Abstract
In the postprandial state, the liver takes up and stores glucose to minimize the fluctuation of glycemia. Elevated insulin concentrations, an increase in the load of glucose reaching the liver, and the oral/enteral/portal vein route of glucose delivery (compared with the peripheral intravenous route) are factors that increase the rate of net hepatic glucose uptake (NHGU). The entry of glucose into the portal vein stimulates a portal glucose signal that not only enhances NHGU but concomitantly reduces muscle glucose uptake to ensure appropriate partitioning of a glucose load. This coordinated regulation of glucose uptake is likely neurally mediated, at least in part, because it is not observed after total hepatic denervation. Moreover, there is evidence that both the sympathetic and the nitrergic innervation of the liver exert a tonic repression of NHGU that is relieved under feeding conditions. Further, the energy sensor 5′AMP-activated protein kinase appears to be involved in regulation of NHGU and glycogen storage. Consumption of a high-fat and high-fructose diet impairs NHGU and glycogen storage in association with a reduction in glucokinase protein and activity. An understanding of the impact of nutrients themselves and the route of nutrient delivery on liver carbohydrate metabolism is fundamental to the development of therapies for impaired postprandial glucoregulation.
Introduction
The liver plays a unique role in postprandial nutrient metabolism because it has first access to most ingested nutrients by virtue of their absorption into the hepatic portal vein. As a result, the liver is exposed to higher nutrient levels than are peripheral tissues. Moreover, it is able both to store and to release glucose to minimize changes in glycemia between the fed and fasted states. In the normal individual, the intake of a mixed meal results in modest hyperglycemia, accompanied by substantial storage of glycogen in the liver. The postprandial period is characterized by carefully titrated changes in hormone secretion and neural signals, as well as changes in nonglucose substrates, that combine to direct the partitioning of the glucose load among the various tissues (1–4). In contrast to the individual with normal glucose tolerance, the person with poorly controlled type 1 or 2 diabetes exhibits marked postprandial hyperglycemia and impaired hepatic glycogen accumulation (4–6). This review focuses on the current understanding of the signals involved in the control of hepatic glucose uptake and glycogen synthesis in vivo.
Current status of knowledge
In response to ingestion of glucose or a mixed meal and the resulting hyperinsulinemia and hyperglycemia, the fasting liver shifts from net output to net uptake of glucose. It is clear, however, based on the measurement of net splanchnic glucose balance in humans (6–8) and net hepatic glucose balance in dogs (9, 10), that neither hyperinsulinemia nor hyperglycemia can independently induce much net hepatic glucose uptake (NHGU6). NHGU remains modest (2.8– 11.1 μmol·kg−1·min−1) even when hyperinsulinemia and hyperglycemia (resulting from glucose infusion into a peripheral vein) are combined (6, 7, 9, 11, 12). On the other hand, when similar levels of hyperinsulinemia and hyperglycemia are brought about by oral or enteral glucose delivery, the resulting rates of NHGU are as great as 25–27.8 μmol·kg−1·min−1 (13, 14). Thus, it is clear that oral glucose delivery triggers a unique hepatic response and that the liver has an important role in disposing of ingested glucose.
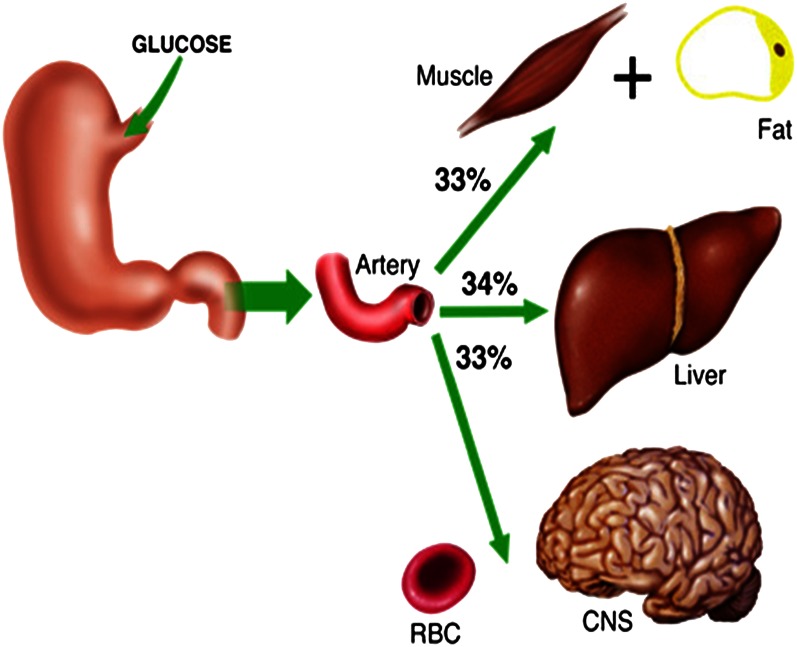
Direct measurement of hepatic glucose uptake in humans is hampered by the technical difficulty and ethical concerns regarding portal vein blood sampling. Using splanchnic balance measurements and tracer techniques, however, investigators have estimated that the human liver disposes of ∼25 to 35% of an oral glucose load (15, 16). In the dog, a model in which it is possible to measure hepatic balance directly, NHGU accounts for 25–40% of the administered glucose, with the exact percentage being determined by the load of glucose and insulin reaching the liver (14, 17). Thus, the data from human and canine experiments are in general agreement that, when presented with a moderately sized oral glucose load, the liver extracts approximately one third of the glucose, together the muscles and fat take up approximately one third, and the noninsulin-sensitive obligate glucose–using tissues dispose of the remaining one third (Fig. 1). In fact, the liver not only takes up glucose but also curtails its release of glucose postprandially. Thus, these proportions underestimate the role of the liver in glycemic control because the glucose consumed by the obligate glucose-requiring tissues has to be derived from the absorbed glucose, as a result of the cessation of hepatic glucose production. Consequently, the liver is actually responsible for the disposal of the equivalent of ∼60–65% of an oral glucose load. Any impairment in its function, therefore, can lead to excessive postprandial glycemia. Because elevated postprandial glucose levels are associated with adverse outcomes including increased risk of death (all cause and cardiovascular), major cardiovascular events, and progression of diabetic retinopathy (18), an understanding of the regulation of hepatic glucose uptake is of great importance.
Figure 1.
Distribution of a glucose load among the liver, insulin-sensitive tissues, and noninsulin-sensitive tissues. CNS, central nervous system; RBC, red blood cell. Reproduced from Reference 97 with permission.
Portal glucose signal
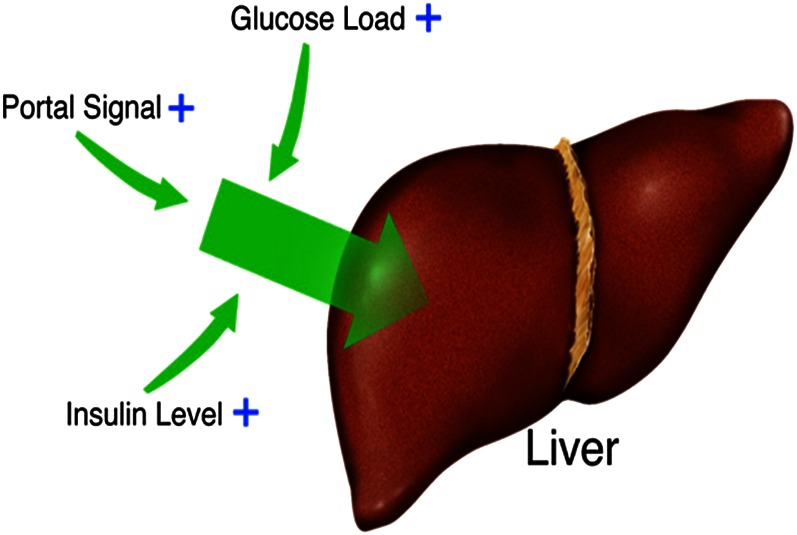
Originally it was postulated that a gut factor could explain the ability of combined increases in insulin and glucose to cause greater splanchnic or hepatic glucose uptake when associated with oral glucose delivery (7). However, such a gut factor was subsequently ruled out by studies in dogs in which hyperglycemia was created via an intraportal glucose infusion that mimicked the absorption profile of oral glucose. In this manner, several laboratories demonstrated that NHGU was not different after intraportal and oral glucose entry (17, 19, 20). Using the hyperglycemic clamp technique along with the pancreatic clamp (basal glucagon replacement with either euinsulinemia or hyperinsulinemia) in dogs, it was possible to ensure that the load of glucose and the pancreatic hormone concentrations at the liver were maintained equivalent, whether glucose was given via a leg vein or the hepatic portal vein. In this way, it was conclusively demonstrated that entry of glucose into the portal vein stimulated NHGU and hepatic glycogen synthesis to a significantly greater extent than glucose delivery via a peripheral vein (12, 21). Thus, a portal vein signal, rather than a gut factor, was demonstrated to be responsible for enhancement of NHGU during oral, enteral, or portal venous glucose delivery. It is this factor, together with the insulin concentration and the load of glucose reaching the liver, that determines the rate of NHGU (12, 22) (Fig. 2). The portal glucose signal does not, however, enhance whole-body glucose clearance (11, 21). Instead, it is associated with a suppression of nonhepatic (primarily muscle) glucose uptake concomitant with the increase in liver glucose uptake (23). Thus, as a result of its reciprocal actions, the portal signal ensures that a glucose load is appropriately distributed among the skeletal muscle, the liver, and the other tissues of the body.
Figure 2.
Factors affecting the magnitude of net hepatic glucose uptake (NHGU). In the physiologic range, increases in the amount of insulin reaching the liver and the hepatic glucose load stimulate NHGU. When the insulin concentrations and hepatic glucose loads are equivalent with the 2 routes of delivery, NHGU is approximately 2-fold greater when glucose is delivered via the portal versus a peripheral vein.
Although the portal glucose signal has been demonstrated to operate in species other than the dog (24, 25), its importance in the human has been more difficult to evaluate because of an inability to catheterize the portal vein and the difficulty in controlling the insulin and glucagon levels reaching the liver. Two investigations in humans are particularly relevant. DeFronzo et al. (7) compared splanchnic glucose uptake in 2 groups of human subjects in whom peripheral vein glucose infusion was used to create a combination of hyperglycemia and hyperinsulinemia. One group then consumed an oral glucose load (1.2 g/kg), with the peripheral glucose infusion rate subsequently being adjusted so that glycemic levels were similar in the presence and absence of the oral glucose load. Glucose ingestion augmented net splanchnic glucose uptake approximately 5-fold, compared with peripheral venous glucose infusion alone. The design of their study was such that the liver in the group receiving oral glucose was exposed to a somewhat larger hepatic glucose load and somewhat higher insulin levels, however, complicating data interpretation. In the second study, Vella et al. (11) used a pancreatic clamp to fix insulin and glucagon concentrations while infusing glucose into either the duodenum or a peripheral vein. Intraduodenal glucose delivery was associated with a 40–125% enhancement of hepatic glucose extraction. It is worth noting that whole-body glucose kinetics in humans did not differ, whether glucose was given via the intravenous or intraduodenal route (26), consistent with findings in the dog and mouse (21, 25). In summary, the available evidence strongly suggests that the portal signal functions across mammalian species.
A meal contains not only carbohydrate but also fat and protein, and thus it is of interest to know what effect these substrates have on hepatic glucose disposal. The impact of lipids on NHGU has been examined with pancreatic hormones clamped and the portal signal present, as well as infusion of nicotinic acid to suppress endogenous lipolysis. Under these conditions, peripheral infusion of a lipid emulsion to maintain nonesterified fatty acid concentrations at their basal levels was associated with a significant (∼50%) reduction in NHGU compared with a control group that received no lipid infusion. The suppression of NHGU in the lipid-infused group was attributable to a combination of stimulation of hepatic glucose production and blunting of hepatic glucose uptake (27). The number and diversity of amino acids have made the examination of interactions between carbohydrate and protein more complex. Under hyperinsulinemic hyperglycemic clamp conditions, portal but not peripheral infusion of a gluconeogenic amino acid mixture (serine, threonine, glutamine, glutamate, glycine, and alanine) significantly blunted NHGU (∼50%) in the presence of the portal glucose signal but not in its absence (28, 29). On the other hand, when a mixture containing the 20 common dietary amino acids was delivered under hyperinsulinemic hyperglycemic clamp conditions in the absence of the portal signal, it brought about a blunting of NHGU (30); interaction between the 20 amino acid mixture and portal glucose delivery has not been examined. Both glucose and amino acids in the hepatoportal region are known to initiate neural signals that are transmitted to the brain, with some of the amino acids having stimulatory effects and others having suppressive effects on afferent firing rates (31–33). It is thus likely that competition or interaction among the various amino acids and glucose alter the transmission of a neural signal responsible for modulating hepatic substrate extraction. In summary, both fat and amino acids affect the liver’s response to glucose delivery, but much work remains to be done to understand the relationships among the macronutrients and their components in the regulation of NHGU.
Mediators of the portal glucose signal.
The liver is innervated by parasympathetic, sympathetic and nonadrenergic, noncholinergic (including nitrergic) nerves (34–37). A role for the central nervous system in the control of liver glucose metabolism is generally supported by the literature (38–40). Electrophysiologic data confirm that glucosensors in the hepatoportal region transmit signals to the hypothalamus (41), and total hepatic denervation ablates both the hepatic and muscle responses to portal glucose delivery (42). The manner in which the portal glucose signal is sensed and signals to muscle and liver remains unclear, however. One possibility is that afferent nerves carry information from the hepatoportal region to the brain, which then signals muscle and liver through efferent nerves. Alternatively, the information sent to the brain could bring about a neural signal to one organ or the other, with the subsequent release of a hepatokine or myokine to coordinate the response between tissues.
In regard to afferent signaling, it has been established that a negative arterial-portal vein glucose gradient (i.e., portal vein glucose concentration higher than that in the artery) triggers the response to portal vein glucose delivery (43). Further, it is clear that the arterial and portal vein glucose levels are compared within the liver and not within the central nervous system (44, 45). Afferent fibers from the hepatoportal region travel with both the spinal and vagus nerves (35). Data from vagal nerve cooling experiments do not support involvement of vagal afferents in the portal glucose signal because inhibition of vagal firing brought about by cooling the vagus nerves in the conscious dog under hyperglycemic, hyperinsulinemic conditions did not lead to a decrease in NHGU, whether portal glucose delivery was present or not (46, 47). The spinal afferent nerves have been shown to function in the detection of hypoglycemia in the portal vein (48), and thus their involvement in the response to a glucose load appears likely, although it has not been examined.
With regard to the efferent limb of the response, again there is little support for a key role for the parasympathetic system. In addition to the evidence from the vagal cooling experiments described earlier, it has been observed that, under hyperinsulinemic euglycemic conditions, hepatic parasympathetic denervation in the rat brings about a reduction in glucose clearance in the skeletal muscle, heart, and kidney but does not affect glucose clearance by the liver (49, 50). On the other hand, data do support a role for sympathetic and nitrergic neural input in regulating NHGU. Surgical ablation of the hepatic sympathetic nerves resulted in an increase in NHGU during glucose infusion into a peripheral vein (51). Likewise, increasing hepatic nitric oxide (NO) using the NO donor 3-morpholinosydnominine HCl (SIN-1) or lowering it using the NO synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (l-NAME) brought about reduced and enhanced NHGU, respectively (52, 53). Taken together, these data suggest that both adrenergic and nitrergic input to the liver exerts a basal restraining effect on NHGU that is removed in response to feeding or portal glucose delivery.
The pathway or pathways bringing about the nonhepatic response to the portal signal remain undefined. Neural signals represent 1 possibility because electrical stimulation of the ventromedial hypothalamus enhances muscle glucose uptake, an effect that can be prevented with sympathetic blockade (54). In addition to receiving neural signals from the periphery, the hypothalamus is sensitive to circulating hormones and substrates, including insulin and glucose (39, 55). Both insulin and glucose modulate the phosphorylation of cerebral 5′AMP-activated protein kinase (AMPK), which can play a role in the regulation of muscle glucose disposal (55).
Glucagon-like peptide 1 (GLP-1) has been suggested as a mediator of the enhancement of NHGU by oral glucose delivery (56). However, physiologic concentrations of GLP-1 have no impact on NHGU when studies are conducted during the infusion of somatostatin (57, 58). Somatostatin prevents endogenous release of GLP-1 as well as glucagon and insulin, eliminating the possibility that differences in concentrations of key glucoregulatory hormones account for the findings. Thus, although numerous possibilities exist with regard to the identities of the afferent and efferent limbs associated with the portal glucose signal, it is unclear at present which are crucial for the response. Nevertheless, the available data point toward the involvement of the nervous system.
NO and hepatic fuel sensing.
AMPK, a metabolic fuel sensor with numerous targets, is activated by an increase in the AMP:ATP ratio, an indicator that tissue energy reserves are low. Activation of AMPK stimulates energy-producing pathways, i.e., glucose utilization and lipid oxidation, while reducing the activity of fuel storage pathways such as glycogenesis and lipogenesis in muscle and other tissues (59). These roles of AMPK suggest that an increase in hepatic glycogen concentrations might be expected to reduce AMPK activity and consequently blunt NHGU and hepatic glycogen storage. Under steady-state conditions and in the presence of physiologic levels of hepatic glycogen (55–72 mg/g tissue), the rate of hepatic glycogen synthesis is directly related to the rate of NHGU (60). However, proposed newer pharmacologic approaches to the management of postprandial hyperglycemia in type 2 diabetes, such as glucokinase (GK) activators, glucagon receptor antagonists, and glycogen phosphorylase inhibitors, might be anticipated to increase hepatic glycogen content. For this reason, studies were carried out on dogs whose livers had been “supercompensated” with glycogen (100 mg/g liver) (61). These high glycogen concentrations were achieved by infusing a small amount of fructose intraportally under hyperglycemic conditions to stimulate hepatic GK. GK is regulated by both long-term and acute mechanisms (reviewed in reference 62). Long-term mechanisms are largely mediated by insulin, which stimulates GK transcription and translation. Acute regulation (inactivation) occurs via binding of GK to its nuclear regulatory protein, GK regulatory protein (GKRP); this binding normally occurs in the presence of low glucose. Under postprandial conditions, elevated glucose levels stimulate dissociation of GK from GKRP, resulting in the translocation of GK in its active form to the cytosol. Enterally or portally delivered fructose is rapidly taken up by the liver and phosphorylated to form fructose-1-P, an extremely potent stimulator of GK/GKRP dissociation and GK translocation. This, in turn, induces supraphysiologic rates of NHGU and glycogen deposition (63). In contrast to a modest increase in the hepatic glycogen content, which had little apparent effect on liver glucose metabolism, the animals with supercompensated hepatic glycogen exhibited reduced glycogen synthesis in response to hyperglycemia, hyperinsulinemia, and the portal glucose signal (60, 61). This was associated with impaired hepatic insulin signaling, increased AMPK phosphorylation, and marked reduction in glycogen synthase (GS) activity coupled with enhanced glycogen phosphorylase activity. McBride and Hardie (64) proposed that glycogen loading increases the binding of AMPK to the nonreducing ends of the glycogen molecule’s outer chains, and this close proximity to GS, which is also glycogen bound, increases the likelihood of GS phosphorylation by AMPK. Thus, although our data do not allow us to draw conclusions about cause-and-effect relationships, they are consistent with a role for AMPK in the regulation of hepatic glucose disposal.
A role for AMPK in the regulation of hepatic energy metabolism has been suggested by a number of different laboratories. In the presence of basal glucagon and high physiologic levels of insulin, whether or not hyperglycemia, euglycemia, or hypoglycemia existed, intraportal infusion of the AMPK activator 5′-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside in dogs led to an increase in hepatic glucose output attributable to an increase in glycogenolysis (65–67). This is in agreement with data indicating that AMPK can activate glycogen phosphorylase and inactivate GS (68), as well as inhibit GK translocation and glucose phosphorylation in hepatocytes (69).
Interaction between AMPK and NOS in the regulation of glucose metabolism has been observed in numerous tissues (70–72). Nevertheless, the nature of this interaction remains unclear. Treatment of isolated mouse or human skeletal muscle with NO donors (sodium nitroprusside or spermine NONOate, respectively) increased glucose transport, concomitant with an increase in activation of the AMPK α-1 subunit (73, 74). Moreover, spermine NONOate increased glycogen synthesis and AMPK Thr172 phosphorylation in L6 myotubes, and the effects were not observed in the presence of a guanylate cyclase inhibitor (73). On the other hand, there is also evidence suggesting that AMPK is an upstream activator of NOS (75, 76). Regardless of the exact relationship between AMPK and NOS, the role of both molecules in the regulation of hepatic glucose metabolism is intriguing and deserves further investigation.
Whole-body insulin sensitivity is decreased by intraportal but not peripheral venous administration of l-NAME, and this effect can be reversed by intraportal but not peripheral delivery of SIN-1 (77–79). As mentioned previously, intraportal infusion of SIN-1 and l-NAME had suppressive and stimulatory effects, respectively, on NHGU (52, 53). Thus, we were interested in determining the mechanisms(s) by which changes in NO levels affected liver glucose uptake.
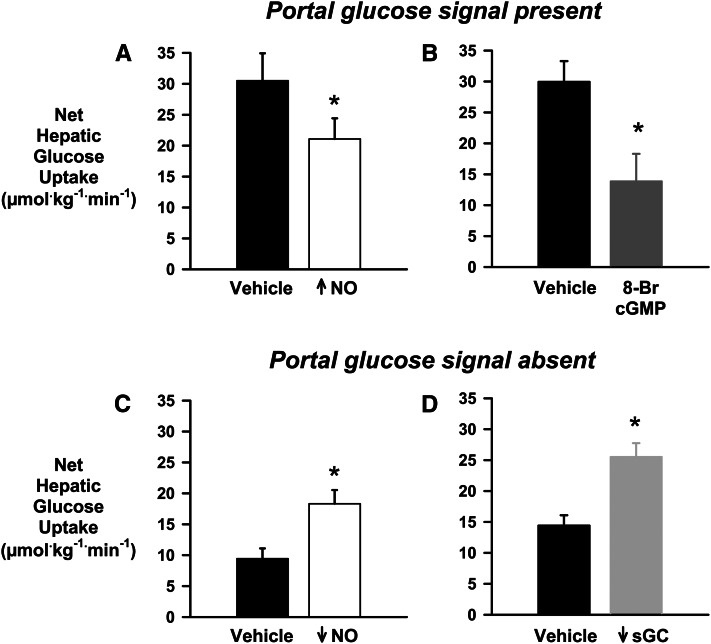
Many of the metabolic actions of NO are mediated via its activation of soluble guanylate cyclase (sGC) and subsequent stimulation of cyclic guanosine monophosphate (cGMP), which modulates the activity of protein kinase G, cGMP-dependent phosphodiesterases, and cyclic nucleotide–gated ion channels (73). Therefore, we infused the sGC inhibitor 1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) intraportally during a hyperinsulinemic, hyperglycemic clamp in the absence of the portal glucose signal in 1 group of dogs, whereas control dogs received the vehicle via intraportal infusion. Two additional groups were examined, 1 receiving vehicle plus intraportal SIN-1 and 1 receiving both ODQ and SIN-1 intraportally. Infusion of the sGC inhibitor resulted in a 55% enhancement of NHGU compared with control, along with a 48% increase in the liver glycogen content at the end of study (Fig. 3). Concomitant infusion of SIN-1 and ODQ did not alter the ODQ-stimulated rate of NHGU. Moreover, intraportal SIN-1 and vehicle administration resulted in NHGU at a rate no different from that in the control dogs. Intraportal ODQ infusion was associated with a 30% decrease in phosphorylation of hepatic AMPK and its downstream target acetyl-CoA carboxylase (ACC), compared with controls, and this was not altered by co-infusion of SIN-1 and ODQ. On the other hand, infusion of SIN-1 plus vehicle resulted in a 25% increase in phosphorylated AMPK/total AMPK and a 30% increase in phosphorylated ACC/total ACC compared with the control group (80). In a follow-up study, the cGMP analogue 8-bromo-cGMP was administered intraportally in the presence of the portal glucose signal, and NHGU was determined to be significantly blunted (81), providing further support for a role of the NO→sGC→cGMP pathway in the regulation of NHGU and glycogen storage (Fig. 3). The data suggest that this pathway could impose an inhibitory signal during fasting that would reduce glucose uptake by the liver. Conversely, a feeding signal that reduced signaling through the pathway might result in enhancement of NHGU and glycogen storage (Fig. 4).
Figure 3.
The relationship of nitric oxide (NO) and net hepatic glucose uptake (NHGU). In the presence of the portal glucose signal, increasing hepatic NO by intraportal infusion of 3-morpholinosydnominine HCl (A) or mimicking NO activation of the soluble guanylate cyclase (sGC)/cyclic guanosine monophosphate (cGMP) pathway by infusing the cGMP analogue 8-Br-cGMP intraportally (B) blunted NHGU. On the other hand, in the absence of the portal glucose signal, reducing hepatic NO by intraportal infusion of the NO synthase inhibitor Nω-nitro-l-arginine methyl ester (C) or blocking the activation of the sGC/cGMP pathway with the sGC inhibitor 1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one (D) enhanced NHGU. *P < 0.05 vs. vehicle. Data from References (52, 53, 80, 81).
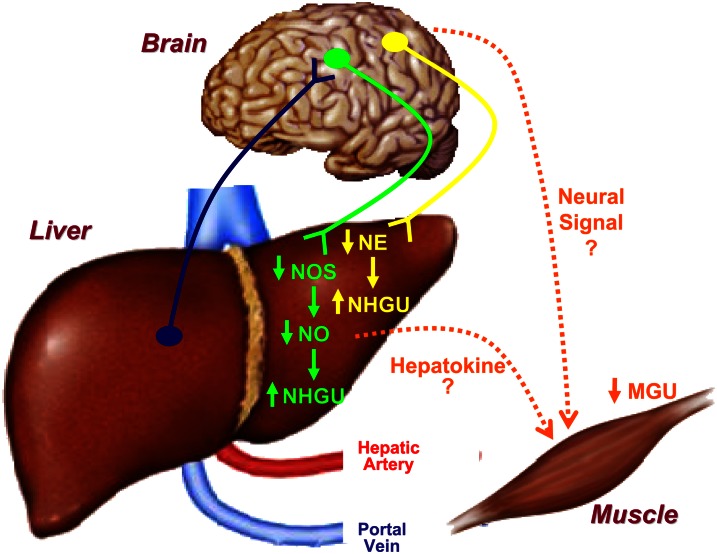
Figure 4.
The distribution of a glucose load between the liver and insulin-sensitive tissues (primarily skeletal muscle) is finely controlled. Ingestion of glucose or infusion of glucose into the portal vein creates a negative arterial-portal glucose gradient (portal vein concentration higher than that in the artery) that is sensed within the liver, giving rise to the portal glucose signal, which is associated with an increase in net hepatic glucose uptake (NHGU) coupled with a decrease in muscle glucose uptake. Afferent signals regarding hepatoportal glucose levels can be transmitted from the liver to the brain, particularly the hypothalamus. The efferent limbs of the response apparently rely on neural and/or humoral signals. Both selective sympathetic denervation of the liver and reduction in hepatic nitric oxide (NO) by inhibition of NO synthase (NOS) activity (mimicking a reduction in nitrergic neural signals) stimulate NHGU in the presence of hyperinsulinemia and hyperglycemia brought about by peripheral glucose infusion. In addition, electrical stimulation of the hypothalamus stimulates muscle glucose uptake, and sympathetic blockade prevents the increase in uptake. It is also possible that a humoral factor released either by the liver or muscle (a hepatokine or myokine) regulates glucose uptake by the opposing tissue. MGU, muscle glucose uptake; NE, norepinephrine.
Impact of long-term consumption of a high-fat and high-fructose diet on NHGU
The U.S. diet is high in fat, particularly saturated fat, and in simple carbohydrates (82, 83). In part because of the increased use of high-fructose corn syrup in beverages and foods, fructose accounts for >10% of energy consumed by the average U.S. child or adult, with the 95th percentile of U.S. fructose intakes totaling ∼20% of total energy (84). Fructose intakes have increased along with increases in the prevalence of obesity, metabolic syndrome, and type 2 diabetes. Epidemiologic and cross-sectional data link high-fat and high-fructose diets (HFFD) with these metabolic derangements (85–87). Although it is not clear that there is a causal relationship between fructose intake and these disorders, there is strong evidence of stimulation of de novo lipogenesis, visceral adipose tissue deposition, and dyslipidemia by high-fructose diets (88, 89). Moreover, women consuming a diet high in fructose versus glucose (25% of total energy intake) for 10 wk exhibited increases in de novo lipogenesis and in fasting plasma glucose and insulin concentrations, along with impairment of glucose tolerance (90).
Animal models exposed to HFFD quickly develop evidence of the metabolic syndrome, including weight gain/overweight, hypertriglyceridemia, and insulin resistance (91–93). The effect of such diets on postprandial hepatic glucose metabolism is incompletely understood, and therefore we examined NHGU and hepatic glycogen deposition in the dog model (94). Adult male dogs were initially fed a balanced meat and chow diet (31% protein, 26% fat, and 43% carbohydrate, virtually all in the form of starch). After baseline assessment, they were either maintained on the meat and chow diet (control group) or switched to an HFFD (HFFD group; energy composition: 22% protein, 52% fat, and 26% carbohydrate, with fructose contributing 17% of the total dietary energy). The insulin and glucose responses to an oral glucose tolerance test conducted at baseline and at 4 and 8 wk of follow-up were stable over time in the control group. Glucose tolerance deteriorated during consumption of the HFFD diet, however, with the area under the curve of the glucose response at both 4 and 8 wk being more than 2-fold that at baseline. Despite the increase in glycemia during oral glucose tolerance testing, there was no compensatory increase in the areas under the curve of the insulin concentrations in the HFFD group. Insulin sensitivity, assessed with a hyperinsulinemic euglycemic clamp at baseline and at 10 wk, also decreased significantly (approximately one third) in the HFFD but not the control dogs. During week 13, a hyperinsulinemic (4 times basal) hyperglycemic (hepatic glucose load 2 times basal) clamp was performed after an overnight fast. For the first 90 min of the clamp, glucose was infused only via a peripheral vein. For the subsequent 90 min, glucose was also infused via the portal vein with the peripheral infusion adjusted as necessary to maintain the hepatic glucose load equivalent in both periods. In response to hyperglycemia of peripheral origin, the control dogs shifted from net hepatic glucose output in the basal state to NHGU at a rate of 10.5 μmol·kg−1·min−1, and during portal glucose infusion, their NHGU nearly doubled (19.4 μmol·kg−1·min−1). In the HFFD group, NHGU did not occur with either route of glucose infusion. Concomitant tracer measurements indicated that this was due to defects in both suppression of hepatic glucose output and stimulation of hepatic glucose uptake. Net hepatic glycogen synthesis was suppressed ∼80% in the HFFD group.
Subsequently, separate groups of control and HFFD (8 wk of HFFD) dogs were studied after ingestion of a liquid mixed meal. Despite the presence of greater hyperinsulinemia and hyperglycemia after the meal in the HFFD versus control dogs, the HFFD group again failed to exhibit any significant NHGU, and glycogen storage was reduced ∼75% in that group (95). Thus, diets rich in fat and fructose impair NHGU and thereby contribute to postprandial hyperglycemia.
Further work has shown that HFFD dogs exhibit substantial decreases in both GK protein and activity in the liver (Δ58% and 71%, respectively) with no decrease in GK mRNA (96), suggesting that the defect in NHGU in the HFFD dogs is likely related to a deficit in GK. This is consistent with an important role for hepatic GK in the regulation of hepatic glucose uptake and glycogen storage (62).
Conclusions
Under normal conditions, the liver plays a critical role in disposing of orally or enterally delivered carbohydrate and therefore in limiting postprandial hyperglycemia. This response involves both a decrease in hepatic glucose production and a stimulation of hepatic glucose uptake. The latter is dependent on a number of inputs: circulating concentrations of glucose, nonesterified fatty acids, and amino acids; hormones (insulin); and neural mediators (NO and norepinephrine). The route of glucose delivery is responsible for determining as much as 50% of NHGU. The oral, enteral, or portal vein route of delivery brings about a negative arterial-portal vein glucose gradient that elicits a coordinated response of liver and muscle in glucose disposal such that NHGU is enhanced and muscle glucose uptake is suppressed. The portal glucose signal appears to be associated with a change in afferent signaling from the liver to the brain, resulting in a modification of efferent signaling to the liver, likely via sympathetic and/or nitrergic innervation. These signals apparently alleviate a tonic inhibition of NHGU. In response to a HFFD, both hepatic glucose production and glucose uptake in the postprandial period are abnormal, in association with a defect in hepatic GK. An improved understanding of the physiologic and pathophysiologic responses in the postprandial period will improve our ability to design appropriate treatments for individuals with impaired glucose tolerance and type 2 diabetes.
Acknowledgments
All authors have read and approved the final manuscript.
Footnotes
Supported by National Institutes of Health DK-43706 and by the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research (A.D.C.). Significant contributions were made by the Metabolic Physiology Core and the Hormone Assay and Analytical Services Core of the Vanderbilt Diabetes Research and Training Center, supported by NIDDK grant DK-20593.
Author disclosures. M.C. Moore, K.C. Coate, J.J. Winnick, Z. An, and A.D. Cherrington, no conflicts of interest.
Abbreviations used: ACC; acetyl-CoA carboxylase; AMPK, 5′AMP-activated protein kinase; cGMP, cyclic guanosine monophosphate; GK, glucokinase; GKRP, glucokinase regulatory protein; GLP-1, glucagon-like peptide 1; GS, glycogen synthase; HFFD, high-fat and high-fructose diet; l-NAME, Nω-nitro-l-arginine methyl ester; NHGU, net hepatic glucose uptake; NO, nitric oxide; NOS, nitric oxide synthase; ODQ, 1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one; sGC, soluble guanylate cyclase; SIN-1, 3-morpholinosydnominine HCl.
Literature Cited
- 1.Fagius J. Sympathetic nerve activity in metabolic control–some basic concepts. Acta Physiol Scand. 2003;177:337–43 [DOI] [PubMed] [Google Scholar]
- 2.Scott EM, Greenwood JP, Vacca G, Stoker JB, Gilbey SG, Mary DA. Carbohydrate ingestion, with transient endogenous insulinaemia, produces both sympathetic activation and vasodilatation in normal humans. Clin Sci (Lond). 2002;102:523–9 [PubMed] [Google Scholar]
- 3.Vollmer K, Gardiwal H, Menge BA, Goetze O, Deacon CF, Schmidt WE, Holst JJ, Meier JJ. Hyperglycemia acutely lowers the postprandial excursions of glucagon-like Peptide-1 and gastric inhibitory polypeptide in humans. J Clin Endocrinol Metab. 2009;94:1379–85 [DOI] [PubMed] [Google Scholar]
- 4.Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhausl W, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53:3048–56 [DOI] [PubMed] [Google Scholar]
- 5.Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest. 1995;95:783–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–83 [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Ferrannini E, Hendler R, Wahren J, Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978;75:5173–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983;32:35–45 [DOI] [PubMed] [Google Scholar]
- 9.Cherrington AD, Williams PE, Abou-Mourad N, Lacy WW, Steiner KE, Liljenquist JE. Insulin as a mediator of hepatic glucose uptake in the conscious dog. Am J Physiol. 1982;242:E97–101 [DOI] [PubMed] [Google Scholar]
- 10.Cherrington AD, Williams PE, Harris MS. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism. 1978;27:787–91 [DOI] [PubMed] [Google Scholar]
- 11.Vella A, Shah P, Basu R, Basu A, Camilleri M, Schwenk WF, Rizza RA. Effect of enteral vs. parenteral glucose delivery on initial splanchnic glucose uptake in nondiabetic humans. Am J Physiol Endocrinol Metab. 2002;283:E259–66 [DOI] [PubMed] [Google Scholar]
- 12.Myers SR, McGuinness OP, Neal DW, Cherrington AD. Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest. 1991;87:930–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abumrad NN, Cherrington AD, Williams PE, Lacy WW, Rabin D. Absorption and disposition of a glucose load in the conscious dog. Am J Physiol. 1982;242:E398–406 [DOI] [PubMed] [Google Scholar]
- 14.Moore MC, Cherrington AD, Cline G, Pagliassotti MJ, Jones EM, Neal DW, Badet C, Shulman GI. Sources of carbon for hepatic glycogen synthesis in the conscious dog. J Clin Invest. 1991;88:578–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrannini E, Bjorkman O, Reichard GA, Jr, Pilo A, Olsson M, Wahren J, DeFronzo RA. The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes. 1985;34:580–8 [DOI] [PubMed] [Google Scholar]
- 16.Mari A, Wahren J, DeFronzo RA, Ferrannini E. Glucose absorption and production following oral glucose: comparison of compartmental and arteriovenous-difference methods. Metabolism. 1994;43:1419–25 [DOI] [PubMed] [Google Scholar]
- 17.Ishida T, Chap Z, Chou J, Lewis R, Hartley C, Entman M, Field JB. Differential effects of oral, peripheral intravenous, and intraportal glucose on hepatic glucose uptake and insulin and glucagon extraction in conscious dogs. J Clin Invest. 1983;72:590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannucci E, Monami M, Lamanna C, Adalsteinsson JE. Post-prandial glucose and diabetic complications: systematic review of observational studies. Acta Diabetol. Epub 2011. Nov 25. [DOI] [PubMed] [Google Scholar]
- 19.Bergman RN, Beir JR, Hourigan PM. Intraportal glucose infusion matched to oral glucose absorption. Lack of evidence for “gut factor” involvement in hepatic glucose storage. Diabetes. 1982;31:27–35 [DOI] [PubMed] [Google Scholar]
- 20.Barrett EJ, Ferrannini E, Gusberg R, Bevilacqua S, DeFronzo RA. Hepatic and extrahepatic splanchnic glucose metabolism in the postabsorptive and glucose fed dog. Metabolism. 1985;34:410–20 [DOI] [PubMed] [Google Scholar]
- 21.Pagliassotti MJ, Holste LC, Moore MC, Neal DW, Cherrington AD. Comparison of the time courses of insulin and the portal signal on hepatic glucose and glycogen metabolism in the conscious dog. J Clin Invest. 1996;97:81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers SR, Biggers DW, Neal DW, Cherrington AD. Intraportal glucose delivery enhances the effects of hepatic glucose load on net hepatic glucose uptake in vivo. J Clin Invest. 1991;88:158–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galassetti P, Shiota M, Zinker BA, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient decreases skeletal muscle glucose uptake. Am J Physiol. 1998;275:E101–11 [DOI] [PubMed] [Google Scholar]
- 24.Ogihara N, Kawamura W, Kasuga K, Hayashi Y, Arakawa H, Kikuchi M. Characterization of the portal signal during 24-h glucose delivery in unrestrained, conscious rats. Am J Physiol Endocrinol Metab. 2004;286:E932–40 [DOI] [PubMed] [Google Scholar]
- 25.Chueh FY, Malabanan C, McGuinness OP. Impact of portal glucose delivery on glucose metabolism in conscious, unrestrained mice. Am J Physiol Endocrinol Metab. 2006;291:E1206–11 [DOI] [PubMed] [Google Scholar]
- 26.Féry F, Deviere J, Balasse EO. Metabolic handling of intraduodenal vs. intravenous glucose in humans. Am J Physiol Endocrinol Metab. 2001;281:E261–8 [DOI] [PubMed] [Google Scholar]
- 27.Moore MC, Satake S, Lautz M, Soleimanpour SA, Neal DW, Smith M, Cherrington AD. Nonesterified fatty acids and hepatic glucose metabolism in the conscious dog. Diabetes. 2004;53:32–40 [DOI] [PubMed] [Google Scholar]
- 28.Moore MC, Flakoll PJ, Hsieh PS, Pagliassotti MJ, Neal DW, Monohan MT, Venable C, Cherrington AD. Hepatic glucose disposition during concomitant portal glucose and amino acid infusions in the dog. Am J Physiol. 1998;274:E893–902 [DOI] [PubMed] [Google Scholar]
- 29.Moore MC, Hsieh PS, Flakoll PJ, Neal DW, Cherrington AD. Differential effect of amino acid infusion route on net hepatic glucose uptake in the dog. Am J Physiol. 1999;276:E295–302 [DOI] [PubMed] [Google Scholar]
- 30.Dardevet D, Kimball SR, Jefferson LS, Cherrington AD, Remond D, DiCostanzo CA, Moore MC. Portal infusion of amino acids is more efficient than peripheral infusion in stimulating liver protein synthesis at the same amino acid load in dogs. Am J Clin Nutr. 2008;88:986–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niijima A. Glucose-sensitive afferent nerve fibers in the liver and their role in food intake and blood glucose regulation. J Auton Nerv Syst. 1983;9:207–20 [DOI] [PubMed] [Google Scholar]
- 32.Niijima A. Reflex control of the autonomic nervous system activity from the glucose sensors in the liver in normal and midpontine-transected animals. J Auton Nerv Syst. 1984;10:279–85 [DOI] [PubMed] [Google Scholar]
- 33.Niijima A, Meguid MM. An electrophysiological study on amino acid sensors in the hepato-portal system in the rat. Obes Res. 1995;3(Suppl 5):741S–5S [DOI] [PubMed] [Google Scholar]
- 34.McCuskey RS. Anatomy of efferent hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:821–6 [DOI] [PubMed] [Google Scholar]
- 35.Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:827–35 [DOI] [PubMed] [Google Scholar]
- 36.Tiniakos DG, Mathew J, Kittas C, Burt AD. Ontogeny of human intrahepatic innervation. Virchows Arch. 2008;452:435–42 [DOI] [PubMed] [Google Scholar]
- 37.Akiyoshi H, Gonda T, Terada T. A comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig, dog and human livers. Liver. 1998;18:352–9 [DOI] [PubMed] [Google Scholar]
- 38.Adachi A, Shimizu N, Oomura Y, Kobashi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46:215–8 [DOI] [PubMed] [Google Scholar]
- 39.Schmitt M. Influences of hepatic portal receptors on hypothalamic feeding and satiety centers. Am J Physiol. 1973;225:1089–95 [DOI] [PubMed] [Google Scholar]
- 40.Jungermann K, Stumpel F. Role of hepatic, intrahepatic and hepatoenteral nerves in the regulation of carbohydrate metabolism and hemodynamics of the liver and intestine. Hepatogastroenterology. 1999;46: Suppl 2:1414–7 [PubMed] [Google Scholar]
- 41.Niijima A. Nervous regulation of metabolism. Prog Neurobiol. 1989;33:135–47 [DOI] [PubMed] [Google Scholar]
- 42.Adkins-Marshall B, Pagliassotti MJ, Asher JR, Connolly CC, Neal DW, Williams PE, Myers SR, Hendrick GK, Adkins RB, Jr, Cherrington AD. Role of hepatic nerves in response of liver to intraportal glucose delivery in dogs. Am J Physiol. 1992;262:E679–86 [DOI] [PubMed] [Google Scholar]
- 43.Pagliassotti MJ, Myers SR, Moore MC, Neal DW, Cherrington AD. Magnitude of negative arterial-portal glucose gradient alters net hepatic glucose balance in conscious dogs. Diabetes. 1991;40:1659–68 [DOI] [PubMed] [Google Scholar]
- 44.Hsieh PS, Moore MC, Marshall B, Pagliassotti MJ, Shay B, Szurkus D, Neal DW, Cherrington AD. The head arterial glucose level is not the reference site for generation of the portal signal in conscious dogs. Am J Physiol. 1999;277:E678–84 [DOI] [PubMed] [Google Scholar]
- 45.Hsieh PS, Moore MC, Neal DW, Cherrington AD. Importance of the hepatic arterial glucose level in generation of the portal signal in conscious dogs. Am J Physiol Endocrinol Metab. 2000;279:E284–92 [DOI] [PubMed] [Google Scholar]
- 46.Cardin S, Pagliassotti MJ, Moore MC, Edgerton DS, Lautz M, Farmer B, Neal DW, Cherrington AD. Vagal cooling and concomitant portal norepinephrine infusion do not reduce net hepatic glucose uptake in the conscious dog. Am J Physiol Regul Integr Comp Physiol. 2004;287:R742–8 [DOI] [PubMed] [Google Scholar]
- 47.DiCostanzo CA, Dardevet DP, Williams PE, Moore MC, Hastings JR, Neal DW, Cherrington AD. The effect of vagal cooling on canine hepatic glucose metabolism in the presence of hyperglycemia of peripheral origin. Metabolism. 2007;56:814–24 [DOI] [PubMed] [Google Scholar]
- 48.Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab. 2007;293:E96–101 [DOI] [PubMed] [Google Scholar]
- 49.Fernandes AB, Patarrao RS, Videira PA, Macedo MP. Understanding postprandial glucose clearance by peripheral organs: the role of the hepatic parasympathetic system. J Neuroendocrinol. 2011;23:1288–95 [DOI] [PubMed] [Google Scholar]
- 50.Xie H, Lautt WW. Insulin resistance of skeletal muscle produced by hepatic parasympathetic interruption. Am J Physiol. 1996;270:E858–63 [DOI] [PubMed] [Google Scholar]
- 51.Dicostanzo CA, Dardevet DP, Neal DW, Lautz M, Allen E, Snead W, Cherrington AD. Role of the hepatic sympathetic nerves in the regulation of net hepatic glucose uptake and the mediation of the portal glucose signal. Am J Physiol Endocrinol Metab. 2006;290:E9–16 [DOI] [PubMed] [Google Scholar]
- 52.Moore MC, Dicostanzo CA, Smith MS, Farmer B, Rodewald TD, Neal DW, Williams PE, Cherrington AD. Hepatic portal venous delivery of a nitric oxide synthase inhibitor enhances net hepatic glucose uptake. Am J Physiol Endocrinol Metab. 2008;294:E768–77 [DOI] [PubMed] [Google Scholar]
- 53.An Z, DiCostanzo CA, Moore MC, Edgerton DS, Dardevet DP, Neal DW, Cherrington AD. Effects of the nitric oxide donor SIN-1 on net hepatic glucose uptake in the conscious dog. Am J Physiol Endocrinol Metab. 2008;294:E300–6 [DOI] [PubMed] [Google Scholar]
- 54.Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res. 1994;649:343–7 [DOI] [PubMed] [Google Scholar]
- 55.Perrin C, Knauf C, Burcelin R. Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, controls muscle glycogen synthesis. Endocrinology. 2004;145:4025–33 [DOI] [PubMed] [Google Scholar]
- 56.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dardevet D, Moore MC, Neal D, DiCostanzo CA, Snead W, Cherrington AD. Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. Am J Physiol Endocrinol Metab. 2004;287:E75–81 [DOI] [PubMed] [Google Scholar]
- 58.Nishizawa M, Moore MC, Shiota M, Gustavson SM, Snead WL, Neal DW, Cherrington AD. Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am J Physiol Endocrinol Metab. 2003;284:E1027–36 [DOI] [PubMed] [Google Scholar]
- 59.Hegarty BD, Turner N, Cooney GJ, Kraegen EW. Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxf). 2009;196:129–45 [DOI] [PubMed] [Google Scholar]
- 60.Winnick JJ, An Z, Moore MC, Ramnanan CJ, Farmer B, Shiota M, Cherrington AD. A physiological increase in the hepatic glycogen level does not affect the response of net hepatic glucose uptake to insulin. Am J Physiol Endocrinol Metab. 2009;297:E358–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winnick JJ, An Z, Ramnanan CJ, Smith M, Irimia JM, Neal DW, Moore MC, Roach PJ, Cherrington AD. Hepatic glycogen supercompensation activates AMP-activated protein kinase, impairs insulin signaling, and reduces glycogen deposition in the liver. Diabetes. 2011;60:398–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matschinsky FM, Zelent B, Doliba NM, Kaestner KH, Vanderkooi JM, Grimsby J, Berthel SJ, Sarabu R. Research and development of glucokinase activators for diabetes therapy: theoretical and practical aspects. Handb Exp Pharmacol . 2011;(203):357–401. [DOI] [PubMed] [Google Scholar]
- 63.Shiota M, Moore MC, Galassetti P, Monohan M, Neal DW, Shulman GI, Cherrington AD. Inclusion of low amounts of fructose with an intraduodenal glucose load markedly reduces postprandial hyperglycemia and hyperinsulinemia in the conscious dog. Diabetes. 2002;51:469–78 [DOI] [PubMed] [Google Scholar]
- 64.McBride A, Hardie DG. AMP-activated protein kinase–a sensor of glycogen as well as AMP and ATP? Acta Physiol (Oxf). 2009;196:99–113 [DOI] [PubMed] [Google Scholar]
- 65.Camacho RC, Lacy DB, James FD, Donahue EP, Wasserman DH. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside renders glucose output by the liver of the dog insensitive to a pharmacological increment in insulin. Am J Physiol Endocrinol Metab. 2005;289:E1039–43 [DOI] [PubMed] [Google Scholar]
- 66.Camacho RC, Pencek RR, Lacy DB, James FD, Donahue EP, Wasserman DH. Portal venous 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion overcomes hyperinsulinemic suppression of endogenous glucose output. Diabetes. 2005;54:373–82 [DOI] [PubMed] [Google Scholar]
- 67.Pencek RR, Shearer J, Camacho RC, James FD, Lacy DB, Fueger PT, Donahue EP, Snead W, Wasserman DH. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside causes acute hepatic insulin resistance in vivo. Diabetes. 2005;54:355–60 [DOI] [PubMed] [Google Scholar]
- 68.Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, et al. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–71 [DOI] [PubMed] [Google Scholar]
- 69.Mukhtar MH, Payne VA, Arden C, Harbottle A, Khan S, Lange AJ, Agius L. Inhibition of glucokinase translocation by AMP-activated protein kinase is associated with phosphorylation of both GKRP and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Am J Physiol Regul Integr Comp Physiol. 2008;294:R766–74 [DOI] [PubMed] [Google Scholar]
- 70.Li J, Hu X, Selvakumar P, Russell RR, 3rd, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–41 [DOI] [PubMed] [Google Scholar]
- 71.Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik G, Menon VP, Bagchi D, Maulik N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J Cell Mol Med. 2008;12:2350–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol. 2011;214:254–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deshmukh AS, Long YC, de Castro Barbosa T, Karlsson HK, Glund S, Zavadoski WJ, Gibbs EM, Koistinen HA, Wallberg-Henriksson H, Zierath JR. Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)alpha1-specific activity and glucose transport in human skeletal muscle. Diabetologia. 2010;53:1142–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001;50:241–7 [DOI] [PubMed] [Google Scholar]
- 75.Fryer LG, Hajduch E, Rencurel F, Salt IP, Hundal HS, Hardie DG, Carling D. Activation of glucose transport by AMP-activated protein kinase via stimulation of nitric oxide synthase. Diabetes. 2000;49:1978–85 [DOI] [PubMed] [Google Scholar]
- 76.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–39 [DOI] [PubMed] [Google Scholar]
- 77.Guarino MP, Correia NC, Lautt WW, Macedo MP. Insulin sensitivity is mediated by the activation of the ACh/NO/cGMP pathway in rat liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G527–32 [DOI] [PubMed] [Google Scholar]
- 78.Guarino MP, Macedo MP. Co-administration of glutathione and nitric oxide enhances insulin sensitivity in Wistar rats. Br J Pharmacol. 2006;147:959–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sadri P, Lautt WW. Blockade of hepatic nitric oxide synthase causes insulin resistance. Am J Physiol. 1999;277:G101–8 [DOI] [PubMed] [Google Scholar]
- 80.An Z, Winnick JJ, Farmer B, Neal D, Lautz M, Irimia JM, Roach PJ, Cherrington AD. A soluble guanylate cyclase-dependent mechanism is involved in the regulation of net hepatic glucose uptake by nitric oxide in vivo. Diabetes. 2010;59:2999–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.An Z, Winnick JJ, Farmer B, Lautz M, Neal D, Snead W, Cherrington AD. The cGMP pathway plays a role in the regulation of net hepatic glucose uptake (NHGU) in conscious dogs. Diabetes. 2010;59:A412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–35S [DOI] [PubMed] [Google Scholar]
- 83.U.S. Department of Agriculture. Agricultural Research Service: nutrient intakes from food: mean amounts consumed per individual, by gender and age. In What we eat in America, NHANES 2007–2008, 2010. Available from: www.ars.usda.gov/ba/bhnrc/fsrg.
- 84.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 85.McAuley K, Mann J. Thematic review series: patient-oriented research. Nutritional determinants of insulin resistance. J Lipid Res. 2006;47:1668–76 [DOI] [PubMed] [Google Scholar]
- 86.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hosseini-Esfahani F, Bahadoran Z, Mirmiran P, Hosseinpour-Niazi S, Hosseinpanah F, Azizi F. Dietary fructose and risk of metabolic syndrome in adults: Tehran Lipid and Glucose study. Nutr Metab (Lond). 2011;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stanhope KL. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med. 2012;63:329–43 [DOI] [PubMed] [Google Scholar]
- 89.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46 [DOI] [PubMed] [Google Scholar]
- 90.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011;4:243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panchal SK, Poudyal H, Iyer A, Nazer R, Alam MA, Diwan V, Kauter K, Sernia C, Campbell F, Ward L, et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol. 2011;57:611–24 [DOI] [PubMed] [Google Scholar]
- 94.Coate KC, Scott M, Farmer B, Moore MC, Smith MS, Roop J, Neal DW, Williams PE, Cherrington AD. Chronic consumption of a high fat/high fructose diet renders the liver incapable of net hepatic glucose uptake. Am J Physiol Endocrinol Metab. 2010;299:E887–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coate KC, Kraft G, Lautz M, Smith M, Neal DW, Cherrington AD. A high-fat, high-fructose diet accelerates nutrient absorption and impairs net hepatic glucose uptake in response to a mixed meal in partially pancreatectomized dogs. J Nutr. 2011;141:1643–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coate KC, Kraft G, Smith MS, Farmer B, Roop JD, Shiota M, Vaughan S, Hajizadeh S, Cherrington AD. Increased dietary fructose markedly reduces hepatic glucose uptake and glycogen synthesis. Diabetes. 2011;60:A454 [Google Scholar]
- 97.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–214 [DOI] [PubMed] [Google Scholar]