Introduction
Helicobacter pylori was definitively identified by culture in 1984 by Robin Warren and Barry Marshall,1 and 10 years later this organism was recognized by the IARC (WHO) as a Type I carcinogen. H. pylori infection is the strongest known risk factor for gastric cancer, and epidemiological studies have estimated that, in the absence of H. pylori infection, 75% of gastric cancers would not exist.2 H. pylori is considered to be the most common etiologic agent of infection-related cancers, and is estimated to be responsible for 5.5% of all cancers world-wide.3 While it is clear that H. pylori is the strongest causative agent for gastric cancer, the precise mechanisms for gastric cancer development in response to H. pylori infection are less well defined, and a complex interplay of strain-specific bacterial constituents, inflammatory responses governed by host genetic diversity, and/or or environmental influences are involved in determining the fate of the host that is persistently colonized by H. pylori.4 This review will focus on specific mechanisms utilized by H. pylori to drive gastric carcinogenesis.
H. pylori virulence factors that mediate carcinogenesis
The H. pylori type IV cag secretion system
The cag pathogenicity island (cag PAI) is a well-characterized and intensively studied H. pylori virulence determinant, and strains that harbor the cag PAI augment the risk for distal gastric cancer compared to strains that lack the cag island.5 Genes within the cag island encode proteins that form a bacterial type IV secretion system (T4SS) that translocates proteins across the bacterial membrane into host gastric epithelial cells.6-8 The terminal gene product of the cag island is CagA, and this is one of the substrates that is translocated into host cells by the T4SS.9 CagA translocation occurs through the interaction of the H. pylori protein CagL, which is located on the distal tip of the T4SS pilus, with integrin α5β1 on host epithelial cells.10 CagI and CagY have also been shown to interact with β1 integrin and mediate CagA translocation,11 and CagL physically associates with CagI and CagH.12 In addition, CagA facilitates its own translocation through specific binding to β1 integrin.11,13 CagA is also reported to be delivered into host epithelial cells by T4SS-induced externalization of phosphatidylserine from the inner leaflet of the cell membrane. The N-terminus of CagA then interacts with phosphatidylserine to gain entry into host epithelial cells.14, 15 Once inside host cells, CagA is tyrosine phosphorylated by Src and Abl kinases at glutamate-proline-isoleucine-tyrosine-alanine (EPIYA) motifs located within the carboxyl-terminus of CagA.
There are four distinct CagA EPIYA motifs (EPIYA-A, -B, -C, or –D) and these are distinguished by different amino acid sequences surrounding the EPIYA motif.16-18 In contrast to EPIYA-A and –B motifs, which are present in strains throughout the world, EPIYA-C is typically found only in strains from Western countries (Europe, North America and Australia), and in these strains, an increased number of CagA EPIYA-C sites confers a heightened risk for developing gastric cancer.19, 20 The EPIYA-D motif is almost exclusively found in East Asian strains.21 Tyrosine phosphorylation of CagA is tightly regulated, and upon injection into the host cell, CagA is immediately phosphorylated on EPIYA-C or EPIYA-D by Src kinase, followed later by phosphorylation on A-B-C or D motifs by Abl.22
Once phosphorylated by members of the Abl and Src family kinases, phospho-CagA targets and interacts with numerous intracellular effectors to lower the threshold for carcinogenesis. Phospho-CagA activates a eukaryotic tyrosine phosphatase (SHP-2), leading to sustained activation of extracellular signal-regulated kinase 1 and 2 (ERK1/2), Crk adaptor, and C-terminal Src kinase, and induces morphological transformations similar to changes induced by growth factor stimulation.23 Interactions of phospho-CagA with C-terminal Src kinase rapidly activates a negative feedback loop to down-regulate Src signaling and subsequently the generation of phospho-CagA.24
The quantity of phospho-CagA is tightly self-regulated; however, non-phosphorylated CagA also exerts effects within the cell that contribute to pathogenesis. Non-phosphorylated CagA interacts with the cell adhesion protein E-cadherin, the hepatocyte growth factor receptor c-Met, the phospholipase PLC-γ, the adaptor protein Grb2, and the kinase PAR1b/MARK2, and activates ß-catenin, 25-28 which culminate in pro-inflammatory and mitogenic responses, disruption of cell-cell junctions, and loss of cell polarity, all of which promote neoplastic progression. Non-phosphorylated CagA also associates with the epithelial tight-junction scaffolding protein ZO-1, and the transmembrane protein, junctional adhesion molecule (JAM)-A, leading to nascent but incomplete assembly of tight-junctions at sites of bacterial attachment distant from sites of cell-cell contacts.29 CagA also directly binds PAR1b/MARK2, a central regulator of cell polarity, inhibits its kinase activity and promotes loss of cell polarity.25, 30, 31 These events will be discussed in more detail (see Manipulation of the apical-junctional complex by H. pylori, and Figure 1).
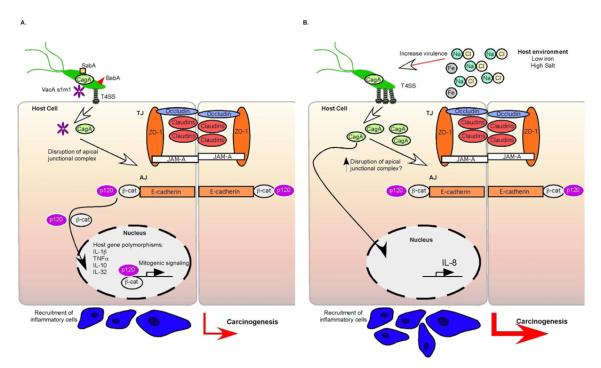
Figure 1.
Gastric cancer is a result of a complex interplay between bacterial virulence factors, host inflammatory responses, and environmental influences. A. H. pylori virulence factors including SabA, BabA, CagA, and VacA influence the outcome of H. pylori infection, with CagA and VacAs1m1 types associated with increased disease severity. H. pylori disrupts the apical-junctional complex at the level of the tight junction (TJ) and adherens junction (AJ), and disrupts cell polarity. Disruption of the adherens junction results in translocation of β-catenin and p120 to the nucleus, altering transcription of genes that promote disease progression. Host genetic diversity also contributes to gastric cancer, including polymorphisms within IL-1β, TNFα, IL-10 and IL-32. B. Host iron (Fe) levels and salt (NaCl) concentrations also impact the virulence of H. pylori. High salt increases CagA production and low iron levels augment assembly of T4SS pili, increase CagA translocation, and augment IL-8 secretion.
Another pathway through which H. pylori CagA can increase the risk for gastric cancer is through manipulation of apoptosis, by increasing spermine oxidase (SMO) production in gastric epithelial cells. This generates oxidative damage and selects for a sub-population of DNA damaged cells that are resistant to apoptosis.32 H. pylori also targets the tumor suppressor p53 to regulate apoptosis in a CagA dependent manner.33, 34 CagA interacts with the apoptosis-stimulating protein of p53 (ASPP2) and prevents ASPP2 from inducing apoptosis through activation of p53. This results in proteasomal degradation of p53 and resistance to apoptosis.33 Recent findings suggest that H. pylori induces specific p53 isoforms that inhibit p53 and p73 activities, induce NF-κB activity, and increase cell survival. 34
CagA is not the only bacterial product delivered through the T4SS, as components of H. pylori peptidoglycan are also delivered into host cells and trigger signaling pathways that lower the threshold for carcinogenesis. Peptidoglycan interacts with the host intracellular pattern recognition molecule Nod1 which leads to activation of NF-κB-dependent pro-inflammatory responses such as secretion of IL-835 or β-defensin-2, as well as production of type I IFN.36, 37 Translocated peptidoglycan can also activate PI3K-AKT signaling, leading to decreased apoptosis, increased proliferation and increased cell migration.38, 39
Vacuolating cytotoxin A (VacA)
Vacuolating cytotoxin A (VacA) is a toxin produced by H. pylori that is associated with increased disease risk.40 VacA exerts multiple effects on epithelial cells including vacuolation as well as inducing apoptosis and suppressing T cell responses, which may contribute to the longevity of infection.41-43
The majority of H. pylori strains possess the vacA gene; however, there is considerable variation in vacA gene structures within the signal (s) region, the middle (m) region, and the intermediate (i) region.44 The s-region and m-region are stratified into s1 or s2 and m1 or m2 alleles respectively. vacA s1/m1 strains induce greater vacuolation than s1/m2 strains, and there is typically no vacuolating activity in s2/m2 strains.44-47 The vacA s1/m1 allele is strongly associated with duodenal and gastric ulcer disease, and gastric cancer.47,48, 49 There are two i region subtypes, i1 and i2; and the i region plays a functional role in vacuolating activity.44 Colonization with vacA i1 strains is strongly associated with the presence of CagA, vacA s1, and gastric cancer.44, 50
Of great interest are recent reports suggesting that VacA and CagA are able to counter-regulate the effects of each other on the host, representing an effective mechanism to promote persistent colonization of H. pylori.51, 52 Phospho-CagA is able to inhibit trafficking of VacA and in this way prevents VacA from reaching its intracellular targets and inducing vacuoles. Non-phosphorylated CagA is also able to oppose vacuolation by blocking VacA activity at the mitochondria.51 Another example of the antagonistic effects of CagA and VacA is CagA activation of the NFAT (nuclear factor of activated T cells) family of transcription factors. CagA induces translocation of NFAT from the cytoplasm to the nucleus while VacA prevents the translocation of NFAT.53 VacA is also able to counteract the effects of CagA by inactivating EGFR, and suppressing activation of ERK1/2 MAP kinase and preventing CagA-mediated cellular elongation.52 Recent work has identified another mechanism for regulation of CagA by VacA, whereby VacA induces autophagy and degradation of CagA through specific binding of VacA m1 to low-density lipoprotein receptor-related preotein-1 (LRP1) on epithelial cells.54 Interestingly, in cells expressing a marker of stem cells, CD44 variant 9 (CD44v9) VacA-induced degradation of CagA is circumvented due to resistance to reactive oxygen species.54 These findings further highlight mechanisms through which H. pylori can avoid the induction of excess cellular damage and maintain long-term persistence in the gastric niche.
Although, CagA and VacA clearly counteract the effects of each other, one exception is facilitation of iron uptake from polarized host epithelial monolayers. In this situation, CagA and VacA act synergistically to create a replicative niche on the apical surface of host epithelial cells by inducing apical mislocalization of transferrin receptors to sites of bacterial attachment.55
Adhesins and outer membrane proteins
In order for H. pylori to colonize, deploy virulence factors, and persist within the gastric niche, adherence of H. pylori to gastric epithelium is required. Sequence analyses have revealed that an unusually high proportion of the H. pylori genome is predicted to encode outer membrane proteins (OMPs), and OMP expression is associated with gastroduodenal ulceration and may heighten the risk for developing gastric cancer (Figure 1).56
Blood group antigen binding adhesin (BabA) is an OMP encoded by the babA2 gene and binds to fucosylated Lewisb antigen (Leb) on the surface of gastric epithelial cells.57-59 Leb-mediated colonization may increase the pathogenic potential of H. pylori 60 and H. pylori babA2+ strains are associated with an increased risk of developing gastric cancer, especially when found in conjunction with cagA and vacA s1 alleles.57
Another H. pylori adhesin is Sialic acid-binding adhesin (SabA). SabA binds to the carbohydrate structure sialyl-Lewisx antigen expressed on gastric epithelium, and is associated with increased gastric cancer risk.61 Sialyl-Lewisx expression is induced during chronic gastric inflammation, suggesting that H. pylori modulates host cell glycosylation patterns to enhance attachment and colonization.62
Outer inflammatory protein (OipA) is an inflammation-related outer membrane protein,63 and the presence of a functional oipA gene is associated with more severe disease outcome and gastric cancer.61, 64 OipA expression is linked to increased production of pro-inflammatory cytokines including IL-8, IL-1, IL-17, and TNFα, 65, 66 as well as other host effector proteins such as upregulation of MMP-1, an MMP associated with gastric cancer, and induction of OipA can result in activation of β-catenin.67,64
Effects of H. pylori on the host immune response that mediate carcinogenesis
Infection with H. pylori invariably results in chronic gastric inflammation, and this occurs through a variety of pathways.68 As discussed above, bacterial factors play an important role in determining the severity of disease outcome; however, these alone are not sufficient to dictate the outcome of H. pylori infection. The immune response of the host is a key determinant of the development of gastric cancer. H. pylori upregulates several inflammatory molecules including IL-1β, IL-32, IL-10, and TNFα and this plays a key role in H. pylori-induced disease progression.5
IL-1ß is a Th1 cytokine that inhibits acid secretion and is increased within gastric mucosa of H. pylori–infected persons.69 Polymorphisms in the IL-1ß gene cluster, specifically IL-1ß-31 and IL-1ß-511, are associated with increased IL-1ß production, and are associated with a significantly increased risk for hypochlorhydria, gastric atrophy, and distal gastric adenocarcinoma compared to persons with genotypes that limit IL-1ß expression, but only among persons infected with H. pylori.70-72 Given that IL-1ß is a potent inhibitor of acid secretion, is profoundly pro-inflammatory, and is up-regulated by H. pylori, colonized individuals harboring high-expression IL-1ß polymorphisms are at increased risk for the development of gastric cancer.
Another cytokine that may increase the risk for gastric cancer is TNF-α. TNF-α is a pro-inflammatory, acid-suppressive cytokine that is increased within H. pylori-colonized human gastric mucosa.73 TNF-α polymorphisms that increase TNF-α production are associated with an increased risk of gastric cancer and its precursors.72, 74 Interestingly, TNF-α expression has been linked to increased ß-catenin signaling through inhibition of GSK3β through the use of transgenic mice that over-express the ß-catenin agonist Wnt1 and these mice develop gastric dysplasia. In vitro studies have revealed that supernatants from activated macrophages promote ß-catenin signaling in gastric epithelial cells, which is attenuated by inhibition of binding of TNF-α to its receptor on gastric epithelial cells, providing a potential mechanism through which enhanced levels of TNF-α may augment the risk for gastric cancer.75
In contrast to IL-1ß and TNF-α polymorphisms for which polymorphisms that increase cytokine production are associated with increased gastric cancer risk, polymorphisms that decrease the production of the anti-inflammatory cytokine IL-10 reciprocally increase the risk for distal gastric cancer.74 Investigations into the combinatorial effects of IL-1ß, TNF-α, and IL-10 polymorphisms on the development of cancer have revealed that the risk of cancer increases progressively with an increasing number of pro-inflammatory polymorphisms and three high-risk polymorphisms increased the risk of cancer 27-fold over baseline.74
The role of IL-32, a recently described pro-inflammatory cytokine that is over-expressed in various inflammatory diseases and cancer has also been investigated in H. pylori infection.76 Expression of IL-32 parallels the severity of gastric pathology, with elevated expression in gastritis and gastric cancer compared to uninfected gastric mucosa. H. pylori-induced IL-32 expression is cagPAI-dependent and requires activation of NFκB. Interestingly, within the context of H. pylori infection, IL-32 expression is linked with expression of the cytokines CXCL1, CXCL2, and IL-8, suggesting that IL-32 may function as a master regulatory protein that controls cytokine expression in H. pylori infection.76
Manipulation of the apical-junctional complex by H. pylori
Gastric mucosal barrier function is controlled by the apical-junctional complex and is essential for preventing potentially immunogenic elements present in the gastric lumen from gaining access to the gastric mucosa.77 The apical-junctional complex is composed of tight junctions and adherens junctions, and studies have revealed that H. pylori targets many of the host molecules that form apical-junctional complexes with the resulting effect being a lowering of the threshold for carcinogenesis (Figure 1).
Studies focused on tight junction proteins have revealed that H. pylori recruits the tight junction proteins ZO-1 and JAM-A to the site of bacterial attachment,29 and disrupts occludin localization at the tight junction.78-80 H. pylori also induces redistribution of claudin-4 and claudin-5 and disrupts barrier function.80
One mechanism through which H. pylori can disrupt the tight junction is via the interaction of CagA with partitioning-defective 1b (PAR1b)/microtubule affinity-regulating kinase 2 (MARK2). PAR1b is a member of the PAR1 family of kinases, and has an essential role in maintaining epithelial cell polarity.25, 81-83 The PAR1b-binding region of CagA is a 16-amino-acid sequence known as the CagA-multimerization (CM) sequence, which is involved in CagA dimerization.84 The CM motif binds to the MARK2 kinase substrate binding site and mimics a host cell substrate that inactivates the kinase activity of PAR1, leading to defects in epithelial cell polarity and disruption of tight junctions.85,25 Dysregulation of tight junctions also permits H. pylori to gain access into sites previously deemed sanctuary sites, such as intercellular spaces and the lamina propria. H. pylori also targets specific components that comprise the adherens junction to promote progression towards gastric carcinogenesis (Figure 1). Adherens junctions are required for maintenance of adhesive cell-cell contacts, cell polarity, and for signal transduction to the nucleus to regulate transcription. E-cadherin is one adherens junction protein that H. pylori dysregulates via methylating the E-cadherin gene promoter, thereby reducing E-cadherin expression.86-88 Loss of E-cadherin function is associated with gastric cancer,86-88 and hypermethylation of the E-cadherin promoter can be reversed by eradication of H. pylori.87-89
H. pylori infection also disrupts the adherens junction through inducing translocation of membranous E-cadherin, β-catenin, and p120 to the cytoplasm of epithelial cells.90-93 Specifically, non-phosphorylated CagA interacts with E-cadherin,26, 94 leading to destabilization of the E-cadherin/β-catenin complex, and accumulation of cytoplasmic and nuclear β-catenin, which subsequently transactivates β-catenin-dependent genes that may promote carcinogenesis.26, 95 Through activation of PI3-K/Akt signaling by non-phosphorylated CagA, H. pylori inactivates GSK-3β which results in increased cytoplasmic expression of β-catenin.96, 97 H. pylori closely regulates β-catenin activation within host cells through an inhibitory domain within the N-terminus of CagA.98 Interestingly, the N-terminus of CagA counteracts effects exerted by the C-terminus of CagA to reduce host-cell responses by strengthening cell-cell contacts and decreasing CagA-induced β-catenin activity.98
H. pylori can also cleave E-cadherin through the actions of the secreted virulence factor high-temperature requirement A (HtrA).99 Loss of E-cadherin from the adherens junction is associated with dissociation and movement of β-catenin and p120 from the adherens junction into the cytosol. Under normal physiological conditions, nuclear expression of p120 is low; however, in transformed cells, expression of p120 is elevated.100-102 H. pylori is associated with mislocalization of p120 to the nucleus in human gastric epithelia and in infected murine primary gastric epithelial cells.93, 103 Further analysis of downstream signaling pathways has determined that p120 mis-localized to the nucleus in response to H. pylori acts to relieve transcriptional repression of mmp-7, a matrix metalloproteinase implicated in gastric tumorogenesis, by an interaction with Kaiso.93 Nagy et al. have also reported that a p120- and β-catenin target gene, PPARδ, regulates gastric epithelial proliferation via activation of cyclin E, representing another important mechanism through which H. pylori may lower the threshold for developing gastric cancer.38
The role of environmental influences
As discussed in this review, there are multiple ways in which H. pylori manipulates the host to lower the threshold for carcinogenesis and, conversely, the host can also signal to and alter the bacterium. Recent work has demonstrated that CagA expression is significantly upregulated when certain strains of H. pylori are cultured in a medium containing high salt concentrations, an epidemiologically defined risk factor for gastric cancer (Figure 1).106 Using sequence analysis and site-directed mutagenesis, it was determined that salt-responsive strains of H. pylori are more likely to contain two copies of a TAATGA motif within the cagA promoter, while strains containing only a single copy of this motif are less likely to possess properties of salt-responsive CagA expression.104
Host iron levels have also been found to manipulate the virulence potential of H. pylori. H. pylori harvested from gerbils with low iron levels were found to assemble more T4SS pili per bacterium, translocate increased amounts of CagA, and augment IL-8 secretion compared to H. pylori strains isolated from gerbils with normal iron levels (Figure 1). Furthermore, strains isolated from patients with low ferritin levels induced significantly higher levels of IL-8 compared to strains isolated from patients with the highest ferritin levels, suggesting that iron deficiency in the host increases H. pylori virulence and the risk for developing gastric cancer.105
Summary
H. pylori infection induces chronic inflammation and is the strongest known risk factor for gastric cancer. The genomes of H. pylori are highly diverse and, therefore, bacterial virulence factors play an important role in determining the outcome of H. pylori infection, in combination with host-responses that are augmented by environmental and dietary risk factors. It is important to gain further understanding of the pathogenesis of H. pylori infection in order to develop more effective treatments for this common but deadly malignancy.
Key points.
Infection with Helicobacter pylori plays a central role in the development of gastric cancer.
The pathogenicity of H. pylori infection is attributable to specific interactions between virulence components, variable host inflammatory responses, and environmental factors.
Mechanisms for the carcinogenesis induced by H. pylori include changes in host gene expression, alterations in proliferation and apoptosis, and disruption of apical-junctional complexes
Acknowledgments
Grant support: NIH CA-116087, DK-58404, DK-58587, and CA-77955
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;15(11):971–6. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. The Journal of Clinical Investigation. 2001;107(7):767–73. doi: 10.1172/JCI12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wroblewski LE, Peek RM, Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clinical Microbiology Reviews. 2010;23(4):713–39. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covacci A, Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. The Journal of Experimental Medicine. 2000;191(4):587–92. doi: 10.1084/jem.191.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14648–53. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akopyants NS, Clifton SW, Kersulyte D, et al. Analyses of the cag pathogenicity island of Helicobacter pylori. Molecular Microbiology. 1998;28(1):37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 9.Odenbreit S, Puls J, Sedlmaier B, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science (New York, NY. 2000;287(5457):1497–500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 10.Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449(7164):862–6. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez-Soto LF, Kutter S, Sewald X, et al. Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathogens. 2009;5(12):e1000684. doi: 10.1371/journal.ppat.1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer CL, Gaddy JA, Loh JT, et al. Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria-host cell interface. PLoS Pathogens. 2011;7(9):e1002237. doi: 10.1371/journal.ppat.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan-Turkoz B, Jimenez-Soto LF, Dian C, et al. Structural insights into Helicobacter pylori oncoprotein CagA interaction with beta1 integrin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(36):14640–5. doi: 10.1073/pnas.1206098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata-Kamiya N, Kikuchi K, Hayashi T, et al. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host & Microbe. 2010;7(5):399–411. doi: 10.1016/j.chom.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Senda M, Morohashi H, et al. Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host & Microbe. 2012;12(1):20–33. doi: 10.1016/j.chom.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nature Reviews. 2004;4(9):688–94. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 17.Higashi H, Yokoyama K, Fujii Y, et al. EPIYA motif is a membrane-targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. The Journal of Biological Chemistry. 2005;280(24):23130–7. doi: 10.1074/jbc.M503583200. [DOI] [PubMed] [Google Scholar]
- 18.Naito M, Yamazaki T, Tsutsumi R, et al. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology. 2006;130(4):1181–90. doi: 10.1053/j.gastro.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Basso D, Zambon CF, Letley DP, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135(1):91–9. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira RM, Machado JC, Leite M, et al. The number of Helicobacter pylori CagA EPIYA C tyrosine phosphorylation motifs influences the pattern of gastritis and the development of gastric carcinoma. Histopathology. 2012;60(6):992–8. doi: 10.1111/j.1365-2559.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 21.Argent RH, Hale JL, El-Omar EM, et al. Differences in Helicobacter pylori CagA tyrosine phosphorylation motif patterns between western and East Asian strains, and influences on interleukin-8 secretion. Journal of Medical Microbiology. 2008;57(Pt 9):1062–7. doi: 10.1099/jmm.0.2008/001818-0. [DOI] [PubMed] [Google Scholar]
- 22.Mueller D, Tegtmeyer N, Brandt S, et al. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. The Journal of Clinical Investigation. 2012;122(4):1553–66. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashi H, Tsutsumi R, Muto S, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science (New York, NY. 2002;295(5555):683–6. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi R, Higashi H, Higuchi M, et al. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278(6):3664–70. doi: 10.1074/jbc.M208155200. [DOI] [PubMed] [Google Scholar]
- 25.Saadat I, Higashi H, Obuse C, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447(7142):330–3. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 26.Murata-Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26(32):4617–26. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 27.Churin Y, Al-Ghoul L, Kepp O, et al. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. The Journal of Cell Biology. 2003;161(2):249–55. doi: 10.1083/jcb.200208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco AT, Israel DA, Washington MK, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amieva MR, Vogelmann R, Covacci A, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science (New York, NY. 2003;300(5624):1430–4. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu HS, Saito Y, Umeda M, et al. Structural and functional diversity in the PAR1b/MARK2-binding region of Helicobacter pylori CagA. Cancer Science. 2008;99(10):2004–11. doi: 10.1111/j.1349-7006.2008.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umeda M, Murata-Kamiya N, Saito Y, et al. Helicobacter pylori CagA causes mitotic impairment and induces chromosomal instability. The Journal of Biological Chemistry. 2009;284(33):22166–72. doi: 10.1074/jbc.M109.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaturvedi R, Asim M, Romero-Gallo J, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141(5):1696–708. e1–2. doi: 10.1053/j.gastro.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buti L, Spooner E, Van der Veen AG, et al. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(22):9238–43. doi: 10.1073/pnas.1106200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J, Noto J, Zaika E, et al. Pathogenic bacterium Helicobacter pylori alters the expression profile of p53 protein isoforms and p53 response to cellular stresses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(38):E2543–50. doi: 10.1073/pnas.1205664109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viala J, Chaput C, Boneca IG, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5(11):1166–74. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 36.Boughan PK, Argent RH, Body-Malapel M, et al. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of beta-defensins during Helicobacter pylori infection. The Journal of Biological Chemistry. 2006;281(17):11637–48. doi: 10.1074/jbc.M510275200. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe T, Asano N, Fichtner-Feigl S, et al. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. The Journal of Clinical Investigation. 2010;120(5):1645–62. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagy TA, Wroblewski LE, Wang D, et al. beta-Catenin and p120 mediate PPARdelta-dependent proliferation induced by Helicobacter pylori in human and rodent epithelia. Gastroenterology. 2011;141(2):553–64. doi: 10.1053/j.gastro.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagy TA, Frey MR, Yan F, et al. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. The Journal of Infectious Diseases. 2009;199(5):641–51. doi: 10.1086/596660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3(4):320–32. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 41.Boncristiano M, Paccani SR, Barone S, et al. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. The Journal of Experimental Medicine. 2003;198(12):1887–97. doi: 10.1084/jem.20030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebert B, Fischer W, Weiss E, et al. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science (New York, NY. 2003;301(5636):1099–102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 43.Sundrud MS, Torres VJ, Unutmaz D, et al. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(20):7727–32. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhead JL, Letley DP, Mohammadi M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133(3):926–36. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 45.Van Doorn LJ, Figueiredo C, Megraud F, et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116(4):823–30. doi: 10.1016/s0016-5085(99)70065-x. [DOI] [PubMed] [Google Scholar]
- 46.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. The Journal of Biological Chemistry. 1992;267(15):10570–5. [PubMed] [Google Scholar]
- 47.Atherton JC, Cao P, Peek RM, Jr., et al. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. The Journal of Biological Chemistry. 1995;270(30):17771–7. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 48.Atherton JC, Peek RM, Jr., Tham KT, et al. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112(1):92–9. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 49.Miehlke S, Kirsch C, Agha-Amiri K, et al. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. International Journal of Cancer. 2000;87(3):322–7. [PubMed] [Google Scholar]
- 50.Chung C, Olivares A, Torres E, et al. Diversity of VacA intermediate region among Helicobacter pylori strains from several regions of the world. Journal of Clinical Microbiology. 2010;48(3):690–6. doi: 10.1128/JCM.01815-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldani A, Cormont M, Hofman V, et al. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathogens. 2009;5(10):e1000603. doi: 10.1371/journal.ppat.1000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tegtmeyer N, Zabler D, Schmidt D, et al. Importance of EGF receptor, HER2/Neu and Erk1/2 kinase signalling for host cell elongation and scattering induced by the Helicobacter pylori CagA protein: antagonistic effects of the vacuolating cytotoxin VacA. Cellular Microbiology. 2009;11(3):488–505. doi: 10.1111/j.1462-5822.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 53.Yokoyama K, Higashi H, Ishikawa S, et al. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9661–6. doi: 10.1073/pnas.0502529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host & Microbe. 2012;12 doi: 10.1016/j.chom.2012.10.014. (IN PRESS) [DOI] [PubMed] [Google Scholar]
- 55.Tan S, Noto JM, Romero-Gallo J, et al. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathogens. 2011;7(5):e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dossumbekova A, Prinz C, Gerhard M, et al. Helicobacter pylori outer membrane proteins and gastric inflammation. Gut. 2006;55(9):1360–1. [PMC free article] [PubMed] [Google Scholar]
- 57.Gerhard M, Lehn N, Neumayer N, et al. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(22):12778–83. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ilver D, Arnqvist A, Ogren J, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science (New York, NY. 1998;279(5349):373–7. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 59.Boren T, Falk P, Roth KA, et al. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science (New York, NY. 1993;262(5141):1892–5. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 60.Guruge JL, Falk PG, Lorenz RG, et al. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):3925–30. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaoka Y, Ojo O, Fujimoto S, et al. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55(6):775–81. doi: 10.1136/gut.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahdavi J, Sonden B, Hurtig M, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science (New York, NY. 2002;297(5581):573–8. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7533–8. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68(2):379–87. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaoka Y, Kudo T, Lu H, et al. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126(4):1030–43. doi: 10.1053/j.gastro.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 66.Sugimoto M, Ohno T, Graham DY, et al. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer science. 2009;100(11):2152–9. doi: 10.1111/j.1349-7006.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu JY, Lu H, Sun Y, et al. Balance between polyoma enhancing activator 3 and activator protein 1 regulates Helicobacter pylori-stimulated matrix metalloproteinase 1 expression. Cancer Res. 2006;66(10):5111–20. doi: 10.1158/0008-5472.CAN-06-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48(6):743–7. doi: 10.1136/gut.48.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noach LA, Bosma NB, Jansen J, et al. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29(5):425–9. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 70.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 71.Figueiredo C, Machado JC, Pharoah P, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94(22):1680–7. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 72.Santos JC, Ladeira MS, Pedrazzoli J, Jr., et al. Relationship of IL-1 and TNF-alpha polymorphisms with Helicobacter pylori in gastric diseases in a Brazilian population. Braz J Med Biol Res. 2012;45(9):811–7. doi: 10.1590/S0100-879X2012007500099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crabtree JE, Shallcross TM, Heatley RV, et al. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32(12):1473–7. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124(5):1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 75.Oguma K, Oshima H, Aoki M, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. The EMBO Journal. 2008;27(12):1671–81. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakitani K, Hirata Y, Hayakawa Y, et al. Role of Interleukin-32 in Helicobacter pylori-Induced Gastric Inflammation. Infection and Immunity. 2012;80(11):3795–803. doi: 10.1128/IAI.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun YQ, Soderholm JD, Petersson F, et al. Long-standing gastric mucosal barrier dysfunction in Helicobacter pylori-induced gastritis in mongolian gerbils. Helicobacter. 2004;9(3):217–27. doi: 10.1111/j.1083-4389.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 78.Wroblewski LE, Shen L, Ogden S, et al. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136(1):236–46. doi: 10.1053/j.gastro.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki K, Kokai Y, Sawada N, et al. SS1 Helicobacter pylori disrupts the paracellular barrier of the gastric mucosa and leads to neutrophilic gastritis in mice. Virchows Arch. 2002;440(3):318–24. doi: 10.1007/s004280100430. [DOI] [PubMed] [Google Scholar]
- 80.Fedwick JP, Lapointe TK, Meddings JB, et al. Helicobacter pylori activates myosin light-chain kinase to disrupt claudin-4 and claudin-5 and increase epithelial permeability. Infection and Immunity. 2005;73(12):7844–52. doi: 10.1128/IAI.73.12.7844-7852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen D, Brennwald PJ, Rodriguez-Boulan E, et al. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J Cell Biol. 2004;164(5):717–27. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeaiter Z, Cohen D, Musch A, et al. Analysis of detergent-resistant membranes of Helicobacter pylori infected gastric adenocarcinoma cells reveals a role for MARK2/Par1b in CagA-mediated disruption of cellular polarity. Cell Microbiol. 2008;10(3):781–94. doi: 10.1111/j.1462-5822.2007.01084.x. [DOI] [PubMed] [Google Scholar]
- 83.Drewes G, Ebneth A, Preuss U, et al. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89(2):297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 84.Ren S, Higashi H, Lu H, et al. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. The Journal of Biological Chemistry. 2006;281(43):32344–52. doi: 10.1074/jbc.M606172200. [DOI] [PubMed] [Google Scholar]
- 85.Ne Sbreve Ic D, Miller MC, Quinkert ZT, et al. Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nature Structural & Molecular Biology. 2009 doi: 10.1038/nsmb.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan AO, Lam SK, Wong BC, et al. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut. 2003;52(4):502–6. doi: 10.1136/gut.52.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leung WK, Man EP, Yu J, et al. Effects of Helicobacter pylori eradication on methylation status of E-cadherin gene in noncancerous stomach. Clin Cancer Res. 2006;12(10):3216–21. doi: 10.1158/1078-0432.CCR-05-2442. [DOI] [PubMed] [Google Scholar]
- 88.Perri F, Cotugno R, Piepoli A, et al. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. Pylori infected patients and effect of eradication. The American Journal of Gastroenterology. 2007;102(7):1361–71. doi: 10.1111/j.1572-0241.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- 89.Chan AO, Peng JZ, Lam SK, et al. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006;55(4):463–8. doi: 10.1136/gut.2005.077776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conlin VS, Curtis SB, Zhao Y, et al. Helicobacter pylori infection targets adherens junction regulatory proteins and results in increased rates of migration in human gastric epithelial cells. Infection and Immunity. 2004;72(9):5181–92. doi: 10.1128/IAI.72.9.5181-5192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki M, Mimuro H, Suzuki T, et al. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. The Journal of Experimental Medicine. 2005;202(9):1235–47. doi: 10.1084/jem.20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weydig C, Starzinski-Powitz A, Carra G, et al. CagA-independent disruption of adherence junction complexes involves E-cadherin shedding and implies multiple steps in Helicobacter pylori pathogenicity. Experimental Cell Research. 2007;313(16):3459–71. doi: 10.1016/j.yexcr.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 93.Ogden SR, Wroblewski LE, Weydig C, et al. p120 and Kaiso regulate Helicobacter pylori-induced expression of matrix metalloproteinase-7. Molecular Biology of the Cell. 2008;19(10):4110–21. doi: 10.1091/mbc.E08-03-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurashima Y, Murata-Kamiya N, Kikuchi K, et al. Deregulation of beta-catenin signal by Helicobacter pylori CagA requires the CagA-multimerization sequence. International Journal of Cancer. 2008;122(4):823–31. doi: 10.1002/ijc.23190. [DOI] [PubMed] [Google Scholar]
- 95.Oliveira MJ, Costa AM, Costa AC, et al. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. The Journal of Infectious Diseases. 2009;200(5):745–55. doi: 10.1086/604727. [DOI] [PubMed] [Google Scholar]
- 96.Nakayama M, Hisatsune J, Yamasaki E, et al. Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. The Journal of Biological Chemistry. 2009;284(3):1612–9. doi: 10.1074/jbc.M806981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki M, Mimuro H, Kiga K, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host & Microbe. 2009;5(1):23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Pelz C, Steininger S, Weiss C, et al. A novel inhibitory domain of Helicobacter pylori protein CagA reduces CagA effects on host cell biology. J Biol Chem. 2011;286(11):8999–9008. doi: 10.1074/jbc.M110.166504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoy B, Lower M, Weydig C, et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11(10):798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayerle J, Friess H, Buchler MW, et al. Up-regulation, nuclear import, and tumor growth stimulation of the adhesion protein p120 in pancreatic cancer. Gastroenterology. 2003;124(4):949–60. doi: 10.1053/gast.2003.50142. [DOI] [PubMed] [Google Scholar]
- 101.Wijnhoven BP, Pignatelli M, Dinjens WN, et al. Reduced p120ctn expression correlates with poor survival in patients with adenocarcinoma of the gastroesophageal junction. Journal of Surgical Oncology. 2005;92(2):116–23. doi: 10.1002/jso.20344. [DOI] [PubMed] [Google Scholar]
- 102.Sarrio D, Moreno-Bueno G, Sanchez-Estevez C, et al. Expression of cadherins and catenins correlates with distinct histologic types of ovarian carcinomas. Human pathology. 2006;37(8):1042–9. doi: 10.1016/j.humpath.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Krueger S, Hundertmark T, Kuester D, et al. Helicobacter pylori alters the distribution of ZO-1 and p120ctn in primary human gastric epithelial cells. Pathology, Research and Practice. 2007;203(6):433–44. doi: 10.1016/j.prp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Loh JT, Friedman DB, Piazuelo MB, et al. Analysis of Helicobacter pylori cagA promoter elements required for salt-induced upregulation of CagA expression. Infection and Immunity. 2012;80(9):3094–106. doi: 10.1128/IAI.00232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, Tan S, Douglas M, Wilson KT, Bravo LE, Correa P, Cover TL, Amieva MR, Peek RM., Jr Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. Journal of Clinical Investigation. 2013 doi: 10.1172/JCI64373. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loh JT, Torres V, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67(10):4709. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]