Abstract
Childhood obesity rates have reached epidemic proportions. Excessive weight gain in infancy is associated with persistence of elevated weight status and later obesity. In this review, we make the case that weight gain in the first 6 mo is especially predictive of later obesity risk due to the metabolic programming that can occur early postpartum. The current state of knowledge regarding the biological determinants of excess infant weight gain is reviewed, with particular focus on infant feeding choice. Potential mechanisms by which different feeding approaches may program the metabolic profile of the infant, causing the link between early weight gain and later obesity are proposed. These mechanisms are likely highly complex and involve synergistic interactions between endocrine effects and factors that alter the inflammatory and oxidative stress status of the infant. Gaps in current knowledge are highlighted. These include a lack of data describing 1) what type of infant body fat distribution may impart risk and 2) how maternal metabolic dysfunction (obesity and/or diabetes) may affect milk composition and exert downstream effects on infant metabolism. Improved understanding and management of these early postnatal determinants of childhood obesity may have great impact on reducing its prevalence.
Introduction
In ancient times, humanity resembled other species whose survival was often threatened by starvation and malnutrition. In modern times, however, the most common threat to human life expectancy is excess adiposity and the accompanying comorbidities. This crisis is not restricted to affluent nations, but has become a global public health concern. It is currently estimated that 68.8% of American adults 20 y and older are overweight (BMI ≥25 kg/m2), and 35.7% are obese (BMI ≥30 kg/m2) (1). The obesity epidemic has spread to the pediatric population; ∼31.8% of American children ages 2–19 y are considered overweight, and 16.9% meet the criteria for obesity (2). Even at birth, 7.5% of American infants are macrosomic, and 9.7% of infants and toddlers are overweight (2, 3). Treatment of overweight and obesity is notoriously difficult and often unsuccessful. As such, public health efforts have increasingly concentrated on prevention-based strategies focused on younger and younger age groups (2).
With regard to childhood obesity prevention, research to understand and interventions to prevent rapid and excess weight gain in infancy may be the most critical for several reasons. Although the in utero environment plays a crucial role in offspring’s future health outcomes, intervention during pregnancy may be unavailable or unfeasible. The first few weeks of life represent the first postnatal opportunity to influence offspring health. Second, intervention during infancy is simpler compared with older ages because dietary intake is homogeneous and many common child feeding issues have not yet manifested. Finally, and most importantly, there is an emerging consensus in the scientific literature regarding the rate of weight gain during early infancy and the risk of obesity and related comorbidities later in life (4–17). Systematic reviews estimate that rapid growth in the first 1–2 y of life is associated with an OR of later obesity that ranges from 1.4 to 5.7 (5, 6). Comprehensive reviews describing this body of literature were recently performed (5–7). Yet to be explained are the biological underpinnings of why early excess weight gain in infancy predisposes to later obesity. The objectives of this review are to 1) summarize what is known regarding the postnatal biological determinants of early weight gain related to infant feeding and 2) propose potential mechanisms whereby these exposures may affect adiposity over the life course. Although intrauterine and behavioral determinants undoubtedly contribute to regulation of infant weight gain and obesity risk, these are only briefly mentioned in this review.
The first 6 mo are a critical window
The literature addressing the association between early infant growth and later risk of overweight is characterized by large variations in the definition and assessment of rapid growth, later obesity, and related outcomes; the time period over which growth was assessed; and the time elapsed before the outcome was measured. This variation complicates interpretation of the data. Definitions of exposure, or excess infant growth, include measures of infant gains in linear growth [centimeters/month and length-for-age Z-score (LAZ)3]; weight [grams/day and weight-for-age Z-score (WAZ)]; and weight relative to length [weight-for-length Z-score (WLZ)]; and BMI Z-score (BMI-Z) for children older than 2 y). Although these measures all assess some aspect of infant growth, they represent distinct biological processes that may not track consistently over time. Furthermore, measures used as proxies for infant adiposity (WLZ, BMI, and BMI-Z) do not actually measure body composition (percentage of body fat) but are often interpreted as such. Additionally, even accurate measures of body composition do not typically address the distribution of infant body fat, such as visceral versus subcutaneous fat or intrahepatocellular lipid, which may play a critical role in the development of metabolic dysfunction (18). Despite these inconsistencies in classification of exposure and outcome, study results consistently support a link between rapid early infant growth and later obesity. The “growth acceleration hypothesis” attempts to explain this link by suggesting that early and rapid growth during infancy programs the infant metabolic profile to be susceptible to obesity and the other components of metabolic syndrome (19). This brief review presents data from relevant studies according to the time period in which infant weight gain was assessed. Overall, these data support the premise that the first 6 mo of life represent a particular time when the growth acceleration hypothesis specifically applies.
When growth was measured in the first 2 y of life, infants who exhibited rapid growth (gain in WAZ ≥0.67 units) were heavier, had a higher BMI, and a higher percentage of body fat at 5 y of age (9) and continued to exhibit increased total and abdominal adiposity in adulthood (20). When gain in BMI-Z in the first 24 mo was considered the exposure, this gain was positively correlated with increased BMI, and specifically with the percentage of body fat, at 7 y of age (21). Infant weight gain (grams/day) over the first 12 mo has also been associated with an increased risk of obesity later in childhood (11, 22). A meta-analysis of 10 cohort studies estimated the OR for obesity in childhood increased by 1.97 for every unit of WAZ gained during the first 12 mo (6).
Other studies have measured infant weight gain over successively smaller intervals of time to identify the most critical window of exposure. Weight gain from birth to 9 mo was positively associated with WAZ at 7 y of age (23). The increase in WLZ and WAZ during the first 6 mo has been correlated with BMI-Z, skinfold thicknesses, and odds of obesity at 3 y of age (24, 25). Absolute weight gain over the first 6 mo was also correlated with overweight in offspring at age 4 y (26) and with BMI, absolute and relative fat mass, and clustered metabolic risk score at 17 y of age (27, 28). In children assessed at 17 y, weight gain during childhood (from 3 to 6 y) was not associated with clustered metabolic risk score at 17 y, suggesting that early infancy is a more crucial window of metabolic programming (27). Excess gain even before 6 mo has also been studied. African American infants gaining ≥1 WAZ unit in the first 4 mo were significantly more likely to be obese by age 20 (OR = 5.22) (10). In a multicenter U.S. cohort, weight gain in the first 4 mo was also associated with OR of obesity at 7 y (16). In a Chinese cohort, increase in WAZ in the first 3 mo was associated with BMI-Z at 7 y (17). Even increases in WAZ as early as the first 8 d of life have been associated with an increased risk of overweight and obesity in adulthood (12).
The studies referenced suggest that the first few weeks and months of life are particularly associated with later weight status (29). However, few studies have actually compared the strength of the relationship across different time intervals during the infant period. Taveras et al. (30) recently showed that crossing ≥2 major weight-for-length percentiles in the first 6 mo was associated with a significantly higher risk of obesity at 5 y than if the gain occurred during any other 6-mo window in the first 2 y. Figure 1 shows previously unpublished data from a Denver birth cohort born between 1999 and 2004 (N = 15,552). All infants were born at term (≥37 wk and <42 wk gestation) and had a birth weight >2.5 kg. Logistic regression was used to compare the risk of overweight (weight for length ≥95th percentile) at 18–24 mo attributed to macrosomia (birth weight >4000 g) or excess weight gain (defined as gaining ≥0.5 WAZ) from birth to 2 mo, 2 to 4 mo, and 4 to 6 mo. These time intervals were chosen based on the timing of well-child visits. The OR of overweight at 18–24 mo attributed to birth weight and weight gain across these individual time intervals were all significant (P < 0.0001). Controlling for birth weight, the OR attributed to excess weight gain between 2 and 4 mo was significantly higher than the OR of overweight from gaining excessively during the other 2 intervals (P < 0.001). Together these studies suggest that weight gain during the first 6 mo merits particular attention in relation to later obesity risk.
Figure 1.
Infant weight gain in the first 6 mo is associated with increased odds of overweight at 18–24 mo. Infants born in Denver, Colorado, between 1999 and 2004 (N = 15,552) were followed through the first 2 y of life. All infants were born at term (≥37 wk and <42 wk gestation) and had a birth weight >2.5 kg; 2636 infants had a birth weight <3.0 kg, and 1234 infants had a birth weight >4.0 kg. Weight gain was monitored bimonthly and weight for age Z-scores (WAZ) generated from the 2006 WHO growth curves. Logistic regression was used to generate the OR of overweight (achieving a weight-for-length ≥95th percentile) at 18–24 mo based on birth weight and gaining ≥0.5 WAZ units between 0 and 2, 2 and 4, or 4 and 6 mo. Birth weight ≥3.0 and ≤4.0 kg (N = 11,682) was used as the reference group, as indicated by the dashed line. Error bars represent 95% CI. The OR attributed to each category (birth weight <3.0 kg or >4.0 kg or gaining ≥0.5 WAZ units at any time point) was significant (P < 0.0001). *ΔWAZ controlled for birth weight. **OR of overweight at 18–24 mo associated with gaining ≥0.5 WAZ between 2 and 4 mo was significantly larger than the OR associated with gaining ≥0.5 WAZ between 0 and 2 or 4 and 6 mo (P < 0.0001).
With that background in mind, the remainder of this review focuses on the first 6 mo after birth. These months are a critical time when metabolic programming can occur, similar to the in utero period, because infants’ organ systems still maintain considerable plasticity for adaptation to nutritional and environmental exposures. Additionally, the first 6 mo are a clinically relevant time when infants are seen frequently by physicians. The mechanism(s) driving the correlation between excess weight gain in the first 6 mo and later obesity remain unknown. This knowledge gap results in part from the inconsistencies in study design, but also from a lack of longitudinal and intervention studies. Furthermore, previous research has emphasized absolute weight gain, rarely addressing changes in body composition and failing to address fat distribution (subcutaneous vs. visceral). To understand the earliest postnatal etiologies of obesity, both the causes and characteristics of risky infant gain must be considered. In the following sections, we review how infant feeding mode [human milk (HM) vs. formula in various formulations] affects early weight gain and child obesity and explore biological mechanisms whereby metabolic programming may occur.
The in utero environment
Although we focus on the first 6 mo postpartum, it would be remiss to ignore the contributions of the in utero environment to the milieu of factors that contribute to infant growth and childhood obesity. Beginning with the work of Barker (31), it has become well accepted that the in utero environment programs infant metabolic profile and affects future chronic disease risk. Birth weight is considered a clinical outcome representative of the summation of exposures and insults that occurred in utero. Birth weight and size within the normal range are linearly associated with the risk of obesity in adulthood (13, 17, 32, 33), and extremes in birth weight, both large and small for gestational age (SGA), increase the risk of later obesity and metabolic disease (34).
Maternal BMI, adiposity, gestational weight gain, circulating triglyceride concentrations, and degree of inflammation during pregnancy are associated with increased birth weight and neonatal adiposity (11, 31, 34–40). Maternal diabetes is linked with higher offspring fat mass at birth (41), increased BMI, and risk of type 2 diabetes (T2D) in childhood and beyond (42). Maternal smoking during pregnancy is associated with offspring risk of overweight and obesity at 5–7 y (11, 43, 44). Various animal models have shown that a high-fat maternal diet during pregnancy causes malprogramming of the fetal liver (45), increased offspring accumulation of fat (46), and development of features of metabolic syndrome in adulthood (47). Maternal nutritional status preconception also plays an important, but often underappreciated role. In ewes, undernutrition around the time of conception causes increased fetal blood pressure and impaired glucose signaling in adult offspring (48, 49). More comprehensive reviews of the fetal origins of obesity are widely available.
Maternal increased BMI, smoking, and circulating triglyceride concentrations are all also associated with rapid postnatal growth (33, 37, 39, 50). Therefore, these maternal characteristics may illicit a “double-hit” programming effect on offspring’s metabolic profile, increasing the odds of later metabolic dysfunction and obesity in a cumulative or even synergistic manner (51).
What the infant is fed
HM versus formula feeding
What the infant is fed (i.e., HM vs. formula) affects the rate of infant weight gain and later risk of obesity. Breastfeeding is moderately but consistently protective against later obesity. Four systematic reviews of dozens of studies each confirmed the protective effect of breastfeeding against obesity in childhood (52, 53) and adulthood (54–56) with OR ranging between 0.78 and 0.87 (52, 56). Causality is suggested by the dose-dependent characteristic of this association (52, 53, 55, 57, 58), with every additional month of exclusive or predominant breastfeeding linked with a 4% decrease in later risk of overweight/obesity (55). However, the true effect size of breastfeeding on later obesity remains unknown because it is impossible to tease apart effects that may result from underlying parental and socioeconomic characteristics that affect choice to breastfeed and duration of breastfeeding (59, 60). However, although the intricacies remain unclear, the consensus that breastfeeding imparts a consistent but small protective effect against later obesity holds (61).
Breast-fed infants exhibit different weight-gain patterns during the first 6 mo from those of formula-fed infants (62). This altered growth pattern may connect the causal pathway between breastfeeding and reduced obesity (19, 52). Breast-fed infants gain weight more slowly beginning at 3 mo while exhibiting similar gains in stature (length), which translates into a lower mean WLZ detectable by 4 mo (63). To reflect the assumption that breast-fed infants’ growth represents ideal physiologic growth, the CDC and the American Academy of Pediatrics have endorsed the WHO growth standards, which are derived from predominantly breast-fed infants, for children 24 mo of age or younger (62, 64).
Breast-fed infants also exhibit different body composition trajectories than formula-fed infants during the first 6 mo (65–67). This difference is more complicated than the differences in weight gain trajectories. One small study (N = 87) documented a lower percentage of fat by 5 mo, and thinner skinfold thicknesses by ∼10 mo in predominantly breast-fed versus formula-fed infants (66). Another study (N = 76) using dual-energy X-ray absorptiometry for body composition assessment found exclusively breast-fed infants (for ≥4 mo) to have higher percentage of fat mass at 3 and 6 mo compared with formula-fed infants (67). A meta-analysis comparing body composition patterns among HM-fed versus formula-fed infants in the first year of life was recently published (65). Although the criteria defining breast-fed in these studies was heterogeneous, the results indicated that differences in body composition trajectories between breast- and formula-fed infants are complex and change over time (65). Notably, fat deposition as a whole should not necessarily be considered deleterious because breast-fed infants often exhibit larger fat mass than formula-fed infants (65, 67), underscoring the need to investigate the long-term impact of altered body composition and fat distribution during infancy.
Although infant feeding choice is associated with the rate and type of infant weight gain, the overarching association between excess and rapid weight gain and later obesity remains significant regardless of feeding mode. Even exclusively breast-fed infants who gain too much too fast in the first 6 mo are still at increased risk of later obesity (9, 26). However, the details and strength of this relationship may differ depending on feeding choice. Among children who exhibited “risky infant weight gain” (gaining ≥8.15 kg in the first 2 y), those who did not become overweight by 6–8 y were more likely to have been exclusively breast-fed for ≥6 mo (68), suggesting that feeding HM may modulate the impact of excessive weight gain on the risk of childhood obesity (25). Why the relationship between excess infant weight gain and later obesity differs among infants fed HM versus formula remains unknown, but may be underlined by the differences in body composition between these 2 groups (65). Future research should address how infant feeding choice affects the composition of weight gain (fat vs. fat-free mass) and fat deposition (subcutaneous vs. visceral) because these effects are likely critical for the risk of later obesity.
Exclusive versus mixed feeding
Although exclusive breastfeeding for approximately the first 6 mo of life is universally recommended, mixed feeding (feeding both HM and formula) is far more common in the United States. Mixed feeding may still provide a protective, albeit a much smaller, effect against childhood obesity (24, 69, 70), but the duration of partial breastfeeding necessary to impart a protective effect is longer than when breastfeeding is exclusive (71). This longer duration may be difficult to obtain given that mixed feeding is also associated with shorter duration of any breastfeeding (70). The attenuated protection imparted by mixed feeding is still likely mediated via reduced rates of weight gain observed in combination fed versus exclusively formula-fed infants (37, 72).
A critical gap in our understanding of how exclusive versus mixed breastfeeding affects offspring derives from the lack of clarity associated with these terms in the literature. Studies are very heterogeneous in their definition of feeding exposure; any given infant may be classified in completely different feeding groups depending on investigators’ variable definitions of “exclusive,” “predominant,” “any,” or “no” breastfeeding. Studies that more strictly classify exposure are called for to characterize the extent of a dose response between the amount and/or duration of breastfeeding and infant growth.
Milk from obese mothers
The human fetus was previously considered the “perfect parasite,” capable of extracting ideal nutrition from the maternal host regardless of her status. However, we now understand that in utero exposures exert powerful effects on the developmental potential of offspring. This paradigm shift in understanding is applicable to HM. Previously, HM was considered a relatively imperturbable and complex mixture that was universal among women. However, understanding is now emerging that maternal phenotype and behaviors (e.g., obesity and high-fat diet) cause alterations in the nutritional and other bioactive components of HM, which may affect infant growth and adiposity gain, potentially contributing to the heritability of obesity.
Maternal obesity affects milk composition in several ways. In rats, the fatty acid composition and insulin concentrations of milk from genetically obese dams differs from those of the milk of lean dams (73), and diet-induced obesity impairs mammary lipid metabolism and milk fat production and results in reduced milk triglycerides (74). HM from overweight and obese women also has higher total fat, glucose, and insulin concentrations than HM from lean mothers (75, 76). The concentrations of the hormonally active adipokines leptin and adiponectin in HM vary by maternal BMI (77–80). Maternal diet also affects milk composition. Nonhuman primates consuming a high-fat diet produce milk that has lower protein content and reduced DHA and EPA concentrations compared with milk from control animals (81). When consuming a high-fat diet, women also produce HM that has an altered fatty acid profile compared with HM produced when consuming a low-fat diet (82). In a similar study, a high fat maternal diet (55% fat, 30% carbohydrate) during lactation resulted in a higher percentage of milk fat and higher infant total volume and energy intake compared with an isocaloric high carbohydrate diet (25% fat, 60% carbohydrate) (83).
The finding that a high-fat maternal diet resulted in increased energy intake of the breast-fed infant (83) indicates that HM alterations resulting from maternal phenotype/behavior may affect regulation of infant energy balance. Animal data suggest that the milk of obese mothers exerts deleterious effects on infant metabolism, weight gain, and obesity risk. When pups of lean control dams were cross-fostered to diet-induced obese dams, offspring developed increased body weight and a nonalcoholic fatty liver disease phenotype by 3 mo (84). When pups of obesity-resistant dams were cross-fostered to obese dams, they exhibited increased adiposity and reduced insulin sensitivity as adults (5 mo) (73). However, normal species differences in milk composition and offspring maturity at birth necessitate that caution be used when extrapolating these results to humans. In fact, epidemiological data suggest that the protective effect of breastfeeding against childhood obesity does not differ between lean (BMI <25 kg/m2) and overweight mothers (BMI ≥25 kg/m2) (85). Understanding the biochemical impact of maternal obesity on HM composition and infant weight gain warrants further research.
Milk from mothers with diabetes
Current understanding of how maternal diabetes affects HM and infant growth postpartum is muddled by the common practice of combining variations of diabetic disease into a single risk category. Gestational diabetes (GDM), type 1 diabetes (T1D), and T2D are often grouped together despite distinctly different etiologies and pathophysiology. Furthermore, obesity is a common comanifestation and confounding risk factor that is specific to GDM and T2D. Contemporary knowledge regarding the impact of insulin resistance on HM composition is still based on historical studies of women with T1D,. Early milk from women with T1D has altered sodium, glucose, insulin, total fat and fat composition compared with milk from healthy women (86–88). However, if diabetes is tightly controlled, the macronutrient, glucose, and insulin composition of her milk is indistinguishable from that of healthy counterparts (89). These observations would suggest that the glycemic and caloric characteristics of HM may be altered if maternal glucose control remains inadequate in the postpartum period. Limited data exist regarding the composition of HM from women with GDM. One study detected alterations in concentrations of certain bioactive peptides and hormones, including ghrelin (90). Data characterizing HM composition from T2D are notably lacking.
Animal data suggest that the milk of mothers with GDM imparts deleterious programming effects to offspring. Control pups cross-fostered to dams with GDM exhibit malprogramming of the hypothalamic arcuate nucleus post-weaning that may cause a dysregulation of appetite, food intake, and body weight (91). Epidemiological data from humans are difficult to interpret. Among infants of women with T1D and GDM, HM intake in the first week of life was associated with greater infant relative body weight and risk of overweight at 24 mo (92). In a study of mothers with T1D, infant weight and BMI at 12 mo did not differ between infants who were exclusively fed formula versus HM, a difference normally detected in healthy cohorts (93). In contrast, another epidemiological study established that breastfeeding protected against offspring overweight at 9–14 y similarly among lean, overweight, and T1D/GDM women (85). In another GDM/T1D cohort, adequate breastfeeding (defined as HM for ≥6 mo) ameliorated the negative impact of in utero exposure to diabetes on childhood adiposity (94). In that same cohort, infants of diabetic mothers who received adequate HM (HM for ≥6 mo) exhibited a lower BMI trajectory during childhood than infants with inadequate breastfeeding, a difference that approached significance (1.11 kg/m2 less BMI gain, P = 0.07, n = 94) (95). In a group of mother with GDM only (n = 324), breastfeeding was protective against child obesity, although obese mothers needed to breastfeed longer to impart any protection, implying an interactive effect of obesity and insulin resistance (96).
The lack of consistency in these findings may partially result from grouping women with GDM and T1D because these very different etiologies likely exert differential effects on HM composition and infant growth. Furthermore, these studies do not control for alterations that may occur in HM composition/properties as lactation progresses, glucose control is re-established, and the infant gut matures. It has been proposed that “early” diabetic milk may have an obesigenic effect on infant weight gain that decreases over time such that protective effects of breastfeeding will only be observed if exclusive breastfeeding is maintained beyond when this negative effect has diminished (97). Research is urgently needed to determine whether a critical window exists in the case of maternal obesity or each type of diabetes and the impact on HM composition and infant weight gain. Equally necessary are studies designed to investigate effects of GDM separately from those of T1D and obesity separately from T2D. The acuteness of these research needs is underscored by the unprecedented prevalence of maternal obesity and T2D. Our understanding of the biochemical impact on HM and infant growth lags far behind the epidemiological data linking maternal phenotype with offspring obesity risk.
Formula versus formula feeding
Although exclusive breastfeeding is recognized as the ideal mode of infant feeding, nonetheless, many infants are fed formula. Just as HM varies in composition between individuals, infant formulas vary in composition. For example, although the percentage of fat content of infant formula is regulated, the fat source and fatty acid composition vary among brands (98). Variation in fatty acid (particularly long-chain PUFA) content can have biological effects on infant growth. When preterm infants (born <33 wk) were fed formula supplemented with DHA and arachidonic acid, they exhibited decreased fat mass and increased fat-free mass by 12 mo of age compared with infants fed control formula (99). Interestingly, no differences in weight or length were observed (99). Extrapolation of these results to term infants is tempered by the different physiological states and nutritional requirements represented by a preterm population.
Formula protein content is another major component that has been extensively studied with relation to weight gain. In the context of SGA or low birth weight infants, excessive postpartum weight gain that matches in utero rates of growth is prescribed. A Cochrane review of this topic reported that higher protein formula (>3.0 and <4.0 g/kg/d) accelerates weight gain and increases all weight gain parameters in low-birth weight infants (100). However, because the potential for chronic disease risk attributed to rapid postpartum gain is increasingly recognized, the ideal degree of “catch-up growth” has become controversial. In a study of SGA infants from the United Kingdom (N = 153), those randomized to receive a nutrient-enriched formula (28–43% higher protein and 6–12% higher energy content) exhibited a greater change in WAZ, but no difference in change in LAZ at 9 mo and a larger fat mass at 6–8 y (101). Similar effects were observed in healthy term infants who, when randomized to receive a higher protein formula, displayed higher weight gain velocity, WAZ, WLZ, and BMI-Z, but no differences in LAZ by 6 mo compared with controls (102, 103). These trends implied a larger fat mass accrual in the high-protein group, a finding that persisted until study termination at 2 y (102).
Not only the amount, but the type, of protein in formula can affect infant growth. Hydrolyzed protein may be more easily absorbed, metabolized differently, and exert different satiety responses than the modified cow’s milk protein of standard formulas. A recent study that randomized healthy infants to receive either standard (cow’s milk based) or hydrolyzed formula from 0.5 to 7.5 mo found higher WAZ starting at 3.5 mo, and higher WLZ starting at 2.5 mo among infants receiving standard formula, but no differences in LAZ (104). Individual amino acid composition may affect infant metabolism as well. Both hydrolyzed formula and cow’s milk formula supplemented with free glutamate resulted in a higher infant satiety response than cow’s milk formula alone (105). Future research on the biological components of HM that may prevent excessive infant gain may be relevant to optimization of formula composition.
Mechanisms of programming
The first 6 mo are such a critical window of programming because at no other time postnatally is the metabolic environment so malleable and the gut so permeable that milk/formula can elicit significant endocrine responses. There are several different overarching components of HM/formula that may affect infant adipose and weight gain, both nutritive and nonnutritive. Traditionally proposed biological mechanisms of programming are reviewed, and novel pathways proposed in the following.
HM is a heterogeneous mixture that is dynamic on several levels, differing among and within individuals throughout the day and over the course of lactation (106, 107). The complexity of HM and role of hundreds of components found in HM are still to be discovered. The individual factors that differ between HM and formula and may differentially affect infant weight gain and adipose deposition have been extensively studied for years and recently reviewed (108). The primary difference that likely affects weight gain is the higher concentrations and different sources of several macro- and micronutrients found in formula. Formula has higher protein and energy density than HM, and infants consume more of it (19, 109), which may plausibly cause the increased weight gain and increased obesity risk of formula-fed infants (57). Additionally, the amount of fat and the fatty acid profile of HM differ from those of formula and may be protective against inflammation and excessive adipose deposition (52, 98, 110). The amount of fat and fatty acid composition of HM also differs among women and varies by maternal BMI and dietary intake, which may be a mechanism whereby maternal phenotype affects infant weight gain (76, 81–83, 111, 112).
HM also provides countless identified and unidentified nonnutritive bioactive molecules that are absent in formula, including hormones, prostaglandins, neuropeptides, and growth factors (77). Because the newborn gut is highly permeable, hormones in HM such as insulin, leptin, and adiponectin may actually elicit endocrine effects and play a role in the short-term regulation of infant appetite and weight gain (77, 78, 113, 114). Both leptin and adiponectin are detected in HM at concentrations that correspond to circulating maternal concentrations such that the HM concentrations of both of these adipokines are positively associated with maternal BMI (77–80). Interestingly, the HM concentrations of both adiponectin and leptin have been associated with lower infant gains in weight, BMI, WAZ, and WLZ during infancy (113, 115–117). The endocrine impact of these hormones is not well understood, especially in the case of adiponectin. Most of these studies have been conducted in relatively lean populations, and whether these relationships are similar at the upper end of the distribution of maternal BMI is unknown. Furthermore, obesity in adults is often characterized by leptin resistance, and how early exposure to elevated leptin concentrations in HM may affect leptin sensitivity also remains unknown. Insulin is another powerful anabolic hormone present in HM that differs based on maternal phenotype (75). Variation in these hormones resulting from maternal obesity and/or diabetes could affect the metabolic programming and growth/adiposity trajectory of the newborn (108).
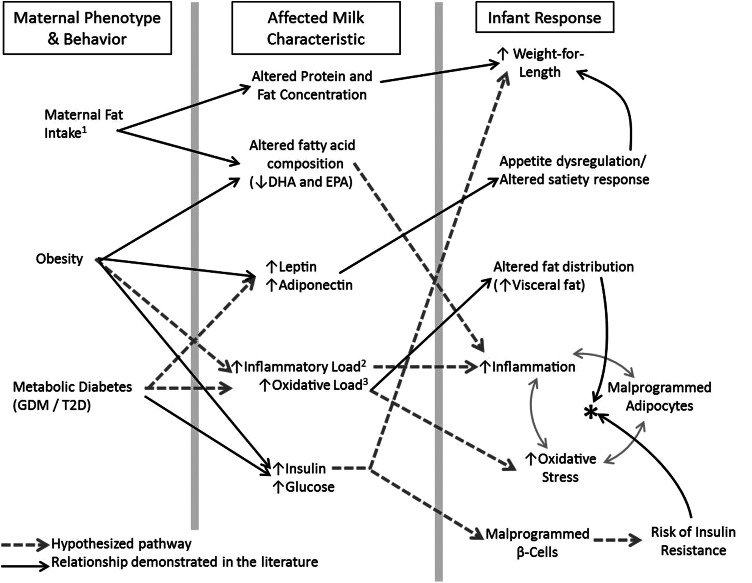
It is likely that bioactive components of HM also affect the type and distribution of adipose tissue laid down by the infant. Obesity and metabolic syndrome in particular are considered states of inflammation and oxidative stress (118–120). Metabolic syndrome is characterized by excess visceral adiposity and a self-perpetuating cycle in which dysfunctional adipocytes both promote and are stimulated by inflammatory cytokines and oxidative stress (Figure 2). The directionality of the causal pathway that links obesity with inflammation and oxidative stress is not fully understood and may be bidirectional. It is possible that when the metabolic profile is susceptible early postpartum, exposure to elevated inflammatory cytokines and/or oxidative stress may mimic the signaling pathways and local environments that characterize the dysfunctional adipocytes of metabolic syndrome and program naïve infant adipocytes to develop as such. Such “stimuli” may be more present in formula, or HM may contain protective factors, likely a combination of both. Similarly, HM of obese or diabetic mothers may contain more inflammatory and oxidative factors and/or less protective factors. A schematic of these proposed mechanisms by which maternal phenotype may affect HM and infant obesity risk is presented in Figure 2.
Figure 2.
Potential biological mechanisms whereby maternal phenotype/behavior may indirectly affect infant metabolic phenotype via alterations in human milk (HM). Shown is a conceptual diagram of potential biological mechanisms by which maternal phenotype may affect infant metabolic phenotype via alterations in HM, affecting weight gain and obesity risk. All infant outcomes listed in the right-hand column may individually or in combination predispose the infant to obesity and metabolic dysfunction later in childhood and adulthood. Solid black lines represent relationships that have been suggested by published data (from animal models and human studies). Dashed gray lines represent hypothesized relationships postulated here. 1Incorporating total fat intake, saturated fat intake, and fatty acid composition of diet, including the n-6:n-3 ratio of dietary fatty acids. 2Inflammatory load incorporates the cumulative effect of both pro- and anti-inflammatory cytokines and other factors of HM. 3Oxidative load incorporates the cumulative effect of both oxidants and the antioxidant capacity of HM. *The cycle of metabolic syndrome in which increased inflammation, and oxidative stress mutually stimulate and respond to malprogrammed adipocytes.
Inflammatory cytokines such as TNF-α and IL-6 play a pivotal role in the signaling that perpetuates the relationship between visceral obesity and increased inflammation (118, 120). Both these and other potent proinflammatory cytokines are detected in HM at widely varied concentrations (121–124). In terms of oxidative stress stimulus, HM also contains a plethora of antioxidant factors that provide breast-fed infants with greater protection against oxidative stress than formula-fed infants (125–127). Antioxidant capacity in the diet has been linked to decreased visceral adiposity in young adults (128) and may be even more critical to infants because healthy term infants normally experience a large degree of oxidative stress as they adjust to ambient oxygen postpartum (129). In contrast to antioxidants, HM can also contain markers of oxidative stress, including isoprostanes. The concentrations of these factors decrease during lactation (130). However, the combination of increased exposure to oxidative stress in HM combined with increased endogenous production of oxidative byproducts that both occur early postpartum suggests that this is a particularly vulnerable time when subtle variation within the normal “oxidative load” delivered to the infant may exert powerful programming effects on developing adipose tissue. It is possible that maternal obesity and/or diabetes may alter the cytokine profile, antioxidant capacity, or amount of oxidative stress in HM, resulting in a higher inflammatory or oxidative “load” than in HM of healthy lean mothers. Although these potential mechanisms are currently speculative, they are based on a contemporary understanding of the complexity of obesity and may expand our understanding of the earliest etiologies of the disorder. The regulatory factors that influence the inflammatory and oxidative load of HM are important areas for future research.
Additional environmental factors that play a role
This review focuses on the biological mechanisms that may link infant nutrition with excessive infant weight gain and predispose to later obesity. Several other environmental and behavioral factors also contribute to the milieu of components that program for metabolic dysfunction and are briefly mentioned in the following.
The method of infant feeding (i.e., suckled directly at the breast or via a bottle) affects infant growth patterns. When feeding at the breast, the pace and volume of intake are controlled by the infant, whereas the caregiver maintains more control when bottle feeding (131). Infants fed from a bottle (vs. fed at the breast) consume more milk, protein, and energy (109, 132, 133), which could potentially result in greater weight gain (66, 109, 134). Removing control from the infant may “malprogram” the infant’s ability to interpret satiety cues and self-regulate food intake accordingly (135–138). These mechanisms are postulated to occur regardless of what is in the bottle (HM vs. formula). Caregiver feeding style may program infants’ ability to self-regulate in a similar manner. Recent reviews suggest that responsive feeding (the ability to identify and appropriately respond to infant hunger cues) is associated with appropriate weight gain and is protective against late obesity (139, 140).
Introduction of complementary foods may also contribute to infant weight gain, although these data are more complicated. Several studies have found that, in formula-fed infants only, early introduction of solid foods (before 4 mo) was associated with excessive infant weight gain and increased risk of obesity at 3 y (37, 141, 142). Other studies failed to detect any relationship between timing of introduction of solid foods and measures of adiposity during infancy and at 5 y (24, 143). Complementary foods are necessary to meet nutritional needs of the ∼6 mo old breast-fed infant. Recent publications have advocated a shift in research focus from the timing to the composition and quality of complementary foods necessary to meet older infant needs and prevent excess gain (144, 145). Beyond feeding, infant sleep patterns also affect early weight gain (7), and <12 h of sleep per day during infancy may increase the risk of overweight later in childhood (146).
Conclusions and research needs
Excessive weight gain, reflected in increasing WLZ, during the first 6 mo of life is predictive of later obesity. A combination of factors including postnatal effects of the intrauterine environment, the putative malleable metabolic environment in early infancy, and the permeability of the infant gut makes this time a vulnerable period. Exclusive breastfeeding is at least modestly protective against excessive early infant gain and later obesity, an effect that may result from differences in composition of weight gain between HM- and formula-fed infants. However, research addressing the impact of infant feeding on infant weight gain is complicated by an inability to completely control for confounding factors, heterogeneous definitions of feeding exposure, and large variation between formulas and HM from different women, all of which likely affect the rate and quality of infant weight gain.
In light of the staggering prevalence of young childhood obesity, the underlying mechanisms linking early weight gain and later obesity urgently need clarification. We speculate here that, in addition to previously proposed mechanisms, exposure to oxidative stress and inflammation may program the infant metabolic profile to be susceptible to excess gain and later obesity. These potential mechanisms (Fig. 2) are by no means a comprehensive summary of all the pathways involved, but provide a framework that incorporates contemporary understanding of the complex pathophysiology of obesity, with which novel hypotheses can be generated. Beyond more descriptive research, both basic mechanistic models and prospective, innovative intervention trials will be needed to characterize adiposity distribution (subcutaneous vs. visceral) that may predispose to obesity, determine the extent to which adipose distribution is driven by feeding mode or maternal phenotype, and to alter current trends and improve the health outcomes of all infants. Although it remains a fundamentally sound premise that mother’s milk is the ideal infant feeding choice, the current prevalence of maternal obesity and T2D is unprecedented. The biochemical impact of maternal metabolic phenotype on HM composition, infant metabolism and growth, and ultimately risk of later obesity represents an additional gap in knowledge. Understanding this interplay may lead to treatments or interventions for at-risk women and infants to optimize maternal health and minimize infant risk of excess gain. Additionally, if there are critical windows during lactation when HM is most affected, targeted breastfeeding support can be provided to at-risk women to ensure that breastfeeding is maintained beyond this time and the infant receives the optimal benefits of HM.
Acknowledgments
We thank Dr. Erin Ross for the statistical analysis of the original data presented. All authors have read and approved the final manuscript.
Footnotes
Supported by grants NIH/NIDDK T32 DK007658-21 and K24 DK083772.
Author disclosures: B. E. Young, S. L. Johnson, and N. F. Krebs, no conflicts of interest.
Literature Cited
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochanek KD, Kirmeyer SE, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2009. Pediatrics. 2012;129:338–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life–a systematic review. Obes Rev. 2005;6:143–54 [DOI] [PubMed] [Google Scholar]
- 5.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Smith GD, Ekelund U, Levy-Marchal C, Jarvelin MR, Kuh D, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26:19–26 [DOI] [PubMed] [Google Scholar]
- 7.Paul IM, Bartok CJ, Downs DS, Stifter CA, Ventura AK, Birch LL. Opportunities for the primary prevention of obesity during infancy. Adv Pediatr. 2009;56:107–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillman MW. Early infancy as a critical period for development of obesity and related conditions. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77:1374–8 [DOI] [PubMed] [Google Scholar]
- 11.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, Steer C, Sherriff A. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, Ziegler EE, Strom BL. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005;111:1897–903 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Matsuzaki A, Kuromaru R, Kinukawa N, Nose Y, Matsumoto T, Hara T. Association between birthweight and body mass index at 3 years of age. Pediatr Int. 2001;43:641–6 [DOI] [PubMed] [Google Scholar]
- 14.Monteiro PO, Victora CG, Barros FC, Monteiro LM. Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27:1274–82 [DOI] [PubMed] [Google Scholar]
- 15.Cameron N, Pettifor J, De Wet T, Norris S. The relationship of rapid weight gain in infancy to obesity and skeletal maturity in childhood. Obes Res. 2003;11:457–60 [DOI] [PubMed] [Google Scholar]
- 16.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–9 [DOI] [PubMed] [Google Scholar]
- 17.Hui LL, Schooling CM, Leung SS, Mak KH, Ho LM, Lam TH, Leung GM. Birth weight, infant growth, and childhood body mass index: Hong Kong's children of 1997 birth cohort. Arch Pediatr Adolesc Med. 2008;162:212–8 [DOI] [PubMed] [Google Scholar]
- 18.Modi N, Murgasova D, Ruager-Martin R, Thomas EL, Hyde MJ, Gale C, Santhakumaran S, Dore CJ, Alavi A, Bell JD. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res. 2011;70:287–91 [DOI] [PubMed] [Google Scholar]
- 19.Singhal A. Does breastfeeding protect from growth acceleration and later obesity? Nestle Nutr Workshop Ser Pediatr Program. 2007;60:15–25 [DOI] [PubMed] [Google Scholar]
- 20.Demerath EW, Reed D, Choh AC, Soloway L, Lee M, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels Longitudinal Study. Obesity (Silver Spring). 2009;17:2060–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karaolis-Danckert N, Buyken AE, Bolzenius K. Perim dF, Lentze MJ, Kroke A. Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr. 2006;84:1449–55 [DOI] [PubMed] [Google Scholar]
- 22.Stettler N, Bovet P, Shamlaye H, Zemel BS, Stallings VA, Paccaud F. Prevalence and risk factors for overweight and obesity in children from Seychelles, a country in rapid transition: the importance of early growth. Int J Obes Relat Metab Disord. 2002;26:214–9 [DOI] [PubMed] [Google Scholar]
- 23.Blair NJ, Thompson JM, Black PN, Becroft DM, Clark PM, Han DY, Robinson E, Waldie KE, Wild CJ, Mitchell EA. Risk factors for obesity in 7-year-old European children: the Auckland Birthweight Collaborative Study. Arch Dis Child. 2007;92:866–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Rossem L, Taveras EM, Gillman MW, Kleinman KP, Rifas-Shiman SL, Raat H, Oken E. Is the association of breastfeeding with child obesity explained by infant weight change? Int J Pediatr Obes. 2011;6:e415–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring). 2006;14:491–9 [DOI] [PubMed] [Google Scholar]
- 27.Ekelund U, Ong KK, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92:98–103 [DOI] [PubMed] [Google Scholar]
- 28.Ekelund U, Ong K, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). Am J Clin Nutr. 2006;83:324–30 [DOI] [PubMed] [Google Scholar]
- 29.Gillman MW. The first months of life: a critical period for development of obesity. Am J Clin Nutr. 2008;87:1587–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, Rich-Edwards JW, Gillman MW. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165:993–8 [DOI] [PubMed] [Google Scholar]
- 31.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond). 1998;95:115–28 [PubMed] [Google Scholar]
- 32.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25:735–40 [DOI] [PubMed] [Google Scholar]
- 33.Eriksson J, Forsen T, Osmond C, Barker D. Obesity from cradle to grave. Int J Obes Relat Metab Disord. 2003;27:722–7 [DOI] [PubMed] [Google Scholar]
- 34.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205–12 [DOI] [PubMed] [Google Scholar]
- 35.Tikellis G, Ponsonby AL, Wells JC, Pezic A, Cochrane J, Dwyer T. Maternal and infant factors associated with neonatal adiposity: Results from the Tasmanian Infant Health Survey (TIHS). Int J Obes.(Lond). 2012;36:496–504 [DOI] [PubMed] [Google Scholar]
- 36.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, Navder K, Yu A, Dorsey K, Gallagher D. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–88 [DOI] [PubMed] [Google Scholar]
- 38.Godfrey KM, Inskip HM, Hanson MA. The long-term effects of prenatal development on growth and metabolism. Semin Reprod Med. 2011;29:257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vrijkotte TG, Algera SJ, Brouwer IA, van Eijsden M, Twickler MB. Maternal triglyceride levels during early pregnancy are associated with birth weight and postnatal growth. J Pediatr. 2011;159:736–42 [DOI] [PubMed] [Google Scholar]
- 40.Lowe LP, Metzger BE, Lowe WL, Jr., Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95:5427–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durnwald C, Huston-Presley L, Amini S, Catalano P. Evaluation of body composition of large-for-gestational-age infants of women with gestational diabetes mellitus compared with women with normal glucose tolerance levels. Am J Obstet Gynecol. 2004;191:804–8 [DOI] [PubMed] [Google Scholar]
- 42.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–11 [DOI] [PubMed] [Google Scholar]
- 43.Toschke AM, Koletzko B, Slikker W, Jr., Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. Eur J Pediatr. 2002;161:445–8 [DOI] [PubMed] [Google Scholar]
- 44.von Kries R, Toschke AM, Koletzko B, Slikker W., Jr Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156:954–61 [DOI] [PubMed] [Google Scholar]
- 45.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krasnow SM, Nguyen ML, Marks DL. Increased maternal fat consumption during pregnancy alters body composition in neonatal mice. Am J Physiol Endocrinol Metab. 2011;301:E1243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006;291:E792–9 [DOI] [PubMed] [Google Scholar]
- 48.Todd SE, Oliver MH, Jaquiery AL, Bloomfield FH, Harding JE. Periconceptional undernutrition of ewes impairs glucose tolerance in their adult offspring. Pediatr Res. 2009;65:409–13 [DOI] [PubMed] [Google Scholar]
- 49.Edwards LJ, McMillen IC. Periconceptional nutrition programs development of the cardiovascular system in the fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R669–79 [DOI] [PubMed] [Google Scholar]
- 50.Sowan N, Stember M. Effect of maternal prenatal smoking on infant growth and development of obesity. J Perinat Educ. 2000;9:22–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat .Endocrinol 2009;5:604–10 [DOI] [PubMed] [Google Scholar]
- 52.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity–a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247–56 [DOI] [PubMed] [Google Scholar]
- 53.Gillman MW, Rifas-Shiman SL, Camargo CA, Jr., Berkey CS, Frazier AL, Rockett HR, Field AE, Colditz GA. Risk of overweight among adolescents who were breastfed as infants. JAMA. 2001;285:2461–7 [DOI] [PubMed] [Google Scholar]
- 54.Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82:1298–307 [DOI] [PubMed] [Google Scholar]
- 55.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162:397–403 [DOI] [PubMed] [Google Scholar]
- 56.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–77 [DOI] [PubMed] [Google Scholar]
- 57.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Anton B, Gruszfeld D, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr. 2009;89:1502S–8S [DOI] [PubMed] [Google Scholar]
- 58.von Kries R, Koletzko B, Sauerwald T, von Mutius E, Barnert D, Grunert V, von Voss H. Breast feeding and obesity: cross sectional study. BMJ. 1999;319:147–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wijlaars LP, Johnson L, van Jaarsveld CH, Wardle J. Socioeconomic status and weight gain in early infancy. Int J Obes .(Lond). 2011;35:963–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butte NF. The role of breastfeeding in obesity. Pediatr Clin North Am. 2001;48:189–98 [DOI] [PubMed] [Google Scholar]
- 61.Butte NF. Impact of infant feeding practices on childhood obesity. J Nutr. 2009;139:412S–6S [DOI] [PubMed] [Google Scholar]
- 62.Grummer-Strawn LM, Reinold C, Krebs NF. Center for Disease Control and Prevention (CDC). Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep. 2010;59:1–15 [PubMed] [Google Scholar]
- 63.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B. Growth of breast-fed and formula-fed infants from 0 to 18 months: the DARLING Study. Pediatrics. 1992;89:1035–41 [PubMed] [Google Scholar]
- 64.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41 [DOI] [PubMed] [Google Scholar]
- 65.Gale C, Logan KM, Santhakumaran S, Parkinson JR, Hyde MJ, Modi N. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:656–69 [DOI] [PubMed] [Google Scholar]
- 66.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B. Breast-fed infants are leaner than formula-fed infants at 1 y of age: the DARLING study. Am J Clin Nutr. 1993;57:140–5 [DOI] [PubMed] [Google Scholar]
- 67.Butte NF, Wong WW, Hopkinson JM, Smith EO, Ellis KJ. Infant feeding mode affects early growth and body composition. Pediatrics. 2000;106:1355–66 [DOI] [PubMed] [Google Scholar]
- 68.Gungor DE, Paul IM, Birch LL, Bartok CJ. Risky vs rapid growth in infancy: refining pediatric screening for childhood overweight. Arch Pediatr Adolesc Med. 2010;164:1091–7 [DOI] [PubMed] [Google Scholar]
- 69.Toschke AM, Vignerova J, Lhotska L, Osancova K, Koletzko B, von Kries R. Overweight and obesity in 6- to 14-year-old Czech children in 1991: protective effect of breast-feeding. J Pediatr. 2002;141:764–9 [DOI] [PubMed] [Google Scholar]
- 70.Holmes AV, Auinger P, Howard CR. Combination feeding of breast milk and formula: evidence for shorter breast-feeding duration from the National Health and Nutrition Examination Survey. J Pediatr. 2011;159:186–91 [DOI] [PubMed] [Google Scholar]
- 71.Bogen DL, Hanusa BH, Whitaker RC. The effect of breast-feeding with and without formula use on the risk of obesity at 4 years of age. Obes Res. 2004;12:1527–35 [DOI] [PubMed] [Google Scholar]
- 72.Mihrshahi S, Battistutta D, Magarey A, Daniels LA. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2006;291:R768–78 [DOI] [PubMed] [Google Scholar]
- 74.Wahlig JL, Bales ES, Jackman MR, Johnson GC, McManaman JL, Maclean PS. Impact of high-fat diet and obesity on energy balance and fuel utilization during the metabolic challenge of lactation. Obesity (Silver Spring) 2012;20:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahuja S, Boylan M, Hart S, Roman-Shriver C, Spallholz J, Pence B, Sawyer B. Glucose and insulin levels are increased in obese and overweight mothers’ breast-milk. Food Nutr Sci. 2011;2:201–6 [Google Scholar]
- 76.Barbosa L, Butte NF, Villalpando S, Wong WW, Smith EO. Maternal energy balance and lactation performance of Mesoamerindians as a function of body mass index. Am J Clin Nutr. 1997;66:575–83 [DOI] [PubMed] [Google Scholar]
- 77.Savino F, Liguori SA. Update on breast milk hormones: leptin, ghrelin and adiponectin. Clin Nutr. 2008;27:42–7 [DOI] [PubMed] [Google Scholar]
- 78.Savino F, Fissore MF, Liguori SA, Oggero R. Can hormones contained in mothers’ milk account for the beneficial effect of breast-feeding on obesity in children? Clin Endocrinol (Oxf). 2009;71:757–65 [DOI] [PubMed] [Google Scholar]
- 79.Aydin S, Ozkan Y, Erman F, Gurates B, Kilic N, Colak R, Gundogan T, Catak Z, Bozkurt M, Akin O, et al. Presence of obestatin in breast milk: relationship among obestatin, ghrelin, and leptin in lactating women. Nutrition. 2008;24:689–93 [DOI] [PubMed] [Google Scholar]
- 80.Newburg DS, Woo JG, Morrow AL. Characteristics and potential functions of human milk adiponectin. J Pediatr. 2010;156:S41–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grant WF, Gillingham MB, Batra AK, Fewkes NM, Comstock SM, Takahashi D, Braun TP, Grove KL, Friedman JE, Marks DL. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS ONE. 2011;6:e17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nasser R, Stephen AM, Goh YK, Clandinin MT. The effect of a controlled manipulation of maternal dietary fat intake on medium and long chain fatty acids in human breast milk in Saskatoon, Canada. Int Breastfeed J. 2010;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohammad MA, Sunehag AL, Haymond MW. Effect of dietary macronutrient composition under moderate hypocaloric intake on maternal adaptation during lactation. Am J Clin Nutr. 2009;89:1821–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–20 [DOI] [PubMed] [Google Scholar]
- 85.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care. 2006;29:2231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bitman J, Hamosh M, Hamosh P, Lutes V, Neville MC, Seacat J, Wood DL. Milk composition and volume during the onset of lactation in a diabetic mother. Am J Clin Nutr. 1989;50:1364–9 [DOI] [PubMed] [Google Scholar]
- 87.Neubauer SH. Lactation in insulin-dependent diabetes. Prog Food Nutr Sci. 1990;14:333–70 [PubMed] [Google Scholar]
- 88.Jovanovic-Peterson L, Fuhrmann K, Hedden K, Walker L, Peterson CM. Maternal milk and plasma glucose and insulin levels: studies in normal and diabetic subjects. J Am Coll Nutr. 1989;8:125–31 [DOI] [PubMed] [Google Scholar]
- 89.van Beusekom CM, Zeegers TA, Martini IA, Velvis HJ, Visser GH, van Doormaal JJ, Muskiet FA. Milk of patients with tightly controlled insulin-dependent diabetes mellitus has normal macronutrient and fatty acid composition. Am J Clin Nutr. 1993;57:938–43 [DOI] [PubMed] [Google Scholar]
- 90.Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31:2236–40 [DOI] [PubMed] [Google Scholar]
- 91.Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, Plagemann A. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. 2004;134:648–54 [DOI] [PubMed] [Google Scholar]
- 92.Rodekamp E, Harder T, Kohlhoff R, Franke K, Dudenhausen JW, Plagemann A. Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: role of the late neonatal period and early infancy. Diabetes Care. 2005;28:1457–62 [DOI] [PubMed] [Google Scholar]
- 93.Kerssen A, Evers IM, de Valk HW, Visser GH. Effect of breast milk of diabetic mothers on bodyweight of the offspring in the first year of life. Eur J Clin Nutr. 2004;58:1429–31 [DOI] [PubMed] [Google Scholar]
- 94.Crume TL, Ogden L, Maligie M, Sheffield S, Bischoff KJ, McDuffie R, Daniels S, Hamman RF, Norris JM, Dabelea D. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34:641–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crume TL, Ogden LG, Mayer-Davis EJ, Hamman RF, Norris JM, Bischoff KJ, McDuffie R, Dabelea D. The impact of neonatal breast-feeding on growth trajectories of youth exposed and unexposed to diabetes in utero: the EPOCH Study. Int J Obes (Lond). 2012;36:529–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schaefer-Graf UM, Hartmann R, Pawliczak J, Passow D, Abou-Dakn M, Vetter K, Kordonouri O. Association of breast-feeding and early childhood overweight in children from mothers with gestational diabetes mellitus. Diabetes Care. 2006;29:1105–7 [DOI] [PubMed] [Google Scholar]
- 97.Plagemann A, Harder T. Fuel-mediated teratogenesis and breastfeeding. Diabetes Care. 2011;34:779–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res. 2006;45:203–36 [DOI] [PubMed] [Google Scholar]
- 99.Groh-Wargo S, Jacobs J, Auestad N, O'Connor DL, Moore JJ, Lerner E. Body composition in preterm infants who are fed long-chain polyunsaturated fatty acids: a prospective, randomized, controlled trial. Pediatr Res. 2005;57:712–8 [DOI] [PubMed] [Google Scholar]
- 100.Premji SS, Fenton TR, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev. 2006;(1):CD003959 [DOI] [PubMed] [Google Scholar]
- 101.Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, Elias-Jones A, Weaver LT, Ibhanesebhor S, MacDonald PD, et al. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. Am J Clin Nutr. 2010;92:1133–44 [DOI] [PubMed] [Google Scholar]
- 102.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Gruszfeld D, Dobrzanska A, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836–45 [DOI] [PubMed] [Google Scholar]
- 103.Escribano J, Luque V, Ferre N, Mendez-Riera G, Koletzko B, Grote V, Demmelmair H, Bluck L, Wright A, Closa-Monasterolo R. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: The EU Childhood Obesity Programme. Int J Obes (Lond) 2012;36:548–53 [DOI] [PubMed] [Google Scholar]
- 104.Mennella JA, Ventura AK, Beauchamp GK. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics. 2011;127:110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ventura AK, Beauchamp GK, Mennella JA. Infant regulation of intake: the effect of free glutamate content in infant formulas. Am J Clin Nutr. 2012;95:875–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Butte NF, Garza C, Johnson CA, Smith EO, Nichols BL. Longitudinal changes in milk composition of mothers delivering preterm and term infants. Early Hum Dev. 1984;9:153–62 [DOI] [PubMed] [Google Scholar]
- 107.Butte NF, Garza C, Smith EO. Variability of macronutrient concentration in human milk. Eur J Clin Nutr. 1988;42:345–9 [PubMed] [Google Scholar]
- 108.Bartok CJ, Ventura AK. Mechanisms underlying the association between breastfeeding and obesity. Int J Pediatr Obes. 2009;4:196–204 [DOI] [PubMed] [Google Scholar]
- 109.Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: the DARLING Study. Am J Clin Nutr. 1993;58:152–61 [DOI] [PubMed] [Google Scholar]
- 110.Ailhaud G, Guesnet P. Fatty acid composition of fats is an early determinant of childhood obesity: a short review and an opinion. Obes Rev. 2004;5:21–6 [DOI] [PubMed] [Google Scholar]
- 111.L'Abbe MR, Friel JK. Superoxide dismutase and glutathione peroxidase content of human milk from mothers of premature and full-term infants during the first 3 months of lactation. J Pediatr Gastroenterol Nutr. 2000;31:270–4 [DOI] [PubMed] [Google Scholar]
- 112.Novak EM, Innis SM. Impact of maternal dietary n-3 and n-6 fatty acids on milk medium chain fatty acids and the implications for neonatal liver metabolism. Am J Physiol Endocrinol Metab. 2011;301:E806–17. [DOI] [PubMed] [Google Scholar]
- 113.Palou A, Pico C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite. 2009;52:249–52 [DOI] [PubMed] [Google Scholar]
- 114.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61:2–8 [DOI] [PubMed] [Google Scholar]
- 115.Miralles O, Sanchez J, Palou A, Pico C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring). 2006;14:1371–7 [DOI] [PubMed] [Google Scholar]
- 116.Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res. 2011;70:633–7 [DOI] [PubMed] [Google Scholar]
- 117.Woo JG, Guerrero ML, Altaye M, Ruiz-Palacios GM, Martin LJ, Dubert-Ferrandon A, Newburg DS, Morrow AL. Human milk adiponectin is associated with infant growth in two independent cohorts. Breastfeed Med. 2009;4:101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16 [DOI] [PubMed] [Google Scholar]
- 119.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6:71–82 [DOI] [PubMed] [Google Scholar]
- 120.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145–56 [DOI] [PubMed] [Google Scholar]
- 121.Tomicić S, Johansson G, Voor T, Bjorksten B, Bottcher MF, Jenmalm MC. Breast milk cytokine and IgA composition differ in Estonian and Swedish mothers-relationship to microbial pressure and infant allergy. Pediatr Res. 2010;68:330–4 [DOI] [PubMed] [Google Scholar]
- 122.Groër MW, Shelton MM. Exercise is associated with elevated proinflammatory cytokines in human milk. J Obstet Gynecol Neonatal Nurs. 2009;38:35–41 [DOI] [PubMed] [Google Scholar]
- 123.Hawkes JS, Bryan DL, Makrides M, Neumann MA, Gibson RA. A randomized trial of supplementation with docosahexaenoic acid-rich tuna oil and its effects on the human milk cytokines interleukin 1 beta, interleukin 6, and tumor necrosis factor alpha. Am J Clin Nutr. 2002;75:754–60 [DOI] [PubMed] [Google Scholar]
- 124.Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I, Wester C, Moffat C, Arimi P, Ndase P, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis. 2007;196:562–9 [DOI] [PubMed] [Google Scholar]
- 125.Hanna N, Ahmed K, Anwar M, Petrova A, Hiatt M, Hegyi T. Effect of storage on breast milk antioxidant activity. Arch Dis Child Fetal Neonatal Ed. 2004;89:F518–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aycicek A, Erel O, Kocyigit A, Selek S, Demirkol MR. Breast milk provides better antioxidant power than does formula. Nutrition. 2006;22:616–9 [DOI] [PubMed] [Google Scholar]
- 127.Friel JK, Martin SM, Langdon M, Herzberg GR, Buettner GR. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr Res. 2002;51:612–8 [DOI] [PubMed] [Google Scholar]
- 128.Hermsdorff HH, Puchau B, Volp AC, Barbosa KB, Bressan J, Zulet MA, Martinez JA. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr Metab (Lond). 2011;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Friel JK, Friesen RW, Harding SV, Roberts LJ. Evidence of oxidative stress in full-term healthy infants. Pediatr Res. 2004;56:878–82 [DOI] [PubMed] [Google Scholar]
- 130.Szlagatys-Sidorkiewicz A, Zagierski M, Jankowska A, Luczak G, Macur K, Baczek T, Korzon M, Krzykowski G, Kaminska B. Longitudinal study of vitamins A, E and lipid oxidative damage in human milk throughout lactation. Early Hum Dev. 2012;88:421– [DOI] [PubMed] [Google Scholar]
- 131.Crow RA, Fawcett JN, Wright P. Maternal behavior during breast- and bottle-feeding. J Behav Med. 1980;3:259–77 [DOI] [PubMed] [Google Scholar]
- 132.Sievers E, Oldigs HD, Santer R, Schaub J. Feeding patterns in breast-fed and formula-fed infants. Ann Nutr Metab. 2002;46:243–8 [DOI] [PubMed] [Google Scholar]
- 133.Isomura H, Takimoto H, Miura F, Kitazawa S, Takeuchi T, Itabashi K, Kato N. Type of milk feeding affects hematological parameters and serum lipid profile in Japanese infants. Pediatr Int. 2011;53:807–13 [DOI] [PubMed] [Google Scholar]
- 134.Haisma H, Coward WA, Albernaz E, Visser GH, Wells JC, Wright A, Victora CG. Breast milk and energy intake in exclusively, predominantly, and partially breast-fed infants. Eur J Clin Nutr. 2003;57:1633–42 [DOI] [PubMed] [Google Scholar]
- 135.Matheny RJ, Birch LL, Picciano MF. Control of intake by human-milk-fed infants: relationships between feeding size and interval. Dev Psychobiol. 1990;23:511–8 [DOI] [PubMed] [Google Scholar]
- 136.Dewey KG, Lonnerdal B. Infant self-regulation of breast milk intake. Acta Paediatr Scand. 1986;75:893–8 [DOI] [PubMed] [Google Scholar]
- 137.Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics. 2010;125:e1386–93 [DOI] [PubMed] [Google Scholar]
- 138.Disantis KI, Collins BN, Fisher JO, Davey A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? Int J Behav Nutr Phys Act. 2011;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hurley KM, Cross MB, Hughes SO. A systematic review of responsive feeding and child obesity in high-income countries. J Nutr. 2011;141:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.DiSantis KI, Hodges EA, Johnson SL, Fisher JO. The role of responsive feeding in overweight during infancy and toddlerhood: a systematic review. Int J Obes (Lond). 2011;35:480–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grote V, Schiess SA, Closa-Monasterolo R, Escribano J, Giovannini M, Scaglioni S, Stolarczyk A, Gruszfeld D, Hoyos J, Poncelet P, et al. The introduction of solid food and growth in the first 2 y of life in formula-fed children: analysis of data from a European cohort study. Am J Clin Nutr. 2011;94:1785S–93S [DOI] [PubMed] [Google Scholar]
- 142.Huh SY, Rifas-Shiman SL, Taveras EM, Oken E, Gillman MW. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics. 2011;127:e544–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burdette HL, Whitaker RC, Hall WC, Daniels SR. Breastfeeding, introduction of complementary foods, and adiposity at 5 y of age. Am J Clin Nutr. 2006;83:550–8 [DOI] [PubMed] [Google Scholar]
- 144.Michaelsen KF, Larnkjaer A, Lauritzen L, Molgaard C. Science base of complementary feeding practice in infancy. Curr Opin Clin Nutr Metab Care. 2010;13:277–83 [DOI] [PubMed] [Google Scholar]
- 145.Grote V, Theurich M, Koletzko B. Do complementary feeding practices predict the later risk of obesity? Curr Opin Clin Nutr Metab Care. 2012;15:293–7 [DOI] [PubMed] [Google Scholar]
- 146.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–11 [DOI] [PMC free article] [PubMed] [Google Scholar]