kir6.2 deletion increases sensitivity of intact cardiomyocytes t o oxidative stress, but does not alter the isoflurane-elicited protective mitochondrial mechanisms, suggesting independent roles for cardiac mitochondria and sarcKATP channels in APC by isoflurane.
Keywords: Kir6.2 KO mouse, ventricular cardiomyocytes, cardiac mitochondria, cardiac KATP channels, isoflurane
Introduction
Anesthetic-induced preconditioning (APC) is an infarct size limiting strategy. A complex network of signaling pathways that are activated in APC1 ultimately converge on two key effectors of protection, the mitochondrial ATP-sensitive K+ (mitoKATP) channels and the sarcolemmal ATP-sensitive K+ (sarcKATP) channels.2, 3 However, it is unclear whether these two effectors of APC operate in a mutually dependent manner or constitute independent cytoprotective mechanisms. The latter would suggest separate, possibly parallel mechanisms of protection that facilitate the survival of cardiomyocytes even when some effectors of protection are dysfunctional.
The mitoKATP channels play an important role both as triggers and end-effectors of the volatile anesthetic-induced APC.2 Opening of this channel limits mitochondrial Ca2+ loading via a mild depolarization of the mitochondrial membrane potential that decreases driving force for Ca2+ influx. The channel participates also in the cellular signal transduction by inducing a small burst of the mitochondrial reactive oxygen species (ROS) that function as signaling molecules in APC. Previous studies have suggested that communication between mitochondria and the sarcKATP channels is important for cardiomyocyte survival, and demonstrated that uncoupled mitochondria may activate the sarcKATP channels in the myocardial cells.4
It is generally accepted that opening of sarcKATP channels is cardioprotective by inducing hyperpolarization of the cell membrane and therefore limiting cytosolic Ca2+ influx and mitochondrial Ca2+ overload, two important noxious stimuli.5,6 But, it is less clear whether opening of the sarcKATP channel could be protective by other, yet unknown mechanisms, such as facilitating the mitoKATP channel opening.
Studies aimed to delineate the role of mitoKATP and sarcKATP channels in protection by APC are hampered by limited selectivity of their pharmacological modulators.7-11 One important reason is that molecular structure of the mitoKATP channel is still undetermined, even though the molecular structure of the cardiac sarcKATP channel has been well characterized. The cardiac sarcKATP channel is formed by association of two subunits, the pore-forming inwardly rectifying Kir6.2 channel, encoded by KCNJ11 gene, and the regulatory sulphonylurea receptor SUR2A, an ATP-binding cassette (ABC) transporter that is encoded by ABCC9 gene.12, 13 The mouse model with knockout of KCNJ11 coding for the inward rectifier Kir6.2 channel (Kir6.2 KO)14 is a genetic model that enables us to more directly assess the role of sarcKATP channels in the anesthetic-induced and mitochondria-mediated APC.
The purpose of the present study was to investigate whether the functional sarcKATP channel is required for isoflurane-elicited activation of specific mitochondrial protective mechanisms that ultimately lead to protection of intact ventricular cardiomyocytes.
Methods
The study was conducted in accordance with guidelines set forth by the Medical College of Wisconsin, the Animal Welfare Act Regulation and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). All experimental protocols of this study were approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical College of Wisconsin, Milwaukee, WI.
Animals
Experiments were performed using 10 to 14 week-old male mice, weighing 25-35g. Wild-type (WT) C57Bl/6 mice were purchased from The Jackson Laboratory (Bar Harbor, MI). The Kir6.2 KO mice were a kind gift from the laboratory of Dr. Susumu Seino (Kobe University, Kobe, Japan) via Dr. John A Auchampach (Medical College of Wisconsin, Milwaukee, WI) and Dr. Richard J Gumina (Ohio State University, Columbus, OH) laboratories. Mice were housed in groups of 4 per cage in the temperature-controlled room with a 12:12 hour dark-light cycle, in Biomedical Resource Center of the Medical College of Wisconsin. Animals were fed standard chow with free access to tap water.
Isolation of mouse ventricular cardiomyocytes
Mouse ventricular cardiomyocytes were isolated as reported previously.15 Each mouse was injected (i.p.) with 100 IU heparin and anesthetized with 3 mg Inactin (sodium thiobutabarbital, Sigma-Aldrich, St Louis, MO). Following thoracotomy, the heart was rapidly excised and arrested in the ice-cold Ca2+−free Tyrode solution containing (in mM): NaCl 135, KCl 4.7, MgCl2 1.2, HEPES 10, glucose 5 and taurine 5, at pH 7.4. The aorta was cannulated under dissecting microscope. The heart was mounted on temperature-controlled (37°C) Langendorff apparatus and perfused at a constant flow of 3.0 ml/min with Ca2+ free Tyrode solution for 4 min, and then with 0.128 mg/ml of Liberase-TM blendzyme (Roche, Indianapolis, IN) in the Ca2+−free Tyrode solution for 8-13 min. Following digestion, the left ventricle was excised and minced in 5 ml of Tyrode solution containing 20% fetal calf serum. Tissue suspension was gently agitated to release single cardiomyocytes. The cells were sedimented for 20 min at room temperature, resuspended in Tyrode solution containing 5% fetal calf serum and allowed to settle for another 20 min during which the extracellular Ca2+ was stepwise increased to 1 mM. Myocytes were stored in the Tyrode solution at room temperature. The rod-shaped cells with distinct cross-striations and intact surface membrane were used for experiments within 5 h after isolation.
Electrophysiolog
The presence of functional cardiac sarcKATP channels in WT myocytes, and their absence in Kir6.2 KO myocytes was confirmed electrophysiologically. Activity of single sarcKATP channels was monitored in the inside-out patch clamp configuration16 at the membrane potential of +40 mV. The extracellular/pipette solution contained (in mM) 145 KCl, 0.5 CaCl2, 0.5 MgCl2, and 10 HEPES at pH 7.4. The intracellular/bath solution contained (in mM) 145 KCl, 0.5 MgCl2, 2 EGTA, 10 HEPES and 0.005 K2ATP at pH 7.2. The channel opener pinacidil (100 μM, Sigma-Aldrich, St Louis, MO), and the channel blockers glibenclamide (1 μM, Sigma-Aldrich, St Louis, MO) and HMR-1098 (30 μM, Sanofi-Aventis Pharma, Frankfurt, Germany) were applied in the bath solution. The heat-polished borosilicate glass patch pipettes (Garner, Claremont, CA) had resistance of 4-7 MΩ when filled with the pipette solution. Recordings were performed at room temperature using EPC-7 amplifier (List, Darmstadt-Eberstadt, Germany) with Digidata 1322A interface (Axon Instruments/Molecular Devices, Union City, CA) and pClamp10 software. Current signal was low-pass filtered at 500 Hz through an eight-pole Bessel filter and sampled at 1 kHz. Single channel data were analyzed with pCLAMP10 (Axon Instruments/Molecular Devices, Union City, CA) and Origin7 (OriginLab, Northampton, MA) software. The all-points amplitude histograms were constructed from 60 second recordings. The channel open probability (Po) was determined from the ratio of the area under the peaks in amplitude histograms fitted by a multi-Gaussian distribution, using the equation Po = [1 –(Pc)1/N] where Pc is the channel closed probability and (N) is the number of channels in the patch.
Laser scanning confocal microscopy
Experiments were conducted on isolated intact cardiomyocytes in the recording chamber mounted on the stage of a laser-scanning confocal microscope (Eclipse TE2000-U, Nikon, Tokyo, Japan) with the x60/1.4 oil-immersion objective (Nikon, Tokyo, Japan). Data were stored on hard disk of PC computer and analyzed off-line using MetaMorph6.2 software (Universal Imaging, West Chester, PA) and NIH ImageJ software. Results are presented as percent change in fluorescence intensity relative to baseline (F0) recorded before the cell exposure to isoflurane (F/F0 × 100).
Analysis of mitochondrial redox state
Isolated cardiomyocytes were placed in the recording chamber and superfused with Tyrode solution. Autofluorescence of endogenous flavoproteins (FP) was excited at 488 nm by an argon laser and the emitted light was collected at 500-530 nm by a photomultiplier and was digitized. To assess the mitochondrial matrix redox state of intact myocytes, FP fluorescence was recorded for 25 min at room temperature as reported previously.17, 18 The FP oxidation, resulting in enhanced autofluorescence signal, is compensatory to mitochondrial uncoupling.3 For statistical analysis, the averaged baseline fluorescence of time points recorded before anesthetic exposure was compared to the averaged fluorescence of time points recorded during exposure to the anesthetic.
Analysis of ROS production
The mitochondrial ROS production was measured using the ROS-sensitive indicator 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester, CM-H2DCFDA (Molecular Probes, Eugene, OR) as described previously.17, 19 CM-H2DCFDA is a membrane-permeable indicator that diffuses into the cell where endogenous esterases cleave acetate groups yielding the membrane impermeable, nonfluorescent CM-H2DCF. In turn, oxidation of deestrified CM-H2DCF by ROS yields a fluorescent CM-DCF. Isolated cardiomyocytes were loaded with 2 μM CM-H2DCFDA for 20 min, followed by a 10-min washout. The cells were superfused with Tyrode solution at room temperature and fluorescence of individual myocytes was recorded for 28 min. CM-DCF fluorescence was excited at 488 nm by an argon laser and emission was collected at 500-550 nm. The neutral density filters (ND32) minimized the dye bleaching. The baseline fluorescence was compared with the averaged fluorescence of time points recorded during the application of isoflurane.
Measurement of mitochondrial membrane potential (ΔΨm)
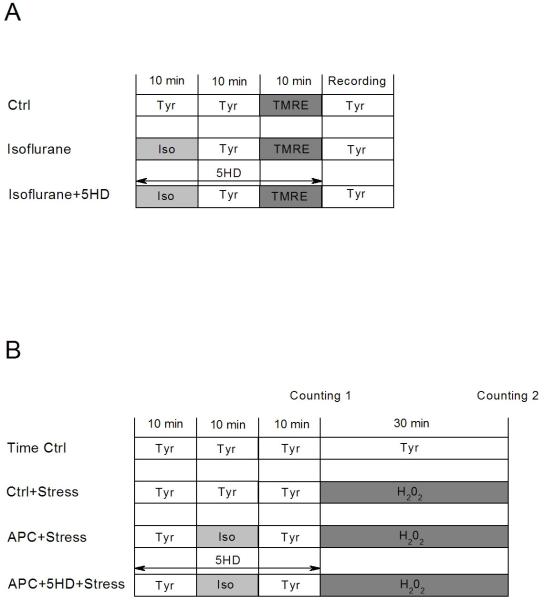
Isolated myocytes were incubated for 10 min with the mitochondrial membrane potential-sensitive fluorescent dye tetramethylrhodamine ethyl ester (TMRE; 100 nM; Invitrogen, Carlsbad, CA) in the absence and the presence of isoflurane. The TMRE fluorescence was monitored at the excitation and emission wavelengths of λex/λem=543/570-610 nm using a green HeNe laser.19 The experimental protocols are shown in Fig. 1A.
Figure 1.
Study protocols. (A) Protocol for mitochondrial membrane potential measurement. (B) Protocols for cell survival experiments. Detailed descriptions are provided in Methods. Ctrl, control; Tyr, Tyrode solution; TMRE, tetramethylrhodamine ethyl ester; Iso, isoflurane; 5HD, 5-hydroxydecanoate; APC, anesthetic preconditioning; Stress, oxidative stress with H2O2.
Assessment of cardiomyocyte response to stress
Myocyte survival was assessed in vitro using taurine-free Tyrode solution, since taurine may have an effect on the key mediators of cardioprotection.20 Approximately 1 ml of cell suspension was placed in a chamber on the stage of an inverted Olympus MT2 microscope (Olympus, Tokyo, Japan), and the cells were allowed to settle for 10 min. Oxidative stress was induced by exposing myocytes to 2.0 mM H2O2 (Calbiochem, LA Jolla, CA) for 30 min at room temperature. In experimental groups that underwent APC, the myocytes were exposed to 0.5 mM isoflurane for 10 min, followed by a 10 min anesthetic washout prior to application of H2O2. In each experiment approximately 100 myocytes were counted by one blinded and one not blinded investigator. The living cell count was based on cell morphological criteria (intact surface membrane, distinct cross-striation, rod shape) and exclusion of Trypan blue.2 Percent cell death was calculated from the living cell count before and after oxidative stress. A labeled grid on the glass floor of the chamber ensured counting the same cardiomyocytes before and after the stress. The experimental protocols are shown in Fig. 1B.
Drugs
The volatile anesthetic isoflurane (Abbott Laboratories, North Chicago, IL) was dispersed in experimental solutions by sonication and delivered to the recording chamber from an airtight glass syringes. At the end of each experiment, samples of the anesthetic-containing solution were taken from the outflow of the recording chamber, and anesthetic concentrations were analyzed by a gas chromatography method using Shimadzu gas chromatograph (Shimadzu, Kyoto, Japan). The mean isoflurane concentration was 0.5±0.04 mM, equivalent to 1 MAC (minimum alveolar concentration) in mice. To investigate the effects of mitoKATP channels on mitochondrial membrane potential, the mitoKATP channel blocker 5-hydroxydecanoate (5HD, 500 μM; Sigma-Aldrich, St. Louis, MO) was used in the confocal microscopy and cell viability experiments. The sarcKATP channel blockers HMR-1098 (30 μM, Sanofi-Aventis Pharma, Frankfurt, Germany) and glibenclamide (1 μM, Sigma-Aldrich, St Louis, MO) were used in electrophysiological experiments.
Statistical analysis
Data are presented as means±SEM, (n) represents the number of cell isolations from individual hearts, i.e. the number of mice. Confocal microscopy data were analyzed with Origin7 software (OriginLab, Northampton, MA). Statistical significance was determined using analysis of variance for multiple comparisons and Student’s t test where appropriate. Patch clamp data were analyzed with pCLAMP10 (Molecular Devices, Union City, CA) and Origin7 (OriginLab, Northampton, MA) software. Statistical significance of results was determined using analysis of variance with Scheffe post hoc test. Differences with at the two-tailed P<0.05 were considered significant.
Results
Activity of single sarcKATP channels in mouse ventricular cardiomyocytes
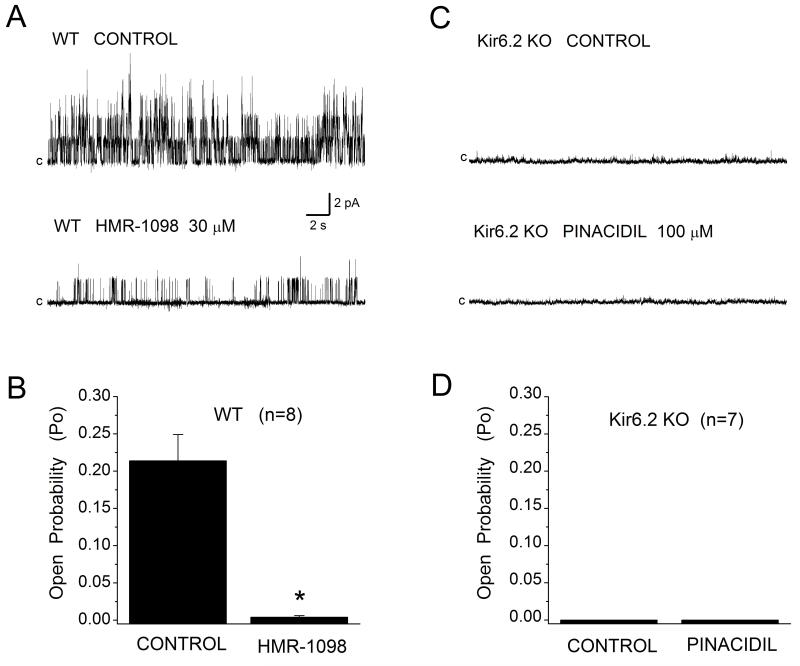
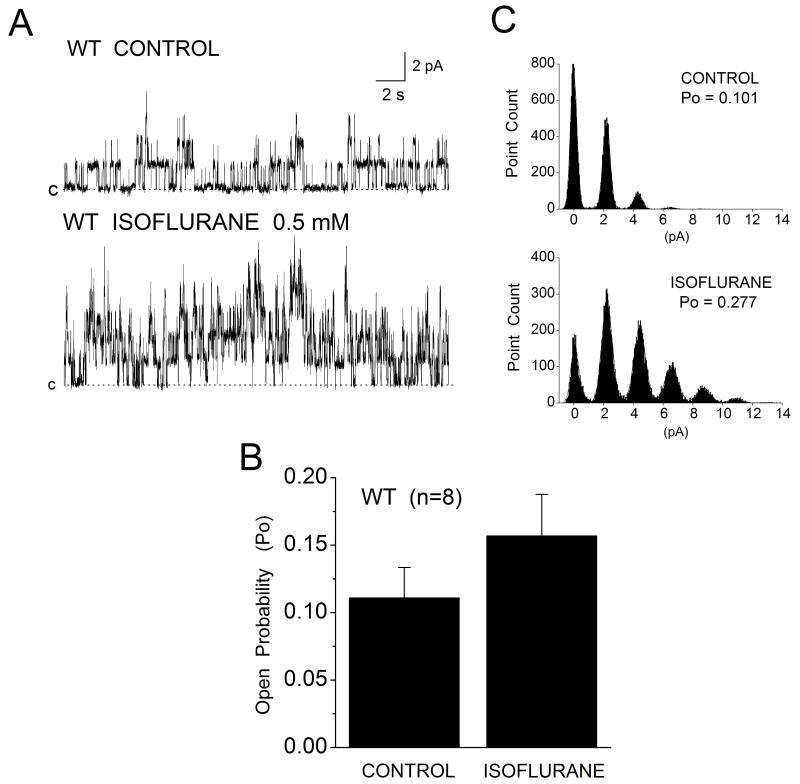
To verify the presence of functional sarcKATP channels in WT mouse cardiomyocytes and their absence in Kir6.2 KO myocytes, single KATP channel activity was monitored from inside-out membrane patches at +40 mV membrane potential in the presence of 5 μM ATP or in the absence of ATP. Figure 2A shows that in patches from WT myocytes the channel activity was high, with Po of 0.214±0.035 (n=8). Po was inhibited by 97% (Po 0.004±0.002, n=8) upon application of 30 μM HMR-1098 (Fig. 2B) or 1 μM glibenclamide, confirming the identity of the channel. By contrast, all patches excised from Kir6.2 KO myocytes were silent (Fig. 2C and 2D). No channel opening was evoked in the absence or presence of low ATP, or on application of the channel opener pinacidil (100 μM). When applied directly to the inside-out membrane patches from WT myocytes isoflurane (0.5 mM) increased channel Po by 33% (Fig. 3A and 3B), from mean control of 0.111±0.022 to 0.157±0.0.031 (n=8), and increased the number of open channels (Fig. 3C, histograms).
Figure 2.
The sarcKATP channel in mouse ventricular cardiomyocytes. (A) Single channel activity was monitored in the inside-out patch configuration at Em +40 mV in the absence (Control) or presence of sarcK channel blocker HMR-1098. (B) HMR-1098 (30 μM) inhibited channel activity by 97%. Data are mean±SEM, n=8. (C, D) No channel activity was detected in membrane patches excised from Kir6.2 KO myocytes when monitored in control conditions or during application of channel opener pinacidil (100 μM), n=7.
Figure 3.
Acute isoflurane enhances sarcKATP channel opening in inside-out patches from mouse WT myocytes. (A) Example recordings of single sarcKATP channel activity monitored from the same patch in the absence (CONTROL) and the presence of 0.5 mM isoflurane (ISOFLURANE). Isoflurane increased the probability of channel opening (Po) by 33% (B) and increased the number of open channels (C). Data are mean±SEM, n=8.
Effect of isoflurane on FP fluorescence in WT and Kir6.2 KO cardiomyocytes
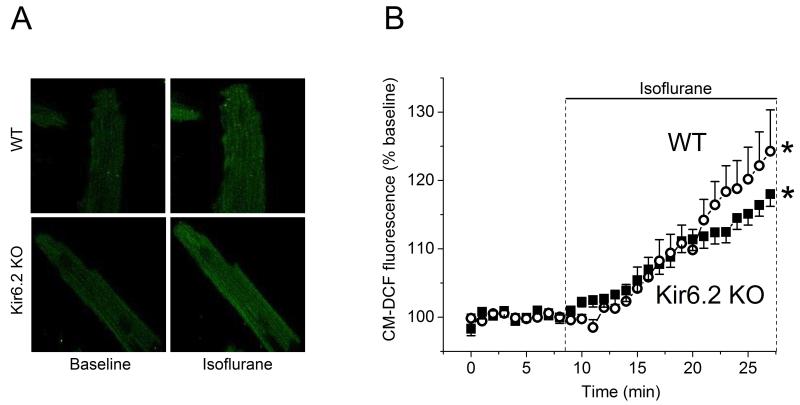
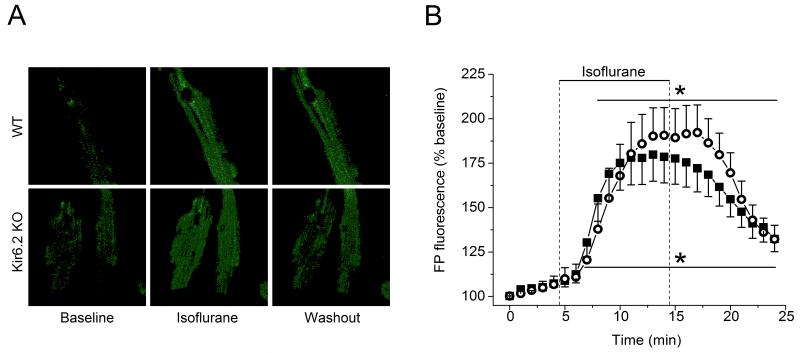
In the present study, FP autofluorescence was used to assess the mitochondrial uncoupling. Figure 4 shows that isoflurane significantly enhanced FP fluorescence to 180±14% and 190±15% of baseline (100%) in cardiomyocytes from WT (n=6) and Kir6.2 KO (n=5) hearts, respectively. Similar responses in both groups suggested the absence of functional sarcKATP channels does not alter isoflurane-induced enhancement of FP oxidation and mitochondrial uncoupling.
Figure 4.
Acute isoflurane alters the mitochondrial redox state of intact mouse ventricular cardiomyocytes. Autofluorescence of mitochondrial flavoproteins (FP) was monitored as the indicator of mitochondrial redox state. (A) Example images of FP fluorescence captured in WT and Kir6.2 KO cardiomyocytes before (Baseline), during (Isoflurane) and after (Washout) exposure to 0.5 mM isoflurane. (B) Time course of changes in FP fluorescence during application of isoflurane. Data are expressed as change from the baseline (100%), data points are mean±SEM. Isoflurane increased oxidation of mitochondrial FP in cardiomyocytes from WT (closed symbols, n=6) and Kir6.2 KO (open symbols, n=5) hearts. *P< 0.05 vs. baseline. Washout between 14 and 25 min.
Effect of isoflurane on mitochondrial ROS production in WT and Kir6.2 KO cardiomyocytes
Fig. 5 shows the time course of changes in mitochondrial ROS generation quantified by CM-DCF fluorescence intensity before and during acute application of isoflurane. During 18 min exposure to isoflurane, the CM-DCF fluorescence significantly increased to 118±2% and 124±6% of baseline (100%) in cardiomyocytes from WT (n=6) and Kir6.2 KO (n=5) hearts, respectively. Acute isoflurane increased the mitochondrial ROS production in both WT and KO myocytes, suggesting mitochondrial ROS generation by isoflurane is preserved in the absence of functional sarcKATP channels.
Figure 5.
Acute isoflurane increases generation of reactive oxygen species (ROS) in isolated intact mouse cardiomyocytes. (A) Representative images of CM-DCF fluorescence captured in WT and Kir6.2 KO cardiomyocytes before and during exposure to 0.5 mM isoflurane. The CM-DCF fluorescence was increased by isoflurane. (B) Summary of time dependent changes in CM-DCF fluorescence. Data were normalized to the baseline and presented as mean±SEM. Isoflurane effects on ROS production were similar in cardiomyocytes from WT (n=6) and Kir6.2 KO (n=5) mouse hearts. *P< 0.05 vs. baseline.
Mitochondrial membrane depolarization by isoflurane in WT and Kir6.2 KO cardiomyocytes
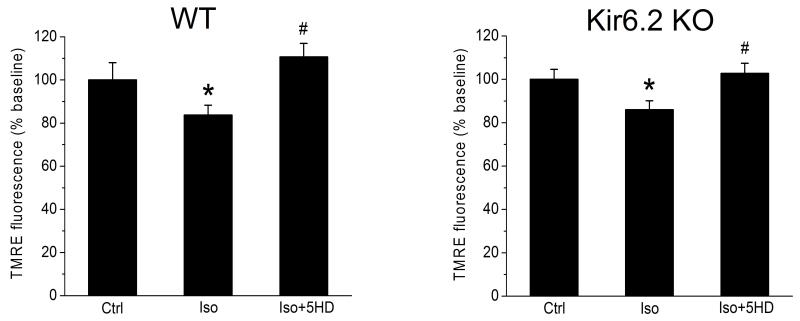
Volatile anesthetics cause a partial depolarization of the mitochondrial membrane potential which contributes to protection afforded by APC and may in part be mediated by mitoKATP channel opening.3, 17 We tested the effects of isoflurane on mitochondrial membrane potential in cardiomyocytes from WT (n=6) and Kir6.2 KO (n=5) hearts using TMRE. Fig. 6A shows that acute isoflurane decreased TMRE fluorescence to 84±6% of control (100%) in WT cardiomyocytes, suggesting partial mitochondrial depolarization. This effect was abolished by the mitoKATP channel blocker 5HD. A similar effect was observed also in Kir6.2 KO myocytes, where isoflurane decreased TMRE fluorescence to 86±4% of control, and 5HD abolished this effect (Fig. 6B), suggesting the function of mitoKATP channel is preserved in Kir6.2 KO myocytes in the absence of functional sarcKATP channel.
Figure 6.
Acute isoflurane induces partial depolarization of mitochondrial membrane potential in cardiomyocytes from WT (n=6) and Kir6.2 KO (n=5) hearts. Isoflurane decreased intensity of TMRE fluorescence in WT and Kir6.2 KO cardiomyocytes. The effect was abolished in the presence of 5HD, a blocker of mitoKATP channels. Ctrl, Control; Iso, isoflurane; 5HD, 5-hydroxydecanoate. Data are mean±SEM, *P< 0.05 Ctrl vs. Iso; #P< 0.05 Iso vs. Iso+5HD. Left panel: Summary data from WT cardiomyocytes. Right panel: Summary data from Kir6.2 KO cardiomyocytes.
APC with isoflurane alters cardiomyocyte response to stress
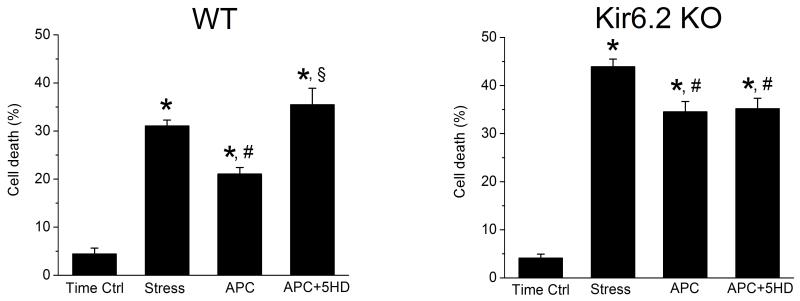
Oxidative stress, due in part to enhanced generation of H2O2 during reperfusion period causes cardiomyocyte death.21 We investigated whether APC with isoflurane may protect WT and Kir6.2KO cardiomyocytes from cell death by H2O2. Fig. 7 shows that in the time control group, where myocytes were superfused only with taurine-free Tyrode solution, the cell death among myocytes from WT (n=9) and Kir6.2 KO (n=6) hearts was relatively low (4±1% and 4±2%, respectively). H2O2 increased cell death to 31±1% in WT myocytes (Fig. 7A) and myocyte pretreatment with isoflurane (APC) attenuated this effect to 21±1%. Bracketing APC with 5HD abolished isoflurane protection and cell death increased to 35±3%. By contrast, H2O2 increased Kir6.2 KO myocyte death to 44±2% and APC with isoflurane attenuated this effect to 35±2%. Interestingly, blocking the mitoKATP channels with 5HD had no significant effect on survival of Kir6.2 KO myocytes (Fig. 7B, APC vs. APC+5HD). The cell death among Kir6.2 KO myocytes exposed to H2O2 in the absence or presence of APC was significantly higher than among WT myocytes (Fig. 7 A and B). Thus APC with isoflurane can elicit protection of both WT and Kir6.2 KO myocytes, in the presence and absence of functional sarcKATP channel. However, susceptibility to stress-induced cell injury is significantly greater in Kir6.2 KO, which confirms the importance of sarcKATP channels for myocyte protection from stress.
Figure 7.
Effect of APC with isoflurane on H2O2 induced stress and survival of isolated cardiomyocytes from WT (n=9) and Kir6.2 KO (n=6) hearts. Percent cell death was determined in four experimental groups: time control (Time Ctrl), oxidative stress with H2O2 (Stress), stress following cell exposure to isoflurane (APC), and stress in isoflurane-pretreated myocytes during blockade of KATP channels with 5HD (APC+5HD). Data are mean±SEM, *P< 0.05 vs. Time Ctrl; #P< 0.05 vs. Stress; §P< 0.05 vs. APC. Left panel: Pretreatment with isoflurane protected WT myocytes from stress. Blockade of mitoKATP channels with 5HD abolished this protection. Right panel: Pretreatment with isoflurane protected Kir6.2 KO myocytes from stress. However, isoflurane-induced protection of Kir6.2 KO myocytes was not altered by 5HD (APC vs. APC+5HD).
Discussion
The present study demonstrates that in isolated intact mouse cardiomyocytes a volatile anesthetic isoflurane elicits competent mitochondrial protective events characteristic for APC signaling.17 These include: (i) oxidation of mitochondrial flavoproteins, an indicator of mitochondrial uncoupling; (ii) generation of small bursts of ROS that function as signaling molecules in APC; and (iii) partial depolarization of mitochondrial membrane potential via opening of mitoKATP channel. Although isoflurane elicited these protective events in both WT and Kir6.2-KO cardiomyocytes, the latter appear more sensitive to oxidative stress than WT myocytes with the functional sarcKATP channels.
The effect of isoflurane on mitochondrial redox state was evaluated based on changes in native FP fluorescence. Increase in flavoprotein oxidation, due to accelerated electron flow through the respiratory chain, is a compensatory response to mitochondrial membrane depolarization and uncoupling, which occur in acutely isolated cardiomyocytes18, 22 and cultured cells 23 during exposure to anesthetics. The mitoKATP channels, previously identified as effectors of protection by preconditioning, appear to mediate APC-induced mitochondrial depolarization and compensatory flavoprotein oxidation.23,24 Isoflurane-induced increase in FP fluorescence in rat cardiomyocytes is sensitive to mitoKATP channel blockade by 5HD and could be mimicked by the mitoKATP channel opener diazoxide3 Our study shows that isoflurane increases FP fluorescence similar in mouse WT and Kir6.2 KO cardiomyocytes. This suggests that isoflurane activation of the signaling cascade that leads to mitoKATP channel opening does not rely on the sarcKATP channel opening and therefore may constitute an independent pathway. Our data support findings by Suzuki et al.25 showing that oxidation of flavoproteins by diazoxide is preserved in Kir6.2 KO cardiomyocytes, and confirm the presence of functional mitoKATP channels. It also suggests that Kir6.2 is not a subunit of the mitoKATP channel.
A moderate increase in ROS generation appears critical for initiating the APC signaling cascade.26, 27 Our study demonstrated that isoflurane elicits a burst of ROS in mouse WT and Kir6.2 KO cardiomyocytes. A similar effect of isoflurane was reported in rat cardiomyocytes.19 The mechanism by which isoflurane induces a burst of ROS involves opening of mitoKATP channels28 and/or partial inhibition of the electron transport chain.19 Thus, mitochondria appear to be the primary source of ROS generated in cardiomyocytes during exposure to isoflurane.19 These findings correlate with FP fluorescence data, showing both WT and Kir6.2 KO myocytes exhibit a similar mitochondrial response to isoflurane. Furthermore, ROS may directly activate the sarcKATP channel29, 30 possibly by modifying its sensitivity to ATP.31 In addition, ROS generated in response to volatile anesthetics can activate PKC,32 which in turn may enhance opening of sarcKATP channel.33, 34 Taken together these findings suggest the opening of sarcKATP channel is downstream of a signaling burst of ROS, and likely downstream or parallel to mitoKATP channel opening.
Modulation of the mitochondrial membrane potential by volatile anesthetics plays an important role in the mechanism of cardioprotection and may involve the mitoKATP channel opening.18, 23, 24 Indeed, isoflurane elicits partial depolarization of cardiac mitochondria in both WT and KO myocytes. These findings corroborate the results from our previous studies in rats and the human embryonic stem cell-derived cardiomyocytes3, 35 underscoring similarities in the mechanisms of APC signaling among different species. Application of 5HD together with isoflurane abolished its effect on mitochondrial membrane potential in WT and Kir6.2 KO myocytes supporting a notion that this effect occurs via activation of the mitoKATP channel. This suggests that Kir6.2 KO myocytes exhibit normal mitochondrial responses to isoflurane, including opening of the mitoKATP channels.
Our cell survival experiments showed that compared to WT the Kir6.2 KO myocytes are more sensitive to stress-induced cell death. This is in line with findings by Suzuki et al.25who showed that basal ischemic damage is greater in Kir6.2 KO than WT hearts, as judged by greater magnitude of ischemic contracture and poorer recovery of ventricular function after reperfusion. Thus, the functional sarcKATP channel is important endogenous effector of protection activated in response to noxious stimuli. Genetic disruption of sarcKATP channel may enhance susceptibility toward stress in KO mice due to increased intracellular Ca2+ loading36 or disruption of metabolic networks.37
Interestingly, APC with isoflurane attenuated cell death of both WT and Kir6.2 KO myocytes, suggesting the APC protective signaling pathways may function in parallel. Intact mitochondrial pathways (mitochondrial redox state, ROS burst and mitoKATP channel opening) in Kir6.2 KO myocytes could likely be the parallel mediators of protection. That would imply the existence of multiple but independent pathways that can afford protection even if other pathways are dysfunctional, suggesting an evolutionary mechanism of a vital importance that ensures survival of cardiomyocytes, the terminally differentiated cells, that cannot be regenerated following detrimental ischemic events.
Previous studies demonstrated that diazoxide-induced preconditioning is abolished in the hearts from Kir6.2 KO mice.38 Hu et al.39 reported that Kir6.2 knockout impairs the left ventricular response to stress: systolic overload following chronic transverse aortic constriction. Furthermore, the hearts of transgenic mice expressing a mutant Kir6.2 channel with reduced ATP sensitivity exhibited incompetent protection by ischemic preconditioning and poor recovery after metabolic inhibition.40
When investigating the cellular mechanisms of APC with isoflurane we tested the possibility of a cross-talk between two molecular signaling pathways that contribute to preconditioning, the sarcKATP channels and mitochondria. After initial reports by Gross and Auchampach41 and Auchampach et al.42 numerous studies focused on the role of sarcKATP channel in cardioprotection. Opening of these channels decreases action potential duration and cytosolic Ca2+ influx, thus protecting the heart from ischemic damage.43, 44 The significance of mitochondrial pathways, including the opening of mitoKATP channels also has been documented. Communication between mitochondria and sarcKATP channel is important for myocyte survival.4 However, the interdependence of these two end-effectors of protection is not completely understood, except for indications that they may be activated at different stages of APC.2, 45, 46 The present study suggests for the first time that the sarcKATP channels and mitochondria might be independent, but complimentary effectors of protection by APC.
In conclusion, knockout of Kir6.2 subunit, the pore of cardiac sarcKATP channel does not perturb the protective mitochondrial responses elicited by isoflurane: uncoupling, mitochondrial ROS burst and mitochondrial membrane depolarization, and does not abolish cellular protection by APC in mouse myocytes. This suggests that mitochondria and sarcKATP channels may function independently, in parallel cellular protective pathways that complement each other. Increased susceptibility of Kir6.2 KO cardiomyocytes to H2O2-induced cell death supports the importance of sarcKATP channels for protection from stress.
Acknowledgements
The authors thank Dr. Susumu Seino (Kobe University, Kobe, Japan), Dr. John A Auchampach (Medical College of Wisconsin, Milwaukee, WI) and Dr. Richard J Gumina (Ohio State University, Columbus, OH) for providing Kir6.2 KO mice.
We thank Mary B Ziebell, Research Technologist in the Department of Anesthesiology, Medical College of Wisconsin, for isoflurane measurements.
Sources of Funding:
This study was supported in part by the National Institutes of Health grants P01GM066730 and R01HL034708 (to ZJB), Bethesda, Maryland.
Footnotes
Conflict of Interest statement: The authors declare no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003. 2003;91:551–565. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- 2.Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology. 2006;105:98–104. doi: 10.1097/00000542-200607000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber MW. Isoflurane preconditioning uncouples mitochondria and protects from hypoxia/reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–C1590. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki N, Sato T, Marban E, O’Rourke B. ATP consumption by uncoupled mitochondria activates sarcolemmal KATP channels in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1882–H1888. doi: 10.1152/ajpheart.2001.280.4.H1882. [DOI] [PubMed] [Google Scholar]
- 5.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 6.Flagg TP, Nichols CG. Sarcolemmal K(ATP) channels: what do we really know? J Mol Cell Cardiol. 2005;39:61–70. doi: 10.1016/j.yjmcc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riess ML, Camara AKS, Heinen A, Eells JT, Henry MM, Stowe DF. KATP channel openers have opposite effects on mitochondrial respiration under different energetic conditions. J Cardiovasc Pharmacol. 2008;51:483–491. doi: 10.1097/FJC.0b013e31816bf4a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Rapedius M, Baukrowitz T, Liu GX, Srivastava DK, Daut J, Hanley PJ. 5-Hydroxydecanoate and coenzyme A are inhibitors of native sarcolemmal KATP channels in inside-out patches. Biochim Biophys Acta. 2010;1800:385–391. doi: 10.1016/j.bbagen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang HX, Akrouh A, Kurata HT, Remedi MS, Lawton JS, Nichols CG. HMR1098 is not an SUR isotype specific inhibitor of heterologous or sarcolemmal KATP channels. J Mol Cell Cardiol. 2011;50:552–560. doi: 10.1016/j.yjmcc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 14.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyzaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorometry. Am J Physiol Heart Circ Physiol. 1996;271:H1250–H1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 16.Stadnicka A, Marinovic J, Bosnjak ZJ. Impact of in vivo preconditioning by isoflurane on sarcolemmal KATP channel in the rat heart: long-term modulation of nucleotide sensitivity during early memory phase of cardioprotection. Anesthesiology. 2006;104:503–510. doi: 10.1097/00000542-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Sedlic F, Pravdic D, Ljubkovic M, Marinovic-Ljubkovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in preconditioning signaling cascade between desflurane and sevoflurane. Anesth Analg. 2009;109:405–411. doi: 10.1213/ane.0b013e3181a93ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedlic F, Pravdic D, Hirata N, Mio Y, Sepac A, Camara AK, Wakatsuki T, Bosnjak ZJ, Bienengraeber M. Monitoring mitochondrial electron fluxes using NAD(P)H-flavoprotein fluorometry reveals complex action of isoflurane on cardiomyocytes. Biochim Biophys Acta. 2010a;1797:1749–1758. doi: 10.1016/j.bbabio.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T, Bosnjak ZJ. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+ Am J Physiol Cell Physiol. 2010b;299:C506–C515. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffer SW, Jong CJ, Ramila KC, Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010;17(Suppl 1):S2. doi: 10.1186/1423-0127-17-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monassier JP. Reperfusion injury in acute myocardial infarction. From bench to cath lab. Part I: Basic considerations. Arch Cardiovasc Dis. 2008;100:491–500. doi: 10.1016/j.acvd.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Nakae Y, Kohro S, Hogan QH, Bosnjak ZJ. Intracellular mechanism of mitochondrial adenosine triphosphate-sensitive potassium channel activation with isoflurane. Anesth Analg. 2003;97:1025–1032. doi: 10.1213/01.ANE.0000077072.67502.CC. [DOI] [PubMed] [Google Scholar]
- 23.Minners J, Lacerda L, McCarthy J, Meiring JJ, Yellon DM, Sack MN. Ischemic and pharmacological preconditioning in Girardi cells and C2C12 myotubes induce mitochondrial uncoupling. Circ Res. 2001;89:787–792. doi: 10.1161/hh2101.098372. [DOI] [PubMed] [Google Scholar]
- 24.Costa AD, Jacob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;28:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullenheim J, Ebel D, Frassdorf J, Preckel B, Thamer V, Schlack W. Isoflurane preconditions myocardium against infarction via release of free radicals. Anesthesiology. 2002;96:934–940. doi: 10.1097/00000542-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Weihrauch D, Kehl F, Ludwig LM, LaDisa JF, Kersten JR, Pagel PS, Warltier DC. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology. 2002;97:1485–1490. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–943. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Tokube K, Kiyasue T, Arita M. Opening of cardiac KATP channel by oxygen free radicals produced by xanthine oxidase reaction. Am J Physiol Heart Circ Physiol. 1996;271:H478–H489. doi: 10.1152/ajpheart.1996.271.2.H478. [DOI] [PubMed] [Google Scholar]
- 30.Ichinari K, Kokei M, Matsuoka T, Nakashima H, Tomaka H. Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea pig ventricular cells: its potentiation by MgADP. J Mol Cell Cardiol. 1996;28:1867–1877. doi: 10.1006/jmcc.1996.0179. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L, Cortassa S, Wei AC, Aon MA, Winslow RL, O’Rourke B. Modeling cardiac action potential shortening driven by oxidative stress-induced mitochondrial oscillations in guinea pig cardiomyocytes. Biophys J. 2009;97:1843–1852. doi: 10.1016/j.bpj.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novalija E, Kevin LG, Camara AK, Bosnjak ZJ, Kampine JP, Stowe DF. Reactive oxygen species precede the epsilon isoform of protein kinase C in the anesthetic preconditioning signaling cascade. Anesthesiology. 2003;99:421–428. doi: 10.1097/00000542-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Light P, Bladen C, Winkfein R, Walsh M, French R. Molecular basis of protein kinase C induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci USA. 2000;97:9058–9063. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinovic J, Bosnjak ZJ, Stadnicka A. Preconditioning by isoflurane induces lasting sensitization of the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel by a protein kinase C epsilon mediated mechanism. Anesthesiology. 2005;103:540–547. doi: 10.1097/00000542-200509000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane preconditioning elicits competent endogenous mechanisms of protection from oxidative stress in cardiomyocytes derived from human embryonic stem cells. Anesthesiology. 2010;113:906–916. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gumina RJ, O’Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol. 2007;292:H1706–H1713. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- 37.Jilkina O, Kuzio B, Rendell J, Xiang B, Kupriyanov VV. K+ transport and energetics in Kir6.2(−/−) mouse hearts assessed by 87Rb and 31P magnetic resonance and optical spectroscopy. J Mol Cell Cardiol. 2006;41:893–901. doi: 10.1016/j.yjmcc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. 2003. [DOI] [PubMed] [Google Scholar]
- 39.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajashree R, Koster JC, Markova KP, Nichols CG, Hofmann PA. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal K(ATP) channels. Am J Physiol Heart Circ Physiol. 2002;283:H584–H590. doi: 10.1152/ajpheart.00107.2002. [DOI] [PubMed] [Google Scholar]
- 41.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 42.Auchampach J, Grover G, Gross G. Blockade of ischemic preconditioning in dogs by the novel ATP-dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res. 1992;26:1054–1062. doi: 10.1093/cvr/26.11.1054. [DOI] [PubMed] [Google Scholar]
- 43.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 44.Cole WC, McPherson CD, Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 69:571–581. doi: 10.1161/01.res.69.3.571. 199. [DOI] [PubMed] [Google Scholar]
- 45.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234–243. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assad AR, Delou JM, Fonseca LM, Villela NR, Nascimento JH, Verçosa N, Lopes AG, Capella MA. The role of KATP channels on propofol preconditioning in a cellular model of renal ischemia-reperfusion. Anesth Analg. 2009;109:1486–1492. doi: 10.1213/ANE.0b013e3181b76396. [DOI] [PubMed] [Google Scholar]