Abstract
Introduction
Pedicularis sibthorpii and P. wilhelmsiana are endemic species mainly found in North-West of Iran. Plants of genus Pedicularis produce some important poly-phenols and flavonoids. In the present work, total phenol and flavonoid contents of the mentioned species as well as their antioxidant capacity have been evaluated.
Methods
Methanol extract of samples was fractionated by SPE method using an ODS cartridge and their 1H-NMR spectra were recorded. Total phenols and flavonoids of methanol extracts were determined using Folin- Ciocalteu and aluminum chloride methods. For determining antioxidant activity of the extracts and fractions, bleaching of purple color methanol solu-tion of 1, 1-diphenylpycryl hydrazyl (DPPH) was measured by spectrophotometric assay.
Results
Total phenols of Pedicularis sibthorpii and P. wilhelmsiana were in the range of 8-30 mg g-1 and 9-20 mg g-1, respectively. The 40% and 60% fractions of P. sibthorpii and the 20%, 40% and 60% fractions of P. wilhelmsiana showed higher amounts of phenolic compounds. The total flavonoid contents of P. sibthorpii and P. wilhelmsiana were in the range of 0-215 mg g-1 and 0-177 mg g-1, respectively, whereas the 40% and 60% fractions showed higher flavonoid amounts. Antioxidant activity of P. sibthorpii and P. wil-helmsiana were in the range of 0.01-0.7 mg mL-1 and 0.01-1.02 mg mL-1. In the same manner, the 20% and 40% fractions of P. sibthorpii and the 40% and 60% fractions of P. wilhelmsiana had lower RC50 than that of other fractions.
Conclusion
Fractions with lower RC50 had higher contents of phenolic and flavonoid compounds. The results of NMR spectra were parallel with these findings and show that it is worth to do phytochemi-cal studies on P. sibthorpii and P. wilhelmsiana.
Keywords: Pedicularis sibthorpii, Pedicularis wilhelmsiana, Antioxidants, Polyphenols, Flavonoids
Introduction
Free radicals can affect whole body system through contribution to many kinds of degenerative diseases including diabetes and cardiovascular damage (Yang et al 2010), CNS injury (Cadet 1998, Demopoulos et al 1982, Demopoulos et al 1980), cancer (Samra et al 2011, Vera-Ramirez et al 2011), liver and kidney damage (Muriel 2009, Small et al 2012), atherosclerosis, inflammatory joint disease, asthma (Rashidi et al 2010), gastritis (Salim 1992) and so on. Oxidation process is one of the most important routs for producing free radicals in food, drugs and even living systems (Mc Cord 2000).
Discovery of free radicals’ impacts on biological system has led to the free-radical theory of aging which implies that preventing free radicals’ action could influence lifespan. Though the healthy body produces a range of its own antioxidant to overwhelm free radicals, supplement natural antioxidants as free radical scavengers may be needed to boost the defensive system and slow down aging. This postulation has evoked great efforts for finding the powerful free radical scavengers to overcome harmful effects of oxidative stress. Recently attentions have been focused on the therapeutic potential of green foods and medicinal plants which is believed to reduce free radical induced tissue injury by trapping them (Beta et al 2005, Cai et al 2004, Chen et al 2007, Dudonne et al 2009). Higher plants produce a variety of antioxidant compounds of which, polyphenols are assumed to be the most potent one (Wang et al 2007, Guangrog et al 2008).
The genus Pedicularis produces iridoids, phenylethanoids, phenylpropanoids and flavonoids (Akdemir et al 1991, Fujii et al 1995, Liu et al 1991, Su et al 1998) which their antioxidant properties have been established, previously (Scalbert et al 2005). Majority of the Pedicularis species are widely distributed in China (Hanbi et al 1998) and most phytochemical studies have been carried out on these species. Pedicularis species have different therapeutic applications in Traditional Chinese Medicine (Jiang et al 2003, Zhang et al 2008). As part of our ongoing research on bioactive constituents of Iranian medicinal plants, we focused on native Pedicularis species. Pedicularis genus is represented by 9 species in flora of Iran (Wendelbo 1981) which their medicinal uses are unknown. Pedicularis sibthorpii and P. wilhelmsiana grow in Azerbaijan province, Iran and according to the best of our knowledge, their biological effects and chemical constituents are not investigated, yet. In the present work, the results of DPPH assay, total phenol and flavonoid content of P. sibthorpii Boiss and P. wilhelmsiana Fisch. extracts and their relationship with 1 H-NMR spectra have been reported.
Materials and methods
Plant materials
The samples of Pedicularis sibthorpii Boiss. were collected from Lighvan valley -Tabriz and samples of P. wilhelmsiana Fisch. ex M. Bieb. were collected from Arasbaran region in East Azerbaijan province, Iran in 2009. Voucher specimens for these collections (P. wilhelmsiana, TUM- FPh 700; P. sibthorpii, TUM- FPh 701) have been deposited in the Herbarium of the Faculty of Pharmacy, Tabriz - Iran.
Extraction and fractionation
Powdered air-dried aerial parts of plants (200g) were extracted by n-hexane (8 h), dichloromethane (10 h) and methanol (8 h) using a Soxhlet, respectively. Solvents were removed in vacuo by rotary evaporator at an ambient temp. Further studies were carried out on methanol extract fractions prepared by solid phase extraction (SPE) method.
The methanol extract (2 g) was loaded on a Sep-pak (Vac 35 cc; 10g; C18 cartridge) cartridge and fractions were eluted by step gradients of methanol- water mixtures ( fr1,10% methanol; fr2, 20% methanol; fr3, 40% methanol; fr4, 60% methanol; fr5, 80% methanol; fr6, 100% methanol; 200 mL each). The solvents of eluted fractions were removed in vacuo and 40°C.
DPPH assay
Antioxidant activity of the extracts and fractions was measured according to Asnaashari et al (2011) with some modifications. In order to obtain dilutions, different sample concentrations were prepared in methanol and 5 mL of each concentration were added to 5mL of 0.004% methanolic solution of DPPH. After completion of reaction at room temperature for 30 min, bleaching of DPPH was monitored at 517 nm against a blank. Inhibition of DPPH was calculated as RC50, extrapolated from dose-response curve. Tests were carried out in triplicate and the same procedure was applied for the quercetin as a positive control.
Determination of total phenol content
Determination of total phenol content was carried out by Folin- Ciocalteu test according to Chun et al (2003) with some modifications. One mL of the extract and fractions’ sample (solved in 60% acetone, 5 mg/100 mL) was mixed with 200 µl Folin- Ciocalteu reagent and 1 mL of aqueous Na2CO3. The mixtures were left at room temperature for 30 min and the phenol contents were determined by colorimetric method at 715 nm. The calibration curve was prepared using Gallic acid solutions at concentrations of 1- 0.01562 mg/mL in 60% acetone. Total phenol contents were expressed in terms of gallic acid equivalent (mg g- 1 ).
Determination of total flavonoids content
The total flavonoid content was estimated using aluminum chloride colorimetric assay (Zhishen et al 1999, Zou et al 2004). The 0.5 mL of test samples’ solution in methanol (5mg/100mL) were mixed with 2mL of distilled water and 150 µl of 5% sodium nitrate. After 6 min, 150 µl of 10% aluminum chloride and 2mL of 1 M sodium hydroxide were added and left at room temperature for 15 min. Absorbance of the mixtures was measured at 510 nm (UV-Visible Ultraspec 2000 spectrophotometer, England) and total flavonoid contents were calculated as rutoside equivalents from a calibration curve of rutoside. The calibration curve was prepared in the same manner using 0.01562-1 mg/mL of rutoside solutions in methanol.
NMR spectra from methanolic fractions
NMR spectra were recorded in CD3OD on a Bruker 200 MHz NMR spectrometer. TMS was used as the internal standard.
Results and discussion
According to the literature records, chemical constituents and bioactivities of different Pedicularis species have previously been investigated. Iridoids, phenylethanoids, phenylpropanoids and flavonoids are the major secondary metabolites of the genus Pedicularis (Akdemir et al 1991, Fujii et al 1995, Liu et al 1991, Su et al 1998). The antioxidant effects of phenylpropanoid glycosides, widely distributed in genus Pedicularis, were studied in different models (Zheng et al 1993, Scalbert et al 2005, Hosoya et al 2008, Ahmad et al 2009, Biao et al 2009, Zhu et al 2010).
Polyphenols consisting of a wide range of biogenic molecules play numerous roles in living organisms. Flavonoids, as the main class of polyphenols, widely distributed in plants, display numerous pharmacological effects (Rashidi et al 2010). It has been shown that free radical scavenging property of flavonoids is responsible for these effects (Cotelle et al 1996, Brown et al 1998, Seyoum et al 2006, Tsimogiannis et al 2006). A common and rapid method to evaluate free radical scavenging ability of flavonoids involves the use of stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH).
Extracts of P. sibthorpii and P. wilhelmsiana and their fractions exhibited varying degrees of free radical scavenging capacities as determined by DPPH methods (Table 1 and Table 2). Total extract of P. sibthorpii (RC50= 0.033 mg mL_1) showed stronger radical scavenging effect than that of P. wilhelmsiana (RC50= 0.15 mg mL_1). Considering the RC50 of quercetin (2.92 x 10-05 mg mL_1), it could be a moderate effect. The experiments showed that the 20% and 40% Sep-Pak fractions of P. sibthorpii as well as the 40% and 60% Sep-Pak fractions of P. wilhelmsiana have lower RC50 than that of other fractions.
Table 1. Antioxidant, total phenol and flavonoid contents of P. sibthorpii.
| Extract or fractions | Total phenol content (as gallic acid equivalents) mg g- 1 | Flavonoid content mg g- 1 | Antioxidant activitya (RC50; mg mL-1) |
| Total extract | 15.28 | 47.50 | 0.0330 |
| 10% | 08.56 | 01.80 | 00.820 |
| 20% | 12.59 | 00.00 | 00.070 |
| 40% | 30.06 | 118.61 | 00.012 |
| 60% | 25.07 | 214.76 | 00.107 |
| 80% | 10.74 | 00.00 | 00.337 |
| 100% | 10.01 | 00.00 | 00.616 |
aPositive control (quercetin): 2.92 x 10-5
Table 2. Antioxidant, total phenol and flavonoid contents of P. wilhelmsiana.
| Extract or fractions | Total phenol content (as gallic acid equivalents) mg g- 1 | Flavonoid content mg g- 1 | Antioxidant activitya (RC50; mg mL-1 ) |
| Total extract | 12.18 | 35.60 | 00.15 |
| 10% | 09.17 | 01.55 | 03.82 |
| 20% | 17.52 | 00.00 | 00.18 |
| 40% | 19.31 | 154.14 | 0.017 |
| 60% | 18.43 | 176.60 | 00.0740 |
| 80% | 11.32 | 00.00 | 00.1774 |
| 100% | 09.84 | 00.00 | 01.0192 |
aPositive control(quercetin): 2.92 x 10-5
Total phenols were assessed by Folin Ciocalteu method and results were reported as gallic acid equivalents by reference to standard curve (y =0.0282+0.0043, r 2 =0.998). Total phenols of P. sibthorpii and P. wilhelmsiana and their fraction were demonstrated in Table 1 and Table 2. According to these findings, 40% and 60% Sep-Pak fractions revealed the highest amount of total phenols at 30.06 and 25.07 mg g - 1 respectively, approximately 2 times more than methanol extracts.
Flavonoid content was determined by aluminum chloride method and expressed as rutoside equivalents in mg g - 1 dry extracts and fractions using a standard curve of rutoside (y= 2.0903x- 0.0298, r 2 = 0.998). As can be seen from the Table 1 and Table 2, flavonoid content of 40% and 60% Sep-Pak fractions was superior to the flavonoid content of other fractions and extracts. The lowest flavonoid content was found within 20%, 80% and 100% fractions.
The collected data have shown the existence of a good harmony between antioxidant potency of samples and their total phenolic and flavonoid content. The maximum antioxidant, total polyphenols and flavonoid content were seen in 40% and 60% Sep-Pak fractions whereas the other fractions were found to contain nearly low or neglectable amounts. Generally, the higher antioxidant activity of the mentioned fractions might be attributed to their contents of total polyphenols and especially flavonoids. However according to Prior et al (2005), many other compounds may contribute to the reaction and cause a false positive error.
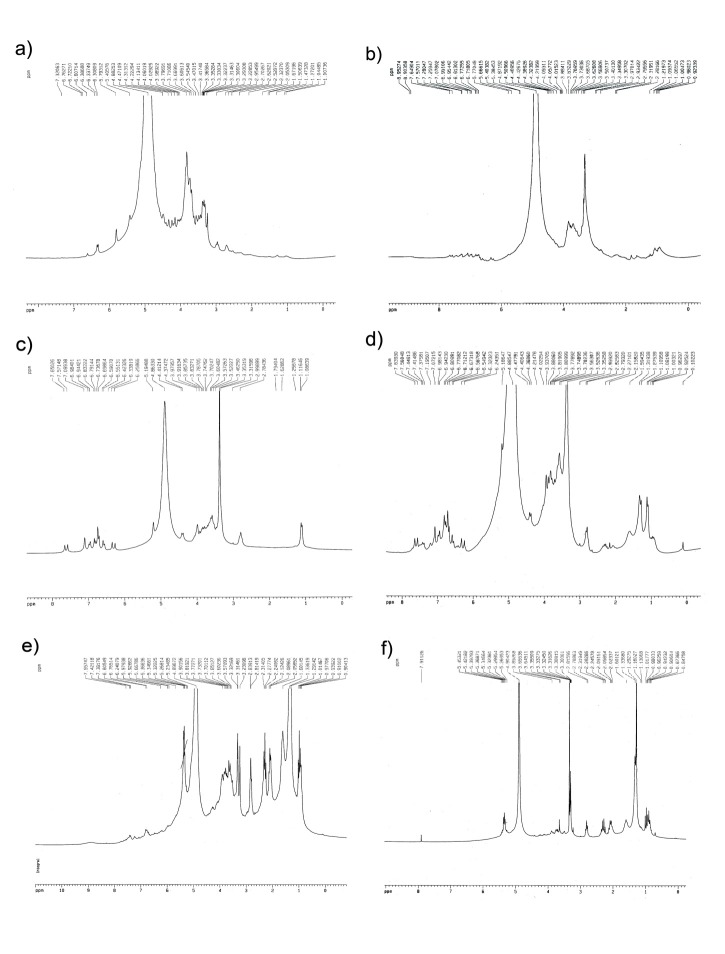
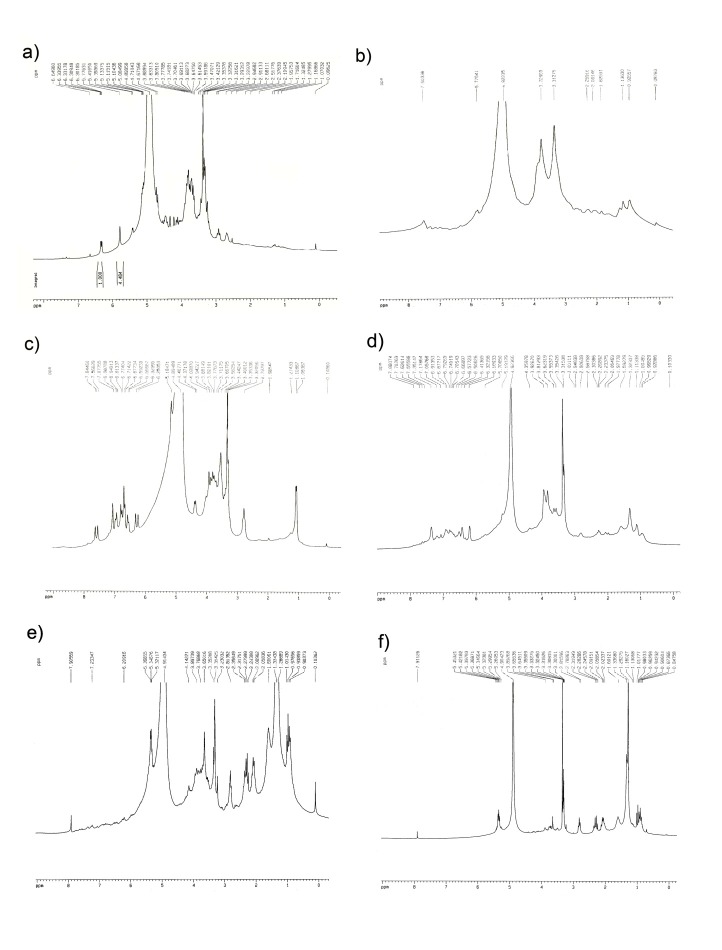
In the present study, the issue was partly clarified by using 1 H- NMR spectra as fingerprints to indicate significant differences between the chemical constituents of fractions (Figures 1 and 2). The 1 H- NMR spectra of 10% Sep-Pak fractions belonging to P. sibthorpii and P. wilhelmsiana (Figures 1-a and 2-a, respectively) have revealed that there is no phenolic compound, but iridoids (signals in the δ 3-6 ppm) in this fraction. In the same way, the Figures 1-b and 2-b show that some phenolic compounds such as phenylethanoids and flavonoids may exist in low concentrations in 20% fractions of P. sibthorpii and P. wilhelmsiana. Verbascoside is a common phenylpropanoid of the Pedicularis genus and its signals have clearly been seen in the 1 H-NMR spectra of 40% fraction (Figures 1-c and 2-c). Flavonoids may exist in 40% fractions in lower concentration, but their signals were covered by verbascoside signals due to its high concentration. Figures 1-d and 2-d, which are NMR spectra of 60% fraction, indicate that phenolic compounds such as phenylethanoids and flavonoids exist in this fraction. Obviously phenolic compounds exist in very low concentrations in 80% fractions (Figures 1-e and 2-e) and there is no sign of similar compounds in 100% fractions (Figures 1-f and 2-f).
Fig. 1 .
1 H-NMR spectra of Sep-Pak fraction obtained from P. sibthropii methanolic extract. The spectra were recorded in CD3OD.
a) 10% fraction, b) 20% fraction, c) 40% fraction, d) 60% fraction, e) 80% fraction and f) 100% fraction.
Fig. 2 .
1 H-NMR spectra of Sep-Pak fraction obtained from P. wilhelmsiana methanolic extract. The spectra were recorded in CD3OD.
a) 10% fraction, b) 20% fraction, c) 40% fraction, d) 60% fraction, e) 80% fraction and f) 100% fraction.
According to these finding, it can be concluded that there is a relationship between antioxidant activity, phenolic and flavonoid content in these two species, because fractions with lower RC50 have higher contents of phenolic and flavonoid compounds. The results of NMR spectroscopy are parallel with these findings, too. Fractions which show signals in aromatic regions (δ 6-8 ppm) possess higher polyphenolic compounds and lower RC50.
Conclusion
Increasing demands for natural antioxidant have provoked great efforts for finding new and potent free radical scavengers from plants. Pedicularis species yield a series of antioxidant, antitumor, antifungal, antidiabetic and anti-inflammatory secondary metabolites (Beta et al 2005, Cai et al 2004). According to some evidences, phenylpropanoids and flavonoids are responsible for antioxidant effects in plants (Choudhary et al 2011).
The results of present work show that it is worth to do phytochemical studies on Iranian Pedicularis species and purify their antioxidant compounds which are estimated to be phenylethanoids and flavonoids according to the 1 H-NMR findings. It could be concluded that fractionation of extracts and running their H-NMR could be a valuable method for predicting and comparing their antioxidant capacity.
Ethical Issues
Not applicable in this research.
Conflict of interests
Authors declared no conflicts of interests.
References
- Ahmad I, Ahmad N and Wang F . 2009 Antioxidant phenylpropanoid glycosides from Buddleja davidii. Journal of Enzyme Inhibition and Medicinal Chemistry, 24(4), 993-997 [DOI] [PubMed] [Google Scholar]
- Akdemir Z, Çaliș I and Junior P . 1991 Iridoids and phenylpropanoid glycosides from Pedicularis nordmanniana. Planta Medica, 57(6), 584-585 [DOI] [PubMed] [Google Scholar]
- Asnaashari S, Dadizadeh E, Talebpour AH, Eskandani M and Nazemiyeh H . 2011 Free Radical Scavenging Potential and Essential Oil Composition of the Dorema glabrum Fisch . C .A . Mey Roots from Iran. BioImpacts, 1(4), 241-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beta T, Names JED and Sapirstein HD . 2005 Phenolic content and antioxidant activity pearled wheat and roller-milled fractions. Cereal Chemistry, 82(4), 390-393 [Google Scholar]
- Biao CH, Hua TN and Sheng PC . 2009 Progress in research on Pedicularis plants. China Journal of Chinese Materia Medica, 34(19), 2536-2546 [PubMed] [Google Scholar]
- Brown JE, Khodr H, Hider RC and Rice-Evans CA . 1998 Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochemical Journal, 15(330), 1173-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL . 1988 Free radical mechanisms in the central nervous system: an overview. International Journal of Neuroscience, 40(1-2), 13-18 [DOI] [PubMed] [Google Scholar]
- Cai Y, Luo Q, Sun M and Corke H . 2004 Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Science, 74, 2157-2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY and Yen GC . 2007 Antioxidant activity and free radical scavenging capacity of extracts from guava (Psidium guajava L .) leaves. Food Chemistry, 101, 686-694 [Google Scholar]
- Choudhary RK and Swarnkar PL . 2011 Antioxidant activity of phenolic and flavonoid compounds in some medicinal plants of India. Natural Product Research, 25(11), 1101-1109 [DOI] [PubMed] [Google Scholar]
- Chun OK, Kim DO and Lee CY . 2003 Superoxide radical scavenging activity of the major polyphenols in fresh plums. Journal o f Agriculture and Food Chemistry, 51, 8067-8072 [DOI] [PubMed] [Google Scholar]
- Cotelle N, Bernier JL, Catteau JP, Pommery J, Wallet JC and Gaydou EM . 1996 Antioxidant properties of hydroxy-flavones. Free Radical Biology and Medicine, 20(1), 35-43 [DOI] [PubMed] [Google Scholar]
- Demopoulos HB, Flamm ES, Pietronigro DD and Seligman ML . 1980 The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiologica Scandinavica Supplement, 492, 91-119 [PubMed] [Google Scholar]
- Demopoulos HB, Flamm ES, Seligman ML, Pietronigro DD, Tomasula J and DeCrescito V . 1982 Further studies on free-radical pathology in the major central nervous system disorders: effect of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Canadian Journal of Physiology and Pharmacology, 60(11), 1415-1424 [DOI] [PubMed] [Google Scholar]
- Dudonne S, Vitrac X, Coutiere P, Woillez M and Merillon JM . 2009 Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Journal of Agriculture and Food Chemistry, 57(5), 1768-1774 [DOI] [PubMed] [Google Scholar]
- Fujii M, Miyaichi Y and Tomimori T . 1995 Flavonoid, phenylethanoid and iridoid constituents of the whole plant of Pedicularis longiflora var . tubiformis. Planta Medica, 61(6), 584 [DOI] [PubMed] [Google Scholar]
- Guangrog H, Jiaxin J and Dehui D . 2008 Antioxidative and antibacterial activity of the methanol extract of Artemisia anomala S . Moore. African Journal of Biotechnology, 7, 1335-1338 [Google Scholar]
- Hanbi Y, Holmgren NH and Mill RR. 1998. Pedicularis. In: Flora of China, Wu CY and Raven PH. (eds.), Vol. 18, Science Press, Beijing, 97-209.
- Hosoya T, Yun YS and Kunugi A . 2008 Antioxidant phenylpropanoid glycosides from the leaves of Wasabia japonica. Phytochemistry, 69, 827-832 [DOI] [PubMed] [Google Scholar]
- Jiang TF, Ou QY and Shi YP . 2003 Separation and determination of phenylpropanoid glycosides from Pedicularis species by capillary electrophoresis. Journal of Chromatography A, 986(1), 163-167 [DOI] [PubMed] [Google Scholar]
- Lee OH and Lee BY . 2010 Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresource Technology, 101(10), 3751-3754 [DOI] [PubMed] [Google Scholar]
- Liu ZM and Jia ZJ . 1991 Phenylpropanoid and iridoid glycosides from Pedicularis striata. Phytochemistry, 30(4), 1341-1344 [DOI] [PubMed] [Google Scholar]
- McCord JM . 2000 The evolution of free radicals and oxidative stress. American Journal of Medicine, 108(8), 652-659 [DOI] [PubMed] [Google Scholar]
- Muriel P . 2009 Role of free radicals in liver diseases. Hepatology International, 3(4), 526-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior RL, Wu X and Schaich K . 2005 Standardized Methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agriculture and Food Chemistry, 53, 4290-4302 [DOI] [PubMed] [Google Scholar]
- Rashidi MR and Nazemiyeh H . 2010 Inhibitory effects of flavonoids on molybdenum hydroxylases activity; a review. Expert Opinion on Drug Metabolism & Toxicolog, 6(2), 133-152 [DOI] [PubMed] [Google Scholar]
- Salim AS . 1992 Oxygen-derived free radical scavengers protect patients against the complications of erosive gastritis. Intensive Care Medicine, 18(1), 61-62 [DOI] [PubMed] [Google Scholar]
- Samra ZQ, Pervaiz S, Shaheen S, Dar N and Athar MA . 2011 Determination of oxygen derived free radicals producer (xanthine oxidase) and scavenger (paraoxonase1) enzymes and lipid parameters in different cancer patients. Clinical Laboratory, 57(9-10), 741-747 [PubMed] [Google Scholar]
- Scalbert A, Johnson IT and Saltmarsh M. 2005Polyphenols: antioxidants and beyond. American Journal of Clinical Nutrition, 81(suppl), 215S-217S [DOI] [PubMed] [Google Scholar]
- Seyoum A, Asres K and El-Fiky FK . 2006Structure–radical scavenging activity relationships of flavonoids. Phytochemistry, 67, 2058-2070 [DOI] [PubMed] [Google Scholar]
- Small DM, Coombes JS, Bennett N, Johnson DW and Gobe GC. 2012. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton), Jan 31, doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed]
- Su BN, Ma LP and Jia ZJ . 1998 Iridoid and phenylpropanoid glycosides from Pedicularis artselaeri. Planta Medica, 64(8), 720-723 [DOI] [PubMed] [Google Scholar]
- Tsimogiannis DI and Oreopoulou V . 2006 The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency . A kinetic approach for the 3’,4’-hydroxy substituted members. Innovative Food Science and Emerging Technologies, 7, 140-146 [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M and Mazur M . 2006 Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico- Biological Interactions, 160(1), 1-40 [DOI] [PubMed] [Google Scholar]
- Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa MC, Ramirez-Tortosa CL, Granados-Principal S, Lorente JA, et al. 2011 Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells . Biological bases to develop oxidative-based therapies. Critical Reviews Oncology / Hematology, 80(3), 347-368 [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin J, Qiao Y, Zhang H and Lu X . 2007 Studies on antioxidant activity and chemical constituents of Artemisia halodendron. Asian Journal of Traditional Medicines, 2, 30-33 [Google Scholar]
- Wendelbo P. 1981. Pedicularis In: Flora Iranica, Rechinger KH (editor), No. 147, Akademische Druk- u. Verlagsanstalt, Graz, 189-207.
- Yang Y, Hayden MR, Sowers S, Bagree SV and Sowers JR . 2010 Retinal redox stress and remodeling in cardiometabolic syndrome and diabetes. Oxidative Medicine and Cellular Longevity, 3(6), 392-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Smith PT, et al. 2011 Antioxidant and Anti-inflammatory Activities of Selected Medicinal Plants Containing Phenolic and Flavonoid Compounds. Journal of Agriculture and Food Chemistry, 59(23), 12361-12367 [DOI] [PubMed] [Google Scholar]
- Zhang ZX, xie WD and Jia ZJ . 2008 Glycosids from two Pedicularis species. Biochemical Systematics and Ecology, 36(5-6), 467-472 [Google Scholar]
- Zheng RL, Wang PF, Li J, Liu ZM and Jia ZJ . 1993 Inhibition of the autoxidation of linoleic acid by phenylpropanoid glycosides from Pedicularis in micelles. Chemistry and Physics of Lipids, 65(2), 151-154 [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T and Jianming W . 1999 The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64, 555-559 [Google Scholar]
- Zhu M, Tan N, Zhu H, Zeng G, He W, Yu B, Chen X . 2010 Anti-sports anaemia effects of verbascoside and martynoside in mice. International Journal of Sports Medicine, 31(8), 537-541 [DOI] [PubMed] [Google Scholar]
- Zou Y, Lu Y and Wei D . 2004 Antioxidant activity of flavonoid-rich extract of Hypericum perforatum L in vitro. Journal of Agriculture and Food Chemistry, 52, 5032-5039 [DOI] [PubMed] [Google Scholar]