Abstract
Introduction
Polyethylenimine (PEI), as a nonviral cationic polymer, has been widely used as gene delivery nanosystem. Although a number of investigations have highlighted its toxic impacts on target cells through induction of apoptosis/necrosis, still it is essential to look at its structural impacts on target cells.
Methods
In this current study, cytogenomic impacts of 25 kD linear and branched PEI (LPEI and BPEI, respectively) in A431 cells are reported to address possible mechanism for induction of apoptosis. At 40-50% confluency, A431 cells were exposed to PEI at a recommended concentration for 4 hr. After 24 hr, to detect apoptosis and DNA damage, the treated cells were subjected to MTT assay, FITC-labeled annexin V flow cytometry and comet assay.
Results
Flow cytometry assessments revealed that the BPEI can result in greater internalization than the linear PEI, which also induced greater cytotoxicity. Annexin V assay confirmed early and late apoptosis by BPEI, imposing somewhat DNA damage detected by comet assay. Western blot analysis resulted in induction of Akt-kinase which is possibly one of biomolecules affected by PEI.
Conclusion
These results highlight that, despite induction of Akt-kinase, the BPEI can elicit apoptosis in target cells.
Keywords: Akt Kinase, Cationic Polymers, Cytotoxicity, Gene Delivery Systems, Polyethylenimine
Introduction
Up until now, a number of gene delivery systems comprising viral and non-viral vectors have been widely exploited for shuttling of nucleic acids in various target cells in vitro and/or in vivo.
Among non-viral vectors, cationic polymers have been successfully used for the delivery of genes, even though these cationic polymers are able to induce inevitable gene expression changes inadvertently. To date, application of dendrimers for macromolecules (antisense, DNA and gene) delivery to modulate biological processes are enormously becoming popular because of their unique characteristics.
Dendrimeric delivery systems include three closely related families prepared by the divergent synthesis. Of cationic polymers widely used for gene delivery in vitro are: polyamidoaimne (PAMAM), polyethylenimine (PEI) and polypropyleneimine(PPI) which are normally branched polymers and the degree of branching is expressed in the generation of dendrimer (Conwell and Huang 2005; Davis 2002; Lungwitz et al. 2005). Of note linear and branched PEI polymers, in fact, among commonly used polymers for gene delivery, are attractive carrier on the subject of gene delivery because of their well-defined characteristics. PEI dendrimers are also found as an appropriate utility in a variety of applications, many of which are biological in nature, however little is known about the biological behaviour (in particular in functional genomics and proteomics) of theses polymers which is critical to their in vivo implementation (Lungwitz et al. 2005).
Numbers of biological properties of cationic polymers such as in vitro and in vivo toxicity, immunogenicity, and biodistribution have been so far investigated. For example, the transfection efficiency toxicity of different molecular weights (MWs) PEIs as DNA complexing agentswere examined in non-differentiated COS-1 (green monkey fibroblasts) and well-differentiated human submucosal airway epithelial cells (Calu-3) (Florea et al. 2002). These researchers showed that transfection efficiency was more dependent upon the cell type than the MWs of the PEI used, so that PEI was 3 orders of magnitude more effective in COS-1 than in Calu-3 cells. It appears that the Calu-3 as differentiated cells can secrete mucins that may impose an additional barrier to gene delivery. Besides transfection efficiency was strongly correlated to PEI cytotoxicity. It appears that the cellular toxicity of polycationic polymers have a strong structural basis rather than being an effect only due to charge. However, no substantial gene expression profiling information is available on the genocompatibility of starburst PEI dendrimers. We have previously reported that cationic lipids and polymers are able to elicit intrinsic cytotoxicity as well as genotoxicity in different types of cells (Hollins et al. 2007; Omidi et al. 2005a; Omidi et al. 2005b; Omidi et al. 2008; Omidi and Barar 2009). In the current investigation, we report the cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells.
Materials and methods
Materials
Low melting point agarose (LMPA), linear and branched polyethylenimine (25 kDa) were obtained from Sigma-Aldrich Co., (Poole, UK). Dulbecco’s modified Eagle’s medium (DMEM) containing 25 mM HEPES, fetal bovine serum (FBS), penicillin G, streptomycin, L-glutamine 200mM (x100), and RNase/DNase free ddH2O were purchased from InVitrogen (Paisley, UK). Tissue culture treated multi-well plates and flasks were obtained from Corning Costar (High Wycombe, UK). The dual-window Comet slide was obtained from Trevigen (Gaithersburg, MD). Human epidermoid carcinoma A431 cell line was purchased from ECACC, (Salisbury, UK).Annexin V-FITC apoptosis detection kit was obtained from Oncogene Research Products, (San Diego, CA, USA). The molecular weight rainbow marker (10kDa-250kDa), Horse-radish peroxidase (HRP) conjugated donkey anti-rabbit antibody NA934V and sheep anti-mouse antibody NA931Vwere obtained from Amersham Biosciences (Bucks, UK). Rabbit anti-EGFR polyclonal antibody 2232 and rabbit anti-Akt antibody 9272 were purchased from Upstate Cell Signaling (Milton Keynes, UK). DC protein assay kit was purchased from Bio-Rad, (Hertfordshire, UK). SuperSignalchemiluminescent substrate was obtained from Perbio Science, (Tattenhall, UK). Kodak autoradiography film was obtained from G.R.I., (Rayne, UK). Protran nitrocellulose membrane was from Schleicher&Schuell, (Dassel, Germany). All other chemical (not mentioned) used in this study were from either Sigma (Poole, UK) or Fisher Scientific (Leicestershire, UK).
Cell culture
A431 cells were cultured at a seeding density of 5 ´ 10 4 cells per cm 2 into 96 or 6-well plates and undergone to a standard transfection using PEI polymers. Briefly, following the manufacturer protocol, designated amount of LPEI or BPEI polymers (without or with salmon testes DNA, 4mg per 1 ml media) was prepared initially in serum-free medium (SFM) and incubated at room temperature for 15 mins and the A431 cells were exposed to the polymers at the 40-50% confluencyand incubated at 37ºC for 4 h. Cells were then replenished with normal growth medium, incubated at 37ºC for 24 h, and then undertaken to cytotoxicity assays.
Fluorescence microscopy
For fluorescence microscopy, cells were cultivated as explained onto the 22-mm2 coverslips. At 40-50% confluency, they were transfected with appropriate ratio of PEI:DNA complexes using FITC-labeledoligodeoxynucleotides (f-ODN) for 4 h. Fixation involved washing the cells 3 times with PBS, followed by 10 min incubation with 2% formaldehyde in PBS at room temperature. After washing cells (X3) with PBS, they were mounted on slides using mounting medium without/with DAPI (50 µM, for 20 min) for nuclear staining. The prepared samples were examined utilizing an Olympus IX81 compound fluorescence microscope equipped with XM10 monochrome camera, Olympus optical Co., Ltd. (Tokyo, Japan) as described previously (Omidi and Barar 2009).
Viability assessment, Zeta potential and particle sizing of polyplexes
To assess the influence of LPEI/BPEI dendrimers on the cellular viability, the A431 or A549 cells were seeded and cultured up to 40-50% confluency in the 96-well plates prior to treatment. Cells were then exposed to a range of concentrations of polymers alone or polyplexes and incubated at 37ºC for 4 h. After which, cells were washed once with phosphate buffered saline (PBS) then replenished with normal culture medium and incubated at 37ºC for 24 h. The normal culture medium was replaced with 200 ml fresh media and then 50 ml MTT reagent (2.5 mg/ml in PBS) was added to each well. Following a 4 h incubation period at 37ºC, medium was removed and cells were exposed to 200 ml DMSO and 50 ml of Sorenson buffer (pH 7.4). Cultures were incubated for 30 min at 37ºC and then UV absorbance was measured at 570 nm using anELx808™ Absorbance Microplate Reader, (BioTek, Winooski, USA).
The zeta potentials of the PEIpolymers alone or as complexed with DNA formed at various mass ratios were determined using a microelectrophoresis, Malvern ZetaSizer 3, Malvern Instruments Ltd., (Malvern, UK). The polyplexes were prepared using varying mass ratios (1:1, 5:1 and 10:1) of PEI polymer and DNA using distilled, degassed biologic-grade water. Samples were consecutively measured ten times with instrument calibration (Malvern AZ55 Electrophoretic Standard) prior to each series of measurements. The DTS1050 was used as a standard control for negative charges. The same sample was then subjected to size analysis by photon correlation spectroscopy (PCS: Malvern ZetaSizer 3) using a 5 mW laser at an angle of incidence of 90°. The experiment was performed as replicates in multimodal analysis. Both zeta potential and size determinations were performed at 25°C, and all glass and plastic wares were pre-washed with filtered water (prior to use)to minimize particulate contamination.
FITC-labeledannexin V flow cytometric apoptosis assay
Flow cytometry (fluorescence-activated cell sorting or FACS) analysis was performed to detect the induction of early and/or late apoptosis phenomena in Lipofectin-treated A431 cells in comparison with control and 5% DMSO. AnnexinV-FITC apoptosis detection assay was exploited following the manufacturer protocol (Peng et al. 2002). Briefly, culture media was removed from each well,gentlywashed with PBS and stored on ice. The cells were then detached by gentle trypsinization using 0.5 ml 0.5x trypsin, and resuspended in the original media to include detached cells in the suspension for analysis. About 1.0×10 6 cells were exposed to 10 ml media binding reagent and 1.25 ml FITC-labeledannexin V and incubated in the dark for 15 min at room temperature. Cell suspension was centrifuged at 1000´g for 5 min, the supernatant was discarded and the cells resuspended in 0.5 ml ice-cold 1X binding buffer. Finally, 10 ml propidium iodide was added, the cells were transferred to FACS tubes (Fahrenheit, UK), placed on ice and analysed immediately. Cell associated fluorescence distributions were obtained from ~20,000 events per cell sample through a FL1 bandpass filter for detection of FITC-labeledannexin V and FL2 bandpass filter for detection of propidium iodideusing a FACScalibur flow cytometer (Becton DickinsonImmunocytometry Systems, San Jose, CA, USA). The fluorescence of cell populations was analyzed using validated analysis software, WinMDI 2.8 (http://facs.scripps.edu/).
Assessment of DNA damage by Comet assay
Comet assay was performed accordance to a quantitation technique for measurement of low levels of DNA damage in individual cells, with a slight modifications as reported previously (Omidi et al. 2008). To quantify the DNA damage, olive tail moment (OTM, i.e. %DNA × distance of center of gravity of DNA) and/or tail moment (TM, i.e. %DNA × tail length) of 30-50 cells from each window of each slide were analysed. Four replicates were performed for all experiments including serum-free medium as negative control and H2O2 treated (100µM) as positive control.
Western blot analysis
Cells were grown to 40-50% confluency and exposed to reagents as described previously. Cells were washed (´3) with ice cold phosphate buffered saline (PBS), then total protein were harvested using 100-200 ml lysis buffer (50mM Tris, 5mM EGTA, 150mM NaCl and 1% Triton) containing protease inhibitors (NaVO4, NaF, PMSF, phenylarsine oxide, sodium molybdate, leupeptin and aprotinin). After 15 min incubation on ice, cell debris was removed by centrifugation at 12,000´g at 4°C for 15 min. The supernatant was analysed for protein content using a standard BSA assay by means of DC protein assay kit (Bio-Rad, Hempstead, UK), and western bloting assay was performed as previously described (Omidi and Barar 2009).
Results
The chemical structures of the linear and branched polyethylenimine are shown in Fig. 1.
Fig. 1 .
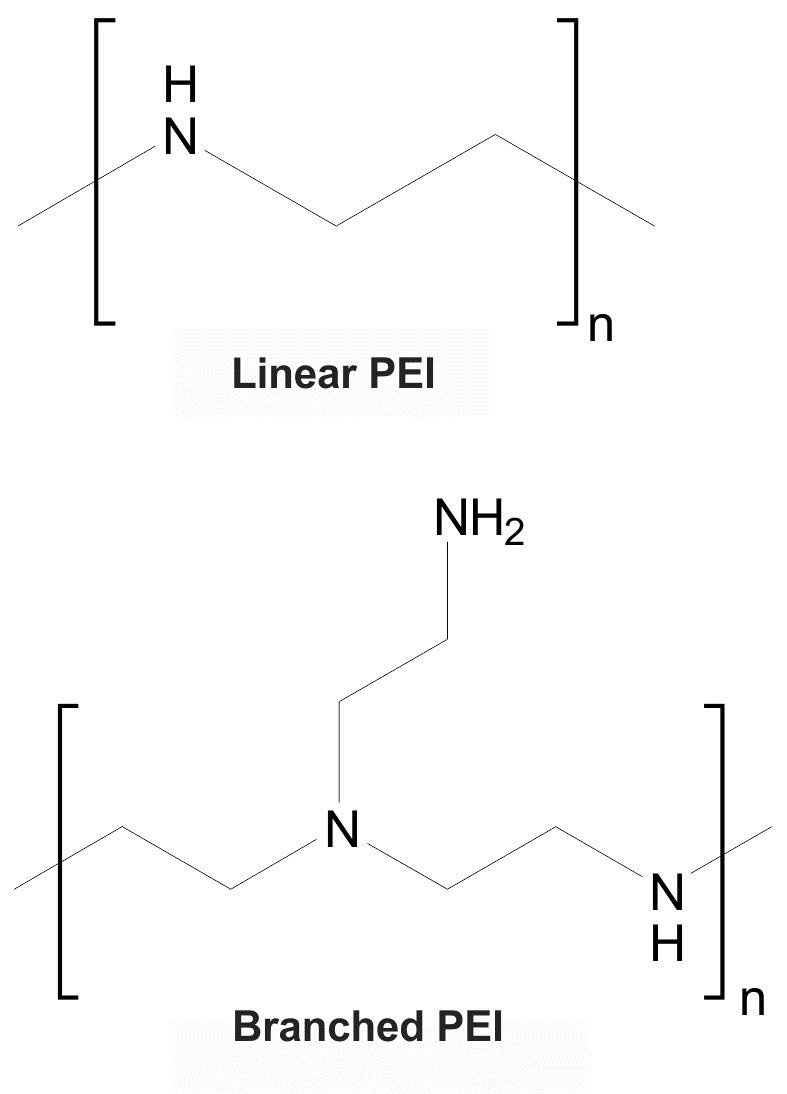
Chemical structures of the linear and branched polyethylenimine (PEI).
Based on physicochemical properties, LPEI and BPEIpossess protonable amine groupsthat indicate the capability of these polymers for DNA condensation and formation of polyplexes.
Fluorescence microscopy
Fluorescently labeled ODN-PEI complexes were incubated with cells in serum-free medium, and the cell associated fluorescence and subcellular distributions were assessed by fluorescent microscopy. DAPI was used to stain the nucleus. Polycationic PEI (in particular BPEI) improved the cellular association of f-ODN in A431 cells (Fig. 2).
Fig. 2 .
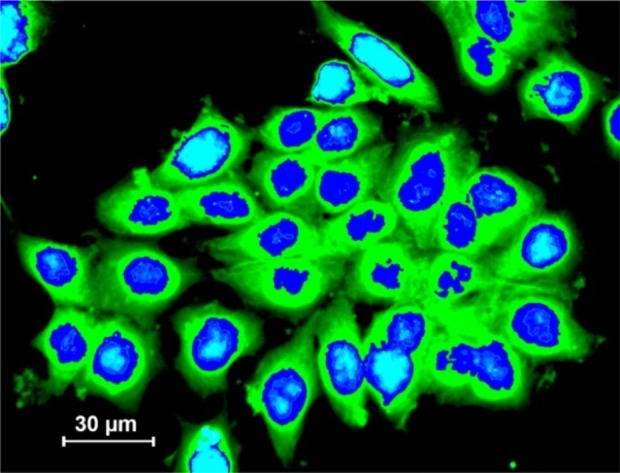
Fluorescent microscopy of transfected A431 cells with branchedpolyethylenimine (BPEI)-AsODNnanopolyplexes (green). Blue color represents nucleus.
The cellular uptake of the naked f-ODN appeared to be very low (data not shown).
MTT survival assay, zeta potential and size of polyplexes
MTT survival assay was undertaken to assess the cytotoxicity potential of the PEIpolymers (alone or as complexed with DNA) within human epithelial A431 cells.
Upon treatments with both LPEI and BPEI, A431 cells showed cytotoxicity that appeared to be largely dependent on concentration andpolymer structure (Fig. 3).
Fig. 3 .
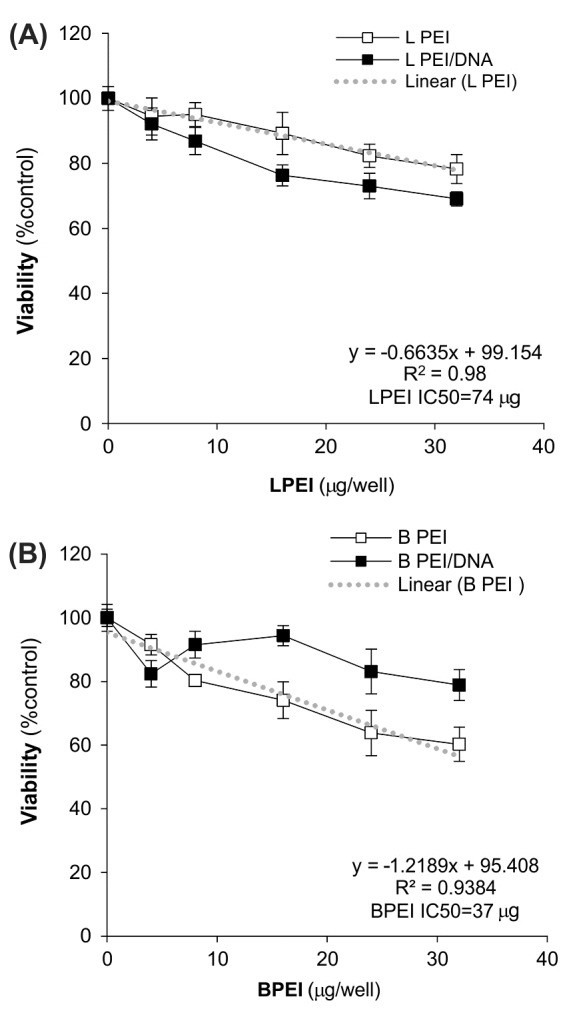
MTT survival assay in A431 cells treated with PEI polymers. A) A431 cells treated with linear polyethylenimine (LPEI) alone or as complexed with DNA (LPEI/DNA). B) A431 cells treated with branched polyethylenimine (BPEI) alone or as complexed with DNA (BPEI/DNA).
The BPEI resulted in higher toxicity than the LPEI within the treated cells.The BPEI:DNA polyplexes displayed significantly reduced cytotoxicity as compared to BPEI alone, however the LPEI:DNA polyplexes showed greater cellular toxicity in comparison with LPEI itself. As shown in Fig. 3, the IC50 for LPEI and BPEI were 74 mg and 37 mg, respectively.
To find possible correlation between surface zeta potential of and cytotoxicity induced by PEIpolymers, the zeta potential of these polymers (alone or as complexed with DNA) were measured. LPEI and BPEI yielded zeta potential of 15.7 ± 4.9 (mV) and 28.7 ± 7.9 (mV), respectively. However, once complexed with DNA, the zeta potential values were significantly (p<0.05) dropped down to approximately 4.9 ± 1.2 (mV) and 14.3 ± 2.1 (mV), respectively. The PEI:DNA polyplexes revealed a particle size distribution that provided mean diameters approximately 80–235 nm.
Apoptosis and DNA fragmentation analyses
To validate the results obtained by using MTT assay, we also exploited annexin V-FITC flow cytometry for detection of early and late apoptosis or even necrosis.
Fig. 4 represents the annexin V-FITC flow cytometry analyses for the untreated control cells (panel A), the treated cells with BPEI (panel B), LPEI (panel C) and 5% DMSO (positive inducer of apoptosis). Treated cells showed significant increases (P < 0.05) in the proportion of cells entering early and/or late apoptotic stages, in which BPEI apoptotic impacts were significantly greater than LPEI’.
Fig. 4 .
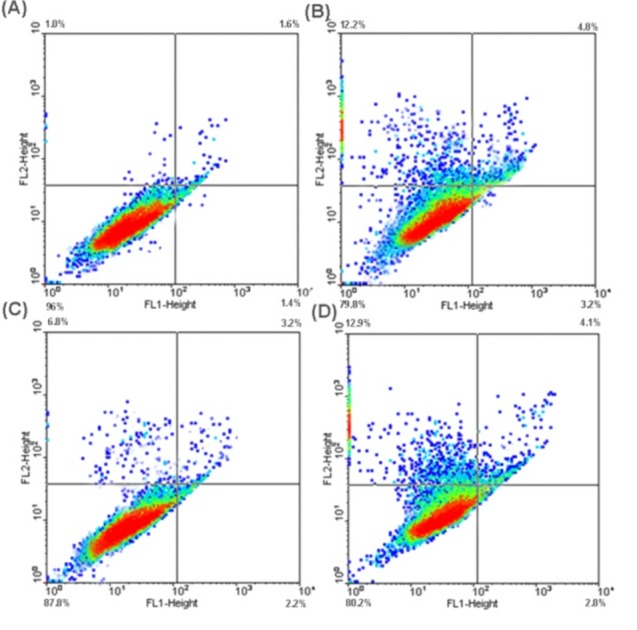
FITC-labeled annexin V flow cytometric detection of apoptosis in A431 cells. A) Untreated control cells. B) Treated cells with branched polyethylenimine (BPEI). C)Treated cells with linear polyethylenimine (LPEI). D) The treated cells with5% DMSO (positive inducer of apoptosis).
To assess the degree of possible DNA fragmentation within treated cells with polymers alone or as complexed with DNA, the widely used single cell electrophoresis (comet assay) was exploited.
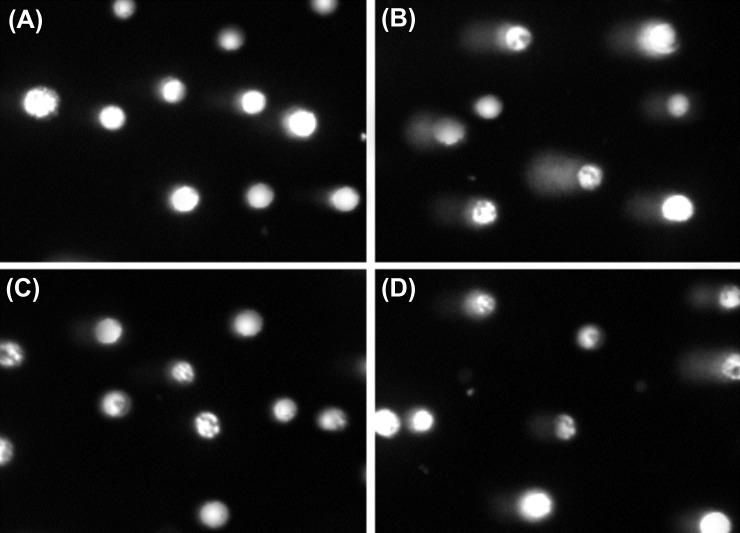
Fig. 5 shows the typical results obtained by means of the comet assay for A431 cells treated with PEI polymers and their polyplexes as well as the hydrogen peroxide, H2O2 (as a positive control known to induce DNA damage).
Fig. 5.
Comet assayfor detection of DNA damage in A431 cells. A) Untreated cells (control). B) Treated cells with hydrogen peroxide, H2O2 (positive control). C) Treated cells with linear polyethylenimine (LPEI).D) Treated cells with branched polyethylenimine (BPEI).
No diffusion of fragmented DNA was observed within untreated A431 cells (Fig. 5A) or those treated with linear PEI polymers (Fig. 5C), however those treated with BPEI (Fig. 5B) or the hydrogen peroxide (Fig. 5D) showed DNA fragmentation to some extent. Statistical analysis confirmed insignificant differences (P > 0.05) between untreated and LPEI or BPEI treated A431 (data not shown).
Western blot analyses
Western blot analysis was further undertaken to substantiate such a cell-dependent effect of PEI polymers on EGFR and Akt1 expression (as one of the major signalling pathway for apoptosis) in the treated cells. Fig. 6 demonstrates protein expressions of EGFR and Akt1 in the A431 cells upon treatment with PEI polymers.
Fig. 6 .
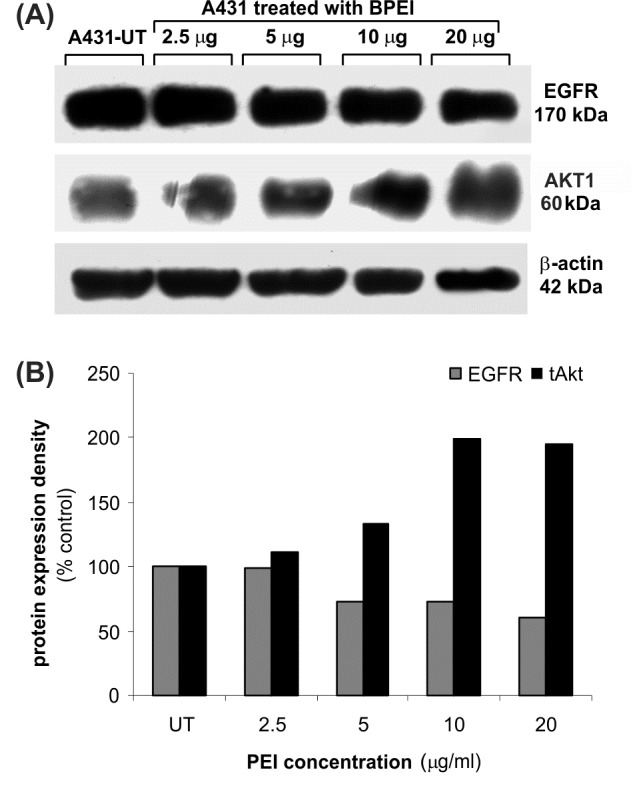
Western blot analysis of EGFR and Akt1 in the A431 cells upon treatment with PEI polymers. A) Protein expression. B) Density percentage.
No significant change in protein expression of EGFR was seen in A431 cells upon treatments with BPEI polymers, while the expression of Akt1 kinase was significantly increased.
Discussion
The immunological and toxicological concerns often limit use of viral vectors for ex vivo and in vivo gene therapy in spite of their high transfection efficiencies. As safer alternatives, non-viral vectors in particular polycationic lipids and polymers have been widely used to transfer genome based therapeutics. The polycations form nanostructures of polyplexe or liposome and entrap DNA through complexation with and condensation of genome-based therapeutics.
Among gene delivery systems, cationic dendrimers have been hugely exploited to deliver antisense, DNA and genes in mammalian cells. But, surprisingly, little information is available in the literature in terms of their genocompatibility and toxicogenomics, of which the extent and/or the nature of undesired gene expression changes that can also inevitably promote some pharmacological consequences. This may also alternatively initiate some gene-based diseases as we previously showed that the non-specific gene expression changes induced by cationic lipids within epithelial A431 cells can direct cells toward apoptosis.The acid-catalyzed polymerization ofaziridine has resulted in formation of PEI, by which highly branched network with ahigh cationic charge-density potential can be yielded. Such cationic structure is deemed to significantly interact with the negatively charged DNA. Since introduction of PEI as a versatile vector for gene therapy in 1995, it has been used as polymeric vector for gene delivery(Boussif et al. 1995). PEI can tightly condense plasmid DNA and is able to promote transgene deliveryto the nucleus of mammalian cells. A merely mechanisticstudy has shown that cationic lipid-DNA complexescan dock and interact with proteoglycans expressedon the surface of mammalian cells (Wiethoff et al. 2001), promotingtheir intracellular uptake, a possible route that might alsobe followed by PEI/DNA complexes.
The high transfectionefficiency of PEI in vitro has been ascribed to itsability to act as a proton sponge that buffers the low pHin the endolysosomal compartments and potentially inducesruptures of the endolysosomal membrane, resultingin the release of PEI/DNA complex into the cytoplasma.Recently, however, their usefulness is tarnished because of less efficiency and importantly their potential to induce cellular toxicity. And accordingly, various modifications have been performed to maximize the transfection efficiency and minimize the undesired adverse subsequent effects. For example, to improvetransfection efficiency of polyethylenimine-based non-viral DNA vectors, dendrimeric vectors were designed possessing an outer oligocation shell, a hydrophobic C-16 alkyl shell, a polycationic core and an acid-cleavable linker (Steele and Shier 2010). It was shown that acid-cleavable linkers to attach an outer shell of short, highly-charged oligocations to a PEI-based dendrimeric vector can substantially increase transfection efficiency without increasing cytotoxicity. Despite high capacity for shuttling of genes in target cells (Fig. 2), the PEI itself can exert significant cellular toxicity so that the IC50 for LPEI and BPEI respectively were 74 mg and 37 mg (Fig. 3). To evaluate transfection efficiency and safety of BPEI for gene delivery, Yoon et al.compared the sonoporation technique with BPEI using CHO, HEK293, and NIH3T3 cells. They showed that transfection efficiency of sonoporation (at 1 MHz intensity) was enhanced by microbubble concentration with no detrimental effects while the BPEI exacerbated cell viability (Yoon et al. 2008).
It has been shownthat, upon entry into the cytoplasm, naked plasmid DNA (pDNA) undergoes a rapid turnover because of degradationby cytosolic nucleases (Lechardeur and Lukacs 2006). Besides, following the internalization of the DNA-polycation complex by endocytosis, a large fraction is targeted to the lysosomal compartment by default. Since the cytosolic release of heterelogous DNA is a prerequisite for nuclear translocation, entrapment and degradation of plasmid DNA in endo-lysosomes constitute a major impediment to efficient gene transfer. This means only a small fraction of internalized plasmid DNA are able penetrate into the cytoplasm, while nuclear translocation of DNA requires either the disassembly of the nuclear envelope or active nuclear transport via the NPC that need an external vehicle such as PEI. Interestingly, Moretet al have shownthat PEI is able to protect pDNA against degradation byserum DNases (Moret et al. 2001). Despite the proton-sponge effect andthe ability to deliver DNA to the nucleus enhance transgeneexpression, in the cellular nucleusPEI may interact with nuclear processes resulting in DNA damage and cell death.
Using FITC-labeledannexin V flow cytometry, we witnessed occurrence of early/late apoptosis and even necrosis within A431 cells treated with BPEI (Fig. 4). To pursue the mechanism behind such cytotoxicity using comet assay, significant fragmentation of DNA was observed in A431 cells treated with BPEI, but not LPEI (Fig. 5). We speculate that there is a possible correlation between cytotoxicity/DNA damage in the treated cells with the surface zeta potential of LPEI (15.7 ± 4.9 mV) and BPEI (28.7 ± 7.9 mV). Because of possessing branches, the BPEI can make a greater interaction with subcellular moieties than the LPEI. This, perhaps, can explain why the BPEI induce significantly greater impacts than the LPEI. Since we had previously experienced induction of human alveolar epithelial cell growth factor receptor (EGFR) and its downstream molecule Akt1by polypropylenimine diaminobutane (DAB) dendrimers in A549 cells (Omidi and Barar 2009), we also aimed to lookat the BPEI polymers impacts on these two important proteins. We found significant increase in protein expression of Akt1, but not EGFR, in A431 cells upon treatments with higher amount (> 10 mg) of BPEI (Fig. 6).It seems the cationic polymers induce Akt1 activity in a similar pattern even though the response of different cells are expected to be different as we observed that the A549 cells were affected by DAB dendrimers. However, the A431 cells treated with BPEI showed no significant changes in expression of these two proteins, perhaps because of the overexpression of these proteins inthe A431 cells.
To study the effect of acyl chain length on transfection efficiency and toxicity of PEI, Aravindan et alacylatedthe amino groups on the polymeric backboneto alter the protonation behavior and the hydrophilic/hydrophobic balance of the polymer. They showed that the acylation reduced the number of primary amines on the polymer and the surface charge, improving haemo-compatibility and reducing cytotoxicity, in which polymers with buffering capacities greater than 50% and less than 80% relative to PEI displayed higher transfection efficiencies than PEI itself. It appeared that such polymeric systems can interactbetter with the cell membrane because of their slightly higher lipophilicity and formed polyplexes which were less cytotoxic than polyplexes of acetic anhydride modified polymers (Aravindan et al. 2009). In fact, the undesired cytotoxic and genotoxic impacts of the PEI polymers have directed scientists to diminish the high cationic chargedensity of PEI to a magnitude that promotes DNA deliverybut decreases the adverse effects of PEI on cell viability (Dai et al. 2011; Forrest et al. 2003; Leclercq et al. 2000; Oskuee et al. 2009; Wen et al. 2009; Xiong et al. 2007).To optimize the polymer length and charge density of PEI analogues, Xiong et alsynthesized a series of PEI analogues with controlled molecular weight and charge density through grafting low-molecular-weight PEI800 (800 Da) to a polyaspartate peptide backbone of varying degrees of polymerization. Examining inflammation and apoptosis/necrosis in the liver and spleen of rodents 24h post-injection, they showed the optimized poly(aspartate-g-PEI800) polymer and PEI800 did not show tissue damage or apoptosis, but not PEI25k (Xiong et al. 2007). Combining the osmotic burst mechanism for lysing endocytotic vesicles with the lipid depletion mechanism is accomplishable via maintaining buffering capacity at the same time as adding a lipid-absorbing hydrophobic shell, upon which PEI transfection efficiency can be improved. This has led Ramazani’s research group to substitute various percentages of the primary amines of PEI with carboxylate-terminated short, moderate and long alkyl chains and showed that the alkylcarboxylate substitution of primary amines on PEI enhanced transfection efficiencies (5-fold) compared to PEI itself. These researchers concluded that an appropriate balance between cationic and hydrophobic regions of alkylated PEI yields the optimal nonviral vector with high transfection efficiency and low toxicity (Oskuee et al. 2009). Dai et al reported establishment of a ternary copolymer PEG-b-PLL-g-lPEI (PPI) using LPEI which was grafted onto the block copolymer (PPL) of poly(l-lysine) (PLL) and poly(ethylene glycol)(PEG). They showed that, given proper control of molecular composition, the copolymers demonstrated lower cytotoxicity, proton buffering capacity, ability to condense pDNA and mediate effective gene transfection in various cell lines. They showed high gene transfection efficiency using PPI with low cytotoxicity and biodegradability (Dai et al. 2011).
Conclusion
So far, most of the investigations have been based on modification of PEI polymers to obtain a gene delivery carrier with maximum transfection efficiency and minimum cytotoxicity. Surprisingly, little is known upon mechanism of cellular toxic effects of these cationic polymers. Thus we aimed to study the cellular toxicity of LPEI and BPEI (25 kDa) to find about the charge and structure impacts of these polymers. Based upon our findings, it can be deduced that LPEI is safer than BPEI even though its transfection is lower than BPEI. However, BPEI can induce greater cytotoxicity than LPEI. Despite induction of Akt-kinase pathway, treated cells with BPEI displayed DNA fragmentation.
Ethical Issues
None to be declared.
Conflict of interests
The authors declare no conflict of interests.
Acknowledgments
Authors are thankful to Professor SaghirAkhtar for his kind support and the Iranian Ministry of Health, Care and Medical Education for the financial support (grant No. 87/p/683).
References
- Aravindan L, Bicknell KA, Brooks G, Khutoryanskiy VV and Williams AC . 2009 Effect of acyl chain length on transfection efficiency and toxicity of polyethylenimine. Int J Pharm, 378(1-2), 201-210 [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. 1995 A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A, 92(16), 7297-7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conwell CC and Huang L . 2005 Recent advances in non-viral gene delivery. Adv Genet, 53, 3-18 [DOI] [PubMed] [Google Scholar]
- Dai J, Zou S, Pei Y, Cheng D, Ai H and Shuai X . 2011 Polyethylenimine-grafted copolymer of poly(l-lysine) and poly(ethylene glycol) for gene delivery. Biomaterials, 32(6), 1694-1705 [DOI] [PubMed] [Google Scholar]
- Davis ME . 2002 Non-viral gene delivery systems. Curr Opin Biotechnol, 13(2), 128-131 [DOI] [PubMed] [Google Scholar]
- Florea BI, Meaney C, Junginger HE and Borchard G . 2002 Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures. AAPS PharmSci, 4(3), E12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest ML, Koerber JT and Pack DW . 2003 A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjug Chem, 14(5), 934-940 [DOI] [PubMed] [Google Scholar]
- Hollins AJ, Omidi Y, Benter IF and Akhtar S . 2007 Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J Drug Target, 15(1), 83-88 [DOI] [PubMed] [Google Scholar]
- Lechardeur D and Lukacs GL . 2006 Nucleocytoplasmic transport of plasmid DNA: a perilous journey from the cytoplasm to the nucleus. Hum Gene Ther, 17(9), 882-889 [DOI] [PubMed] [Google Scholar]
- Leclercq F, Dubertret C, Pitard B, Scherman D and Herscovici J . 2000 Synthesis of glycosylated polyethylenimine with reduced toxicity and high transfecting efficiency. Bioorg Med Chem Lett, 10(11), 1233-1235 [DOI] [PubMed] [Google Scholar]
- Lungwitz U, Breunig M, Blunk T and Gopferich A . 2005 Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm, 60(2), 247-266 [DOI] [PubMed] [Google Scholar]
- Moret I, Esteban PJ, Guillem VM, Benet M, Revert F, Dasi F, et al. 2001 Stability of PEI-DNA and DOTAP-DNA complexes: effect of alkaline pH, heparin and serum. J Control Release, 76(1-2), 169-181 [DOI] [PubMed] [Google Scholar]
- Omidi Y and Barar J . 2009 Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol, 28(2), 113-122 [DOI] [PubMed] [Google Scholar]
- Omidi Y, Barar J and Akhtar S . 2005. a Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv, 2(4), 429-441 [DOI] [PubMed] [Google Scholar]
- Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA and Akhtar S . 2008 Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial A549 cells. Toxicol Mech Methods, 18(4), 369-378 [DOI] [PubMed] [Google Scholar]
- Omidi Y, Hollins AJ, Drayton RM and Akhtar S . 2005. b Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target, 13(7), 431-443 [DOI] [PubMed] [Google Scholar]
- Oskuee RK, Dehshahri A, Shier WT and Ramezani M . 2009 Alkylcarboxylate grafting to polyethylenimine: a simple approach to producing a DNA nanocarrier with low toxicity. J Gene Med, 11(10), 921-932 [DOI] [PubMed] [Google Scholar]
- Peng L, Jiang H and Bradely C . 2002 Detection of B lymphoma cells undergoing apoptosis by Annexin-V assay. Chin Med Sci J, 17(1), 17-21 [PubMed] [Google Scholar]
- Steele TW and Shier WT . 2010 Dendrimeric alkylated polyethylenimine nano-carriers with acid-cleavable outer cationic shells mediate improved transfection efficiency without increasing toxicity. Pharm Res, 27(4), 683-698 [DOI] [PubMed] [Google Scholar]
- Wen Y, Pan S, Luo X, Zhang X, Zhang W and Feng M . 2009 A biodegradable low molecular weight polyethylenimine derivative as low toxicity and efficient gene vector. Bioconjug Chem, 20(2), 322-332 [DOI] [PubMed] [Google Scholar]
- Wiethoff CM, Smith JG, Koe GS and Middaugh CR . 2001 The potential role of proteoglycans in cationic lipid-mediated gene delivery . Studies of the interaction of cationic lipid-DNA complexes with model glycosaminoglycans. J Biol Chem, 276(35), 32806-32813 [DOI] [PubMed] [Google Scholar]
- Xiong MP, Forrest ML, Ton G, Zhao A, Davies NM and Kwon GS . 2007 Poly(aspartate-g-PEI800), a polyethylenimine analogue of low toxicity and high transfection efficiency for gene delivery. Biomaterials, 28(32), 4889-4900 [DOI] [PubMed] [Google Scholar]
- Yoon CS, Jung HS, Kim TK, Kwon MJ, Kim MK, Lee M, et al. 2008 Comparison of the efficiency and toxicity of sonoporation with branched polyethylenimine-mediated gene transfection in various cultured cell lines. J Drug Target, 16(10), 773-779 [DOI] [PubMed] [Google Scholar]