Abstract
Rationale
Glutamate and orexin/hypocretin systems are involved in Pavlovian cue-triggered drug seeking.
Objectives
Here, we asked whether orexin and glutamate interact within ventral tegmental area (VTA) to promote reinstatement of extinguished cocaine seeking in a rat self-administration paradigm.
Methods/results
We first found that bilateral VTA micro-injections of the orexin 1 receptor (OX1R) antagonist SB-334867 (SB) or a cocktail of the AMPA and NMDA glutamate receptor antagonists CNQX/AP-5 reduced reinstatement of cocaine seeking elicited by cues. In contrast, neither of these microinjections nor systemic SB reduced cocaine-primed reinstatement. Additionally, unilateral VTA OX1R blockade combined with contralateral VTA glutamate blockade attenuated cue-induced reinstatement, indicating that VTA orexin and glutamate are simultaneously necessary for cue-induced reinstatement. We further probed the receptor specificity of glutamate actions in VTA, finding that CNQX, but not AP-5, dose-dependently attenuated cue-induced reinstatement, indicating that AMPA but not NMDA receptor transmission is required for this type of cocaine seeking. Given the necessary roles of both OX1 and AMPA receptors in VTA for cue-induced cocaine seeking, we hypothesized that these signaling pathways interact during this behavior. We found that PEPA, a positive allosteric modulator of AMPA receptors, completely reversed the SB-induced attenuation of reinstatement behavior. Intra-VTA PEPA alone did not alter cue-induced reinstatement, indicating that potentiating AMPA activity with this drug specifically compensates for OX1R blockade, rather than simply inducing or enhancing reinstatement itself.
Conclusions
These findings show that cue-induced, but not cocaine-primed, reinstatement of cocaine seeking is dependent upon orexin and AMPA receptor interactions in VTA.
Keywords: AMPA, Self-administration, Relapse, Addiction, Ventral tegmental area, Cocaine, Glutamate, Hypocretin, Conditioned, NMDA
Introduction
Conditioned cues are potent triggers of reward seeking in animals and increase relapse risk in abstinent human addicts (Cardinal and Everitt 2004; Robinson and Berridge 2001). Cues are associated with increased ventral tegmental area (VTA) dopamine cell firing, and concomitant forebrain dopamine release is necessary and sufficient for reward seeking and approach efforts (Berridge and Robinson 1998; Cheer et al. 2007; Fiorillo et al. 2003; Ikemoto and Panksepp 1999; Salamone et al. 2007; Satoh et al. 2003). Accordingly, VTA is required for cue-induced drug and food seeking (Di Ciano and Everitt 2004; Murschall and Hauber 2006), but the neuro-chemical inputs that modulate VTA involvement in conditioned reward seeking are unclear. Orexin and glutamate are two key transmitters involved in cue-induced reward seeking, and it is possible that they act in VTA to modulate this behavior.
Orexin/hypocretin is a hypothalamic peptide neurotransmitter involved in cue-triggered motivation. Lateral hypothalamic (LH) orexin neurons Fos-activate in relation to conditioned place preference (Aston-Jones et al. 2010; Harris et al. 2005), and systemic injections of the orexin 1 receptor (OX1R) antagonist SB-334867 (SB) attenuate cue-, context-, or stress-induced reward seeking for a variety of drugs of abuse and natural rewards (Boutrel et al. 2005; Cason et al. 2010; Harris et al. 2005; Lawrence et al. 2006; Richards et al. 2008; Smith and Aston-Jones in press; Smith et al. 2009, 2010).
In VTA, orexin increases dopamine cell activity and modulates certain reward-seeking behaviors (Espana et al. 2011; Korotkova et al. 2003; Moorman and Aston-Jones 2010; Muschamp et al. 2007; Narita et al. 2006, 2007). Intra-VTA microinjections of orexin reinstate extinguished drug seeking (Harris et al. 2005; Wang et al. 2009), whereas intra-VTA SB attenuates some types of drug-seeking behaviors (Borgland et al. 2006; Espana et al. 2010; Harris et al. 2007; James et al. 2011; Narita et al. 2006). These findings indicate that VTA orexin may be involved in learning about and pursuing drugs, especially in response to external stimuli such as Pavlovian cues.
Similar to orexin, glutamate in VTA promotes dopamine cell firing and modulates reward-seeking behavior (Chergui et al. 1993; Geisler et al. 2008; Harris and Aston-Jones 2003; Wang et al. 1994; Zellner and Ranaldi 2010). AMPA/NMDA ratios are increased following cocaine exposure, and changes in AMPA subunits, in particular, modulate motivation to self-administer cocaine (Borgland et al. 2004; Choi et al. 2011; Saal et al. 2003; Ungless et al. 2001; White et al. 1995; Zhang et al. 1997). However, the role played by VTA glutamate in discrete cue-induced relapse to drug seeking has not been well studied. VTA glutamate neurotransmission is necessary for stress-induced reinstatement, as well as cocaine seeking under extinction conditions (Wang et al. 2005; Wise 2009; You et al. 2007) [though see (Nolan et al. 2010)]. However, as cue-induced reinstatement of extinguished cocaine seeking involves different neural substrates than stress-induced reinstatement or extinction responding (Fuchs et al. 2006; Kalivas 2008; Peters et al. 2008; Shaham et al. 2003), it is important to examine whether VTA glutamate is similarly involved in cue-triggered reinstatement as well.
Recent studies have demonstrated that orexin and glutamate interact at the synaptic level in VTA, and that orexin facilitates glutamate actions on dopamine cell firing. In vitro, orexin potentiates VTA glutamate signaling by increasing the number and function of postsynaptic AMPA and NMDA receptors, as well as glutamate release (Borgland et al. 2009; 2006). In anesthetized animals, orexin also potentiates control of VTA dopamine neurons by prefrontal cortical afferents, further indicating that orexin can amplify concurrent glutamate signaling (Moorman and Aston-Jones 2010). However, the degree to which these transmitters interact during goal-directed behaviors has not been explored.
Here, we hypothesized that an interaction between orexin and glutamate in VTA mediates cue-triggered cocaine seeking in a self-administration/reinstatement model of relapse. We found that OX1R and AMPA transmission in VTA are simultaneously necessary for cues, but not cocaine, to trigger drug seeking. We also show that when VTA OX1R transmission is antagonized, facilitating endogenous AMPA transmission with the positive allosteric modulator PEPA restores cue-induced reinstatement behavior to control levels. These findings point to a synergistic interaction of VTA OX1 and AMPA receptor signaling that is required for drug-associated stimuli to trigger relapse in addiction.
Methods
Subjects
Male Sprague Dawley rats (n=141; arrival weight 250–300 g; Charles River) were single- or double-housed under a reverse 12:12 h light cycle in a standard tub cage with corncob bedding and ad lib food and water. All procedures were approved by the Medical University of South Carolina’s Institutional Animal Care and Use Committee and conducted according to specifications of the Guide for the Care and Use of Laboratory Animals (2011).
Surgery
Intracranial cannulae
Animals were anesthetized with ketamine/xylazine (56.5/8.7 mg/kg) and received the non-opioid analgesic meloxicam (1 mg/kg). Bilateral 23-ga stainless steel guide cannulae were implanted 2 mm above VTA (n=76) or nearby control sites (n=39) with a stereotaxic device (coordinates for VTA (relative to bregma): AP=−5.2 to −6.2; ML=+2.0 to 2.2;DV=−7.0 to −7.2). Cannulae were affixed to the skull with screws and dental cement and occluded with 28-ga stainless steel stylets.
Jugular catheters
In the same surgery, chronic indwelling catheters were inserted into the right jugular vein and exited the body via a port between the scapulae. Animals received prophylactic i.v. cefazolin (10 mg) and heparin (10 U) daily starting 3 days after surgery and continuing throughout self-administration. Rats receiving systemic SB (n=26) received jugular catheters only. Animals were allowed to recover for 1 week prior to self-administration training.
Drugs
Cocaine
Cocaine HCl (NIDA, Research Triangle Park, NC) was dissolved in 0.9% sterile saline.
Orexin antagonist
For systemic administration, the OX1R antagonist SB-334867 [SB; purchased from Tocris (Ellisville, MO), or generously donated by Eli Lilly (Indianapolis, IN)] was suspended in 2% dimethylsulfoxide (DMSO) and 10% 2-hydroxypropyl-beta-cyclodextrin (Sigma; St. Louis, MO) in sterile water; all doses were given in a volume of 4 ml/kg (i.p.). A 30-mg/kg SB dose was chosen based upon our previous finding that this dose abolished cue-induced reinstatement (Smith et al. 2009). For intracranial microinjections, SB was obtained from Tocris and suspended in artificial cerebrospinal fluid with agitation and sonification (ACSF; 1 mM). This concentration of SB was chosen as a relatively low dose based on previous intracranial injection studies (Borgland et al. 2006; Espana et al. 2010; Harris et al. 2007; James et al. 2011). When SB was compared to or mixed with PEPA, 2% DMSO was added to drug and vehicle preparations.
Glutamate antagonists
The AMPA antagonist CNQX (0.7 and 0.35 mM) and the NMDA antagonist AP-5 (1.6 and 0.8 mM) were obtained from Sigma and dissolved in ACSF. These doses were chosen based on previous VTA microinjection studies, and they are about 10× higher concentrations than those used for in vivo electrophysiology in anesthetized preparations and over 100× higher concentrations than used in slice physiology experiments (Georges and Aston-Jones 2002; Harris and Aston-Jones 2003; Harris et al. 2004; Marinelli et al. 2005; Riegel and French 2002). The higher dose of each antagonist was used for the CNQX/AP-5 cocktail.
AMPA allosteric enhancer
PEPA (Tocris) enhances glutamate binding at AMPA recepetors and decreases AMPA desensitization, potentiating functional effects of endogenous glutamate release in vitro and in vivo (Ahmed et al. 2010; LaLumiere et al. 2010; Sekiguchi et al. 1997; Yamada et al. 2009). PEPA was dissolved in 2%DMSO and diluted with ACSF to 0.25 or 0.5 mM, less than 30 min prior to microinjection. These doses were chosen based on previous microinjection studies using PEPA (LaLumiere et al. 2010; Zushida et al. 2007). For PEPA+SB microinjections, both drugs were mixed into a cocktail shortly before microinjection.
Handling and microinjections
Animals were handled for 5 min each day for at least 3 days prior to beginning self-administration training. Before the first microinjection, an injector extending 2 mm past the end of the cannula into the brain structure of interest was inserted to habituate animals to microinjection procedures. Five minutes prior to behavioral testing, microinjections were administered through a 28-ga injector (2mmpast guide cannulae) in a 0.3-μl volume over 70 s by automated syringe pump, and injectors were left in place for 1 additional min to allow diffusion away from injection site. No animal received more than five total microinjections in any experiment.
Behavioral training
Med-Associates experimental chambers (St. Albans, VT) were equipped with two retractable levers with white lights above them, a red houselight, and a tone generator. Intravenous cocaine was administered via a pump located outside a sound-attenuating box. Rats received 10 daily 2-h cocaine self-administration sessions (>10 cocaine infusions/day, 0.2 mg/50 μl infusion). Pressing one lever (active lever; fixed ratio 1 schedule) yielded a 3.6-s cocaine infusion, 2.9-kHz tone, and light presented above the active lever. A 20-s timeout period (signaled by turning off the houselight) followed, during which additional lever presses yielded nothing. Presses on the other lever (inactive lever) had no consequences at any time. By the end of self-administration, animals received 35.7±1.3 (mean±SEM) cocaine infusions/day. No differences in total cocaine intake between experimental groups or VTA vs. control site cannula groups were found.
Animals next received at least 7 days of extinction training, during which active lever presses yielded neither cocaine nor tone/light cues. On the first day of extinction, active and inactive lever presses increased over self-administration levels (active lever: t63=24.2; inactive lever: t63=6.8, ps<0.001), but pressing decreased significantly over the next 6 days of extinction training (Fig. 1). Rats received extinction training until they pressed <25 times on the active lever for 2 or more consecutive days. Between cue-induced or cocaine-primed reinstatement sessions, animals received at least 2 additional extinction training days, until they returned to extinction criterion. No differences in the number of extinction days to criterion were found between animals with VTA or control site cannulae or between experimental groups.
Fig. 1.
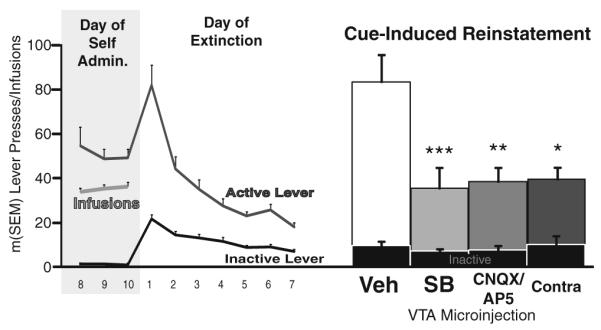
Simultaneous VTA orexin and ionotropic glutamate transmission is necessary for cue-induced reinstatement of cocaine seeking. Day of self-admin: lever presses (active and inactive) and cocaine infusions (with cue presentations) are shown for days 8–10 of self-administration. Day of extinction: lever presses during the first 7 days of extinction (no cocaine, no cues) are shown. Cue-induced reinstatement: cue-induced reinstatement behavior is shown after VTA microinjections of bilateral vehicle (Veh), bilateral orexin blockade with SB-334867 (SB; 1 mM), bilateral glutamate blockade with a microinjection of a cocktail of the NMDA/AMPA antagonists CNQX/AP-5 (0.7 and 1.6 mM), or unilateral VTA SB with simultaneous contralateral VTA CNQX/AP-5 cocktail (Contra). Inactive lever presses on reinstatement days are shown with black bars. *p<0.05; **p<0.01; ***p<0.001; significant difference from vehicle reinstatement day
Reinstatement testing
Cue-induced reinstatement
Animals received three cue-induced reinstatement tests after reaching extinction criterion. During these 2-h sessions, active lever presses yielded the tone/light presentations previously paired with cocaine, but no cocaine. As in self-administration training, each cue presentation was followed by a 20-s timeout period where lever presses were recorded, but yielded no additional cue presentations. Cue-induced reinstatement testing was completed first, followed by cocaine-primed reinstatement testing, in order to prevent potential effects of non-contingent cocaine injections on cue-induced reinstatement behavior (Feltenstein et al. 2009; Kippin et al. 2006; Rogers et al. 2008; See 2009).
Cocaine-primed reinstatement
Animals were injected with cocaine (10 mg/kg; i.p.) immediately prior to being placed in the experimental chamber for a 2-h session. Active and inactive lever presses were recorded, but did not result in cue presentations or cocaine.
Experimental manipulations
Effects of orexin and glutamate antagonists on cue-induced reinstatement
Five min prior to each test, rats received bilateral microinjections (in counterbalanced order) of 0.3-μl ACSF vehicle and two of the following: bilateral SB (1 mM; VTA n=23; control sites n=17), bilateral CNQX/AP-5 cocktail (0.7/1.6mM; VTA n=22; control sites n=14) and unilateral SB+contralateral CNQX/AP-5 (VTA n=16; control sites n=10). All rats received three total cue-induced reinstatement sessions. To examine the relative contributions of VTA NMDA vs. AMPA receptors in cue-induced reinstatement, separate groups of animals received vehicle either CNQX (0.7 and 0.35 mM; VTA n=7) or AP-5 (1.6 and 0.8 mM; VTA n=9; control site n=1) in counterbalanced order prior to cue-induced reinstatement tests.
Effects of orexin and glutamate antagonists on cocaine-primed reinstatement
A subset of the animals tested for cue-induced reinstatement also received cocaine-primed reinstatement tests after bilateral microinjections (in counterbalanced order) of vehicle and one of the following (at the same concentrations and volumes as previously discussed): bilateral SB (VTA n=15, control sites n=14), bilateral cocktail of CNQX/AP-5 (VTA n=15, control sites n=4), bilateral CNQX alone (VTA n=4), and bilateral AP-5 alone (VTA n=4; control sites n=1). No animals received more than five intra-VTA microinjections in total.
Systemic SB effects on cocaine-primed reinstatement
Vehicle or SB (10 mg/kg, n=9; or 30 mg/kg, n=17) was injected (i.p.) in counterbalanced order 30 min prior to each of two cocaine priming reinstatement test sessions per animal.
Effects of SB and PEPA on cued reinstatement
A separate group of animals was assigned to receive, in counterbalanced order, 0.3 μl of 2% DMSO ACSF vehicle and two of the following prior to cue reinstatement tests: SB alone (1 mM; VTA n=20; control sites n=10), a cocktail of 1 mM SB+0.25 mM PEPA (VTA n=6; control sites n=8), 1 mM SB+0.5 mM PEPA (VTA n=18; control sites n=8), and 0.5 mM PEPA alone (VTA n=13; control sites n=3). Some animals also received vehicle and 0.5 mM PEPA alone under extinction conditions (VTA n=9; control sites n=6).
Histology
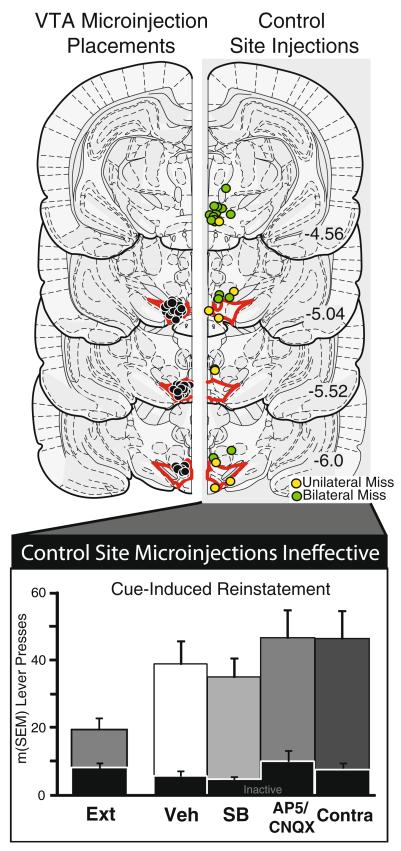
Following behavioral testing, animals were deeply anesthetized with ketamine/xylazine, and brains were removed, frozen, sectioned at 60 μm on a cryostat, and counterstained with neutral red. Cannula sites in and around VTA were compared with Paxinos and Watson (2007) to reconstruct microinjection sites (Fig. 5). Animals with both microinjection sites within the borders of VTA were considered hits, and animals with one or both injection sites in adjacent brain regions were considered control site injections.
Fig. 5.
Control site microinjections are ineffective. VTA microinjection placements (left): microinjection sites for animals with bilateral VTA cannulae (black dots) are represented. Control site injections (right): microinjection sites outside the borders of the VTA are represented, from animals with unilateral (yellow/light gray dots) or bilateral (green/dark gray dots) cannula placements in nearby structures. For VTA and control site animals, bilateral placements were collapsed into a unilateral view for presentation [adapted from Paxinos and Watson (2007)]. Control site microinjections ineffective: cue-triggered reinstatement data for animals with unilateral or bilateral cannula placements outside the VTA are shown, after microinjections of vehicle (Veh), SB-334867 (SB; 1 mM), CNQX/AP-5 (0.7/1.6 mM), or contralateral SB and CNQX/AP-5 (Contra). Inactive lever presses are shown with black bars
Statistics
Reinstatement after vehicle microinjections was verified with paired sample t-tests comparing the average of the last 2 days of extinction pressing to pressing on vehicle reinstatement days. Mixed model ANOVAs tested effects of drug (between-subjects factor) on active and inactive lever pressing (within-subject factor) to examine effects on reinstatement of intra-VTA: (a) vehicle, (b) bilateral SB (1 mM), (c) bilateral AP-5/CNQX (0.7/1.6 mM), and (d) unilateral SB (1 mM) with simultaneous contralateral AP-5/CNQX (0.1/1.6 mM) (Fig. 1). Effects of PEPA on reinstatement, with or without concurrent SB, were also tested with a mixed ANOVA, including a between-subjects factor of drug (vehicle, SB alone, SB+0.25 mM PEPA, SB+0.5 mM PEPA, 0.5 mM PEPA alone), and the within-subjects factor of lever (active, inactive). Fisher’s least significant difference posthoc tests were used to compare cocaine seeking after each drug to vehicle. Mixed ANOVAs were used because animals received three cue-induced reinstatement sessions (of the four or five possible conditions, depending on the experiment), which precluded the use of repeated-measures ANOVA to analyze across all conditions. Effects of PEPA on extinction responding were tested with ANOVAs, including two within-subjects factors: drug (vehicle vs. PEPA) and lever (active, inactive). Repeated-measures ANOVAs (two within-subjects factors: drug×lever) were also used to examine the effects of intra-VTA CNQX (0, 0.35, and 0.7 mM) or AP-5 (0, 0.8, and 1.6 mM) on cue-induced reinstatement. For repeated-measures ANOVAs, Bonferroni-corrected t-test posthocs were employed. To examine effects of systemic SB on cue-induced reinstatement, a mixed model ANOVA tested effects of drug (0 and 10 or 30 mg/kg; between-subjects factor) on active and inactive lever pressing (within-subjects factor).
Results
Simultaneous VTA orexin and glutamate transmission is necessary for cue-induced reinstatement
After vehicle microinjections into VTA animals robustly reinstated active lever, but not inactive lever, pressing for cocaine cues compared to the average of the last 2 extinction training days (Fig. 1; active lever: t34=6.2, p<0.001; inactive lever: n.s.). However, VTA microinjections of bilateral SB, bilateral CNQX/AP-5, or contrahemispheric SB and CNQX/AP-5 all reduced active lever pressing to less than half of vehicle reinstatement levels (Fig. 1; drug×lever interaction: F(3,92)=6.6, p<0.001; LSD posthocs for drug effects—veh vs.: SB: p<0.001; CNQX/AP-5: p<0.01; contralateral CNQX/AP-5 and SB: p=0.02). In contrast, inactive lever presses were not significantly affected by drug microinjections compared to vehicle day.
Neither VTA OX1R nor glutamate receptor signaling is necessary for cocaine-primed reinstatement
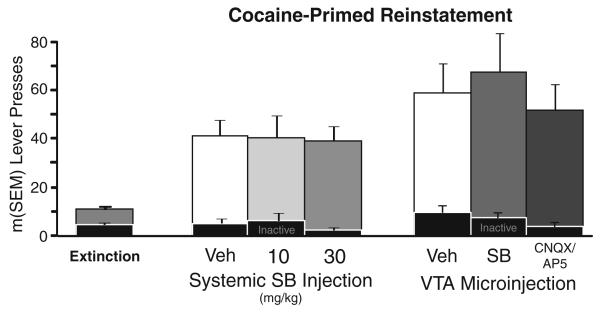
In vehicle-treated animals, cocaine priming injections robustly reinstated pressing on the active lever, but not the inactive lever compared to prior extinction days (active lever: t22=5.5, p<0.001, inactive lever: n.s.). Systemic administration of SB at 10 or 30 mg/kg did not affect cocaine-primed reinstatement of pressing on either the active or inactive lever (Fig. 2), whereas previous studies found that 30-mg/kg SB completely blocked cue-induced reinstatement of cocaine seeking (Smith et al. 2009).
Fig. 2.
Neither VTA orexin nor ionotropic glutamate transmission is necessary for cocaine-primed reinstatement. Extinction (left bar): the average of the last 2 days of extinction responding immediately before reinstatement tests is shown for comparison with cocaine-primed reinstatement. Systemic SB injection: cocaine-primed reinstatement behavior is shown after systemic injections of vehicle or SB-334867 (10 or 30 mg/kg, i.p.). VTA microinjection: cocaine-primed reinstatement behaviors are shown after bilateral intra-VTA SB (1mM) or CNQX/AP-5 (0.7/1.6 mM). Inactive lever presses on reinstatement test days are shown with black bars
Similarly, intra-VTA microinjections of neither SB nor a CNQX/AP-5 cocktail affected cocaine-primed reinstatement (Fig. 2), though the same doses of these antagonists attenuated cue-induced reinstatement (Fig. 1). Inactive lever presses were also unaffected by bilateral intra-VTA orexin or glutamate antagonists after a cocaine prime (Fig. 2). Together, these results reveal specificity for VTA orexin and glutamate involvement in cue-induced vs. cocaine-primed reinstatement.
VTA AMPA, but not NMDA, transmission is necessary for cue-induced reinstatement
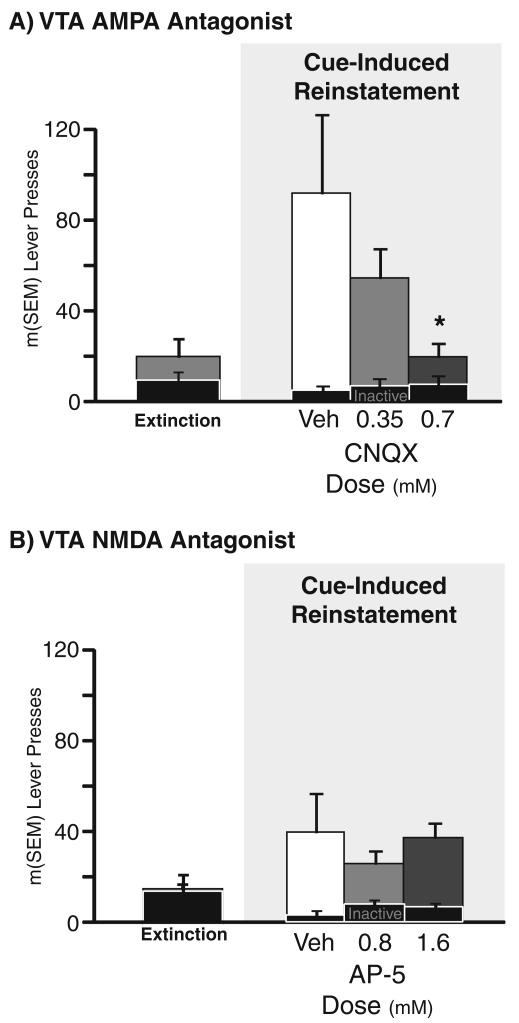
Next, we compared effects of AMPA vs. NMDA antagonists on cue-induced reinstatement (Fig. 3). The AMPA antagonist CNQX dose-dependently attenuated cue-induced reinstatement, as compared to vehicle (drug×lever interaction: F(2,12)=4.7, p<0.05; 0.7 mM: t6=2.5, p<0.05; 0.35 mM: n.s.). In contrast, the NMDA antagonist AP-5 did not affect cue-induced reinstatement at either dose. Neither 0.7 mM CNQX nor 1.6 mM AP-5 affected cocaine-primed reinstatement in a small subset of animals, as expected given the lack of effect of the drug cocktail on cocaine-primed reinstatement. These findings indicate that simultaneous AMPA and OX1R transmission in VTA are required for cues to trigger maximum reinstatement of cocaine seeking.
Fig. 3.
AMPA but not NMDA receptor transmission in VTA is required for cue-induced reinstatement. a VTA AMPA antagonist: cueinduced reinstatement is shown after bilateral VTA microinjections of the AMPA antagonist CNQX (0.7 and 0.35 mM). b VTA NMDA antagonist: cue-induced reinstatement behavior is shown after bilateral VTA microinjections of the NMDA antagonist AP-5 (1.6 and 0.8 mM). Pressing in late extinction is shown at the left in both panels for comparison. Inactive lever presses on reinstatement days are shown with black bars. *Different from vehicle reinstatement day, p<0.05
Potentiation of endogenous glutamate signaling at AMPA receptors compensates for orexin antagonism and restores reinstatement
In a separate group of animals, we asked whether potentiating endogenous glutamate signaling via administration of the allosteric AMPA receptor enhancer PEPA would overcome SB-induced attenuation of cue-induced reinstatement (Fig. 4). Again, we found cue-induced reinstatement of cocaine seeking following VTA vehicle injections, as compared to extinction levels (t23=4.6, p<0.001). Bilateral VTA SB (1 mM) once again reduced reinstatement to half of vehicle levels, but co-administration of 0.5 mM (but not 0.25 mM) PEPA with SB in a cocktail microinjection rescued cue-induced reinstatement to vehicle levels (drug×lever interaction: F(3,64)=4.7, p<0.01, veh vs. SB: p<0.01; SB alone vs. SB+0.5-mM PEPA: p<0.05; SB alone vs. SB+0.25-mM PEPA: n.s.; Fig. 4). Reinstatement was not statistically different after vehicle or SB+0.5-mM PEPA microinjections, demonstrating a complete reversal of intra-VTA SB effects. PEPA alone did not potentiate cue-induced reinstatement over vehicle levels (vehicle vs. 0.5-mMPEPA: n.s.). In addition, the recovery of SB-attenuated reinstatement by 0.5-mM PEPA was not due to a simple additive increase in cocaine seeking, as PEPA alone did not drive reinstatement when given in an extinction test (no main effect of drug or drug×lever interaction). These findings point to a functional interaction of orexin and glutamate inputs in VTA in triggering motivation specifically in response to drug-associated cues.
Fig. 4.
Enhancing endogenous glutamate signaling at VTA AMPA receptors reverses attenuation of reinstatement by OX1R blockade. Extinction: effects of bilateral intra-VTA microinjections of the allosteric AMPA enhancer PEPA (0.5 mM) during late extinction, in the absence of cue presentations. Cue-induced reinstatement: cue-induced reinstatement behavior is shown after bilateral intra-VTA microinjections of vehicle (Veh), SB-334867 (SB; 1 mM), PEPA(0.5 and 0.25 mM) co-administered with SB (1 mM), and PEPA without SB (Veh+0.5-mM PEPA). **p<0.01; significant difference from vehicle reinstatement day
VTA is a critical site for orexin and glutamate promotion of reinstatement
In contrast to the reliable attenuation of cue-induced reinstatement observed with bilateral SB, CNQX, or contrahemispheric SB and glutamate antagonist microinjections into VTA, similar unilateral or bilateral microinjections outside VTA into adjacent structures such as the caudal lateral hypothalamus (n=8), substantia nigra (n=4), mammillary nucleus (n=5), or red nucleus (n=9) were ineffective (Fig. 5, bilateral extra-VTA placements represented with green/dark gray dots). Similarly, PEPA did not significantly affect reinstatement when injected outside VTA, with or without SB co-injection. No consistent differences in drug effects at the different individual control sites were observed. In animals with a unilateral VTA cannula (and the other cannula in a nearby control structure; n=18), microinjected drugs also had no significant effects on cued reinstatement (Fig. 5, yellow/light gray dots; no effect of SB, CNQX/AP-5, or contralateral SB and CNQX/AP-5), indicating that bilateral blockade of orexin or glutamate receptors is required to reduce cue-induced reinstatement. Therefore, VTA is likely to be the main locus for the observed effects of orexin and glutamate receptor drugs on cued reinstatement.
Discussion
Here, we provide the first functional evidence that simultaneous ventral tegmental area (VTA) orexin and glutamate signaling is necessary for cue-induced relapse to drug seeking and that orexin acts to facilitate VTA AMPA responses to cue-related glutamate inputs. We found that bilateral VTA injection of the OX1R antagonist SB, the AMPA antagonist CNQX, or a cocktail of the AMPA/NMDA antagonists CNQX/AP-5 attenuates cue-induced reinstatement of extinguished cocaine seeking. In contrast, none of these manipulations affect cocaine-primed reinstatement. Intra-VTA AP-5 alone failed to affect either cue-induced or cocaine-primed reinstatement, indicating that AMPA, not NMDA receptors, is the critical signaling mechanism for VTA glutamate modulation of cue-induced reinstatement. Unilateral VTA OX1R blockade and simultaneous contrahemispheric VTA glutamate blockade also “attenuates” cue-induced reinstatement, indicating that simultaneous orexin and glutamate transmission in VTA is necessary. Finally, we found that enhancing VTA responses to endogenous glutamate signals with the AMPA allosteric modulator PEPA compensates for antagonizing OX1Rs by restoring cocaine seeking to control levels, although it did not elicit reinstatement on its own. These results show that an interaction of VTA OX1 and AMPA receptor signaling is necessary for reinstatement of cocaine seeking elicited by cues, but not a cocaine prime.
VTA orexin is necessary for cue-induced but not cocaine-primed reinstatement
Orexin plays a crucial role in some, but not all, types of reward seeking. Previous studies showed that OX1R signaling is necessary for reward seeking triggered by cues, contexts, or stressors. Systemic administration of SB reduces discrete cue- and/or context-induced reinstatement of cocaine, ethanol, heroin, and food seeking (Cason et al. 2010; Lawrence et al. 2006; Smith and Aston-Jones in press; Smith et al. 2009, Smith 2010), and stress-induced reinstatement of cocaine and ethanol seeking (Boutrel et al. 2005; Richards et al. 2008). Systemic SB also blunts cocaine self-administration under progressive ratio, but not fixed ratio-1, schedules, indicating that orexin is specifically involved in the motivational and cue-driven aspects of cocaine seeking, but not in primary cocaine reinforcement (Borgland et al. 2009; Espana et al. 2010; Smith et al. 2009). Here, we found that reinstatement of cocaine seeking evoked by a cocaine prime is unaffected by systemic SB, indicating that OX1Rs are also unnecessary for the priming properties of cocaine (Fig. 2). Taken together, these findings show that orexin is specifically involved in cocaine seeking triggered by external stimuli (e.g., cues, contexts, stressors).
The current results also demonstrate that VTA is a critical site for orexin’s role in cue-triggered drug seeking. Previous studies found that intra-VTA SB reduces acquisition of drug place preferences, cocaine sensitization, and motivation to work for cocaine (Borgland et al. 2006; Espana et al. 2010; Harris et al. 2007; Narita et al. 2006). In addition, James et al. (2011) recently demonstrated that a high concentration of SB (~20× higher than used here) injected unilaterally in VTA attenuates reinstatement of cocaine seeking elicited by a discriminative stimulus cue, without affecting locomotor activity. Here, we show that selective OX1R blockade in VTA prevents discrete cocaine-associated cues from serving as effective conditioned reinforcers, as bilateral VTA injections of SB reduced cue-induced reinstatement of cocaine seeking to half of vehicle levels (Fig. 1). In contrast, SB injections nearby, but outside the borders of VTA, failed to affect cocaine seeking; therefore, SB effects are not likely to have been due to diffusion of the drug to adjacent structures (Fig. 5). Importantly, unilateral VTA SB (or glutamate antagonists) did not affect cue-triggered reinstatement, indicating that VTA orexin/glutamate transmission in at least one hemisphere is sufficient for maximal reinstatement to occur.
VTA glutamate is also required for cue-induced but not cocaine-primed reinstatement
We also found that VTA microinjections of a relatively low dose of the specific AMPA antagonist CNQX attenuated the ability of cues to reinstate cocaine seeking (Fig. 3). In contrast, the specific NMDA antagonist AP-5 did not affect cue-induced reinstatement on its own, nor did it potentiate CNQX effects on cue-induced reinstatement. Instead, NMDA receptors in VTA may be required for learning about rewards but not for generating motivation based on prior learning, consistent with the present results (Harris et al. 2004; Ranaldi et al. 2010; Zellner et al. 2009).
Bossert et al. (2004) previously demonstrated an important role for VTA glutamate release in context-induced reinstatement of heroin seeking, and another report showed that the non-selective ionotropic glutamate antagonist kynurenic acid in VTA blocked reinstatement of cocaine seeking by cocaine+discrete cues (Sun et al. 2005). In addition, You et al. (2007) found that low concentrations of “…either CNQX or AP-5” (both at 0.1 mM) blocked cocaine seeking in early extinction, although a later paper by Nolan et al. (2010) failed to replicate this effect of AMPA antagonism with NBQX, a drug with lower affinity for the glycine binding site on the NMDA receptor than CNQX. Here, we demonstrate that VTA AMPA blockade with CNQX dose-dependently attenuated cue-induced cocaine seeking, whereas even relatively high concentrations of the NMDA antagonist AP-5 (up to 1.6mM) failed to do so. The doses of AMPA and NMDA antagonists used here are the same or higher than those previously shown, in VTA, to block drug seeking (Harris and Aston-Jones 2003; Harris et al. 2004; You et al. 2007) and to block increases in PFC or NAc dopamine caused by handling stress, systemic nicotine injection, or glutamate agonist co-infusion (Enrico et al. 1998; Fu et al. 2000; Svensson et al. 1998; Westerink et al. 1998). The present findings therefore indicate that the lack of effect observed for NMDA blockade on cue-induced or cocaine-primed reinstatement is not likely due to insufficient dosing. Instead, although cocaine seeking in early extinction requires both AMPA and NMDA transmission in VTA (You et al. 2007), cue-induced reinstatement of cocaine seeking appears to be dependent upon AMPA transmission alone in VTA.
Like SB, intra-VTA glutamate antagonists failed to reduce cocaine-primed reinstatement at the same doses at which they markedly reduced cue-induced cocaine seeking, indicating a specific role for VTA glutamate in appetitive responses to drug-paired conditioned stimuli. This contrasts with previous studies showing attenuated cocaine-primed reinstatement following intra-VTA administration of kynurenic acid or a high dose of CNQX (~10× higher than the high dose here), though a similar CNQX dose as used here did not affect primed reinstatement (Schmidt et al. 2009;Wise et al. 2008). In addition, Rebec and colleagues found that that intra-VTA kynurenic acid blocked cocaine+cue-induced reinstatement, and the glutamate release inhibitor LY-379268 blocked cocaine-primed reinstatement (Lu et al. 2011; Sun et al. 2005). These previous results may be explained by an attenuation of baseline VTA firing, rather than a specific blockade of reinstatement-related glutamate inputs. High-dose glutamate antagonists can reduce tonic and phasic firing rates of VTA dopamine cells and concomitant forebrain extrasynaptic dopamine levels (Chergui et al. 1993; Christoffersen and Meltzer 1995; Sombers et al. 2009; Takahata and Moghaddam 2000). Because dopamine reuptake blockade by cocaine is impulse dependent, strongly decreased tonic firing of dopamine neurons by these high-dose treatments might limit cocaine’s ability to increase extracellular dopamine and thus decrease drug seeking primed by cocaine (Nomikos et al. 1990; Sombers et al. 2009). In the current studies, cocaine-primed reinstatement was not affected by the same doses of glutamate (and orexin) antagonists that robustly attenuated cue-induced reinstatement, indicating that these doses of antagonists effectively interfered with VTA’s role in cue-induced drug seeking, but likely spared tonic dopamine cell activity necessary for cocaine-primed relapse.
It is unlikely that the reduction in reinstatement behavior observed after orexin and/or glutamate receptor antagonism can be attributed to general effects on locomotion. First, none of the manipulations reduced pressing on an inactive control lever. Second, a previous report also showed that much higher doses of SB microinjected into VTA failed to affect spontaneous locomotor activity (James et al. 2011). Further, a relatively high systemic dose of SB (30 mg/kg) failed to affect lever pressing for food pellets, suggesting a lack of general locomotor suppression (LeSage et al. 2010). Higher systemic doses of SB (up to 30 mg/kg) only modestly reduce activity in a locomotor chamber and have no effect on some types of lever pressing, including cocaine-induced reinstatement (Fig. 2) or cocaine self-administration (Smith et al. 2009).
A synergistic interaction between VTA glutamate and orexin transmission
Previous work has shown that orexin inputs to VTA can enhance dopamine cell activity by potentiating their responses to glutamate inputs. VTA recordings in vitro show that application of orexin promotes synaptic insertion of NMDA receptors and enhanced AMPA and NMDA responses to glutamate agonists (Borgland et al. 2006). Following a history of cocaine or palatable food self-administration, orexin has the additional effect of amplifying presynaptic glutamate release (Borgland et al. 2009). Electrophysiology studies in vivo also indicate that orexin facilitates glutamatergic control of VTA dopamine neurons, as a low concentration of intra-VTA orexinA potentiates dopamine cell firing evoked by stimulation of putatively glutamatergic inputs from medial prefrontal cortex (Moorman and Aston-Jones 2010).
Given these previous reports, as well as the classical role for VTA dopamine neurons in reward seeking and the present findings that both VTA orexin and glutamate are necessary for cue-induced cocaine seeking, we hypothesized that simultaneous orexin and glutamate transmission in VTA promotes cue-triggered reinstatement of extinguished cocaine seeking. Therefore, we first asked whether orexin and glutamate are simultaneously necessary for cue-induced reinstatement. We found that blocking ionotropic glutamate receptors in one VTA, while concurrently blocking OX1Rs in the contralateral VTA, attenuates cue-induced reinstatement. This indicates that neither VTA orexin nor glutamate inputs alone are sufficient for cue-induced reinstatement to occur; instead, maximal cocaine seeking requires both. However, this experiment does not directly test the interaction of orexin and glutamate, but instead confirms that simultaneous orexin and glutamate inputs to VTA are necessary for cues to elicit maximal cocaine seeking.
Therefore, we further tested the hypothesis that orexin potentiates VTA responses to cue-related glutamate by employing the allosteric AMPA receptor modulator PEPA, which prolongs endogenous glutamate-induced AMPA activation by preventing receptor desensitization and increasing glutamate binding affinity (Kessler and Arai 2006; Sekiguchi et al. 1997). When injected into infralimbic cortex, PEPA potentiates extinction of fear and drug memories, showing that PEPA can functionally promote endogenous glutamate transmission in vivo (LaLumiere et al. 2010; Lalumiere et al. 2012; Zushida et al. 2007). Here, we report the first usage of PEPA to examine a functional interaction between AMPA and another receptor system. We found that intra-VTA PEPA reverses effects of OX1R blockade and returns cue-triggered reinstatement to control levels. This suggests that VTA orexin normally potentiates activity at AMPA receptors to drive cue-triggered cocaine seeking, but when orexin is blocked by SB, reinstatement can be rescued by enhancing AMPA activity via a completely different mechanism. Importantly, we found that intra-VTA PEPA alone does not induce reinstatement. This is likely due to the fact that PEPA is an allosteric modulator of AMPA receptors and is relatively ineffective in the absence of significant local glutamate release. Therefore, PEPA has no effect on cocaine seeking during late extinction, when there is no cue-induced VTA glutamate release. However, PEPA alone also fails to enhance cue-induced reinstatement above control levels, indicating a potential ceiling effect for glutamate’s impact on VTA cell firing and/or cue-triggered motivation in the presence of intact orexin neurotransmission. It is only after OX1R blockade that co-administered PEPA has an effect—completely restoring cue-induced reinstatement. These data, in conjunction with related previous findings, strongly point to a crucial interaction between VTA orexin and AMPA receptor signaling that promotes cue-triggered motivation. Previously, Wang et al. (2009) showed that intra-VTA orexinA administration induces reinstatement of cocaine seeking, and this effect is attenuated by concurrent administration of the non-selective ionotropic glutamate antagonist kynurenic acid. The present data suggest that this effect was very likely AMPA mediated. Together, these findings support the hypothesis that orexin potentiation of VTA AMPA signaling is crucial for reinstatement of cocaine-seeking behavior.
We propose that orexin enhances VTA dopamine cell responses to glutamate inputs that convey stimulus information required for transforming cues into potent triggers of cocaine-seeking behavior. These glutamate inputs to VTA may arise from prefrontal cortex, hypothalamus, or other afferents (Geisler et al. 2007; You et al. 2007; Zellner and Ranaldi 2010). We further propose that concurrent cue-related orexin release in VTA [likely originating from lateral portions of the hypothalamic orexin field (Harris and Aston-Jones 2006)] enhances responsiveness of VTA neurons to these glutamate inputs via AMPA receptor recruitment, promoting the incentive salience of encountered cues and leading to relapse (Aston-Jones et al. 2009; Borgland et al. 2006; Moorman and Aston-Jones 2010). This synergistic interaction of orexin and glutamate in VTA may provide a novel pharmacological target for reducing cue-triggered cravings and relapse in human addicts.
Acknowledgments
We would like to thank Phong Do and Lana Zhang for assistance with behavioral testing. These experiments were funded by NIDA P50 DA015369, R37 DA06214, F32 DA026692, and T32 DA007288.
Footnotes
Conflicts of Interest None
References
- Academies NRCotN . Guide for the care and use of laboratory animals. 8th edn National Academies Press; Washington, DC: 2011. [Google Scholar]
- Ahmed AH, Ptak CP, Oswald RE. Molecular mechanism of flop selectivity and subsite recognition for an AMPA receptor allosteric modulator: structures of GluA2 and GluA3 in complexes with PEPA. Biochemistry. 2010;49:2843–2850. doi: 10.1021/bi1000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J. Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, Friedman AK, Walsh JJ, Rahman Z, Monteggia LM, Eisch AJ, Neve RL, Nestler EJ, Han M, Self DW. Reinforcement-related regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci. 2011;31:7927–7937. doi: 10.1523/JNEUROSCI.6014-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen CL, Meltzer LT. Evidence for N-methyl-d-aspartate and AMPA subtypes of the glutamate receptor on substantia nigra dopamine neurons: possible preferential role for N-methyl-d-aspartate receptors. Neuroscience. 1995;67:373–381. doi: 10.1016/0306-4522(95)00047-m. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug-paired conditioned stimulus in rats. Eur J Neurosci. 2004;19:1661–1667. doi: 10.1111/j.1460-9568.2004.03232.x. [DOI] [PubMed] [Google Scholar]
- Enrico P, Bouma M, de Vries JB, Westerink BH. The role of afferents to the ventral tegmental area in the handling stress-induced increase in the release of dopamine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Brain Res. 1998;779:205–213. doi: 10.1016/s0006-8993(97)01132-3. [DOI] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin–orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Do PH, See RE. Repeated aripiprazole administration attenuates cocaine seeking in a rat model of relapse. Psychopharmacology. 2009;207:401–411. doi: 10.1007/s00213-009-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: reevaluation of the role of N-methyl-d-aspartate receptors in the ventral tegmental area. J Pharmacol Exp Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. The Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brain-stem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33:2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J. Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kessler M, Arai AC. Use of [3H]fluorowillardiine to study properties of AMPA receptor allosteric modulators. Brain Res. 2006;1076:25–41. doi: 10.1016/j.brainres.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Xue Y, Steketee JD, Rebec GV, Sun W. Regulation of cocaine-induced reinstatement by group II metabotropic glutamate receptors in the ventral tegmental area. Psychopharmacology. 2011;220:75–85. doi: 10.1007/s00213-011-2455-5. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pascucci T, Bernardi G, Puglisi-Allegra S, Mercuri NB. Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology. 2005;30:864–870. doi: 10.1038/sj.npp.1300615. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learn Mem. 2006;13:123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Miyatake M, Ikegami D, Kurahashi K, Suzuki T. Implication of protein kinase C in the orexin-induced elevation of extracellular dopamine levels and its rewarding effect. Eur J Neurosci. 2007;25:1537–1545. doi: 10.1111/j.1460-9568.2007.05403.x. [DOI] [PubMed] [Google Scholar]
- Nolan BC, Saliba M, Tanchez C, Ranaldi R. Behavioral activating effects of selective AMPA receptor antagonism in the ventral tegmental area. Pharmacology. 2010;86:336–343. doi: 10.1159/000322095. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis. Synapse. 1990;6:106–112. doi: 10.1002/syn.890060113. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edn Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Kest K, Zellner MR, Lubelski D, Muller J, Cruz Y, Saliba M. The effects of VTA NMDA receptor antagonism on reward-related learning and associated c-fos expression in forebrain. Behav Brain Res. 2010;216:424–432. doi: 10.1016/j.bbr.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, French ED. Abused inhalants and central reward pathways: electrophysiological and behavioral studies in the rat. Ann N Y Acad Sci. 2002;965:281–291. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. J Neurosci. 2003;23:9913–9923. doi: 10.1523/JNEUROSCI.23-30-09913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30:1358–1369. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, Wada K. A novel allosteric potentiator of AMPA receptors: 4–2-(phenylsulfonylamino)ethylthio–2,6-difluoro-phenoxyaceta mide. J Neurosci. 1997;17:5760–5771. doi: 10.1523/JNEUROSCI.17-15-05760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. doi: 10.1111/j.1460-9568.2012.08013.x. (in press) DOI: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Mathe JM, Nomikos GG, Schilstrom B. Role of excitatory amino acids in the ventral tegmental area for central actions of non-competitive NMDA-receptor antagonists and nicotine. Amino Acids. 1998;14:51–56. doi: 10.1007/BF01345242. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Target-specific glutamatergic regulation of dopamine neurons in the ventral tegmental area. J Neurochem. 2000;75:1775–1778. doi: 10.1046/j.1471-4159.2000.0751775.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, O’Connor WT, Ungerstedt U, French ED. N-methyl-d-aspartic acid biphasically regulates the biochemical and electro-physiological response of A10 dopamine neurons in the ventral tegmental area: in vivo microdialysis and in vitro electrophysiological studies. Brain Res. 1994;666:255–262. doi: 10.1016/0006-8993(94)90780-3. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Enrico P, Feimann J, De Vries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialsis study in the ventral tegmental area and prefrontal cortex of the rat brain. J Pharmacol Exp Ther. 1998;285:143–154. [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Wise RA. Ventral tegmental glutamate: a role in stress-, cue-, and cocaine-induced reinstatement of cocaine-seeking. Neuropharmacology. 2009;56(Suppl 1):174–176. doi: 10.1016/j.neuropharm.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Zushida K, Wada K, Sekiguchi M. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology. 2009;34:2574–2584. doi: 10.1038/npp.2009.86. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner MR, Ranaldi R. How conditioned stimuli acquire the ability to activate VTA dopamine cells: a proposed neurobiological component of reward-related learning. Neurosci Biobehav Rev. 2010;34:769–780. doi: 10.1016/j.neubiorev.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Zellner MR, Kest K, Ranaldi R. NMDA receptor antagonism in the ventral tegmental area impairs acquisition of reward-related learning. Behav Brain Res. 2009;197:442–449. doi: 10.1016/j.bbr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]