Abstract
The consequences of deep wound infections before, during, and after coronary artery bypass grafting have prompted research to clarify risk factors and explore preventive measures to keep infection rates at an irreducible minimum. An analysis of 42 studies in which investigators used multivariate logistic regression analysis revealed that diabetes mellitus and obesity are by far the chief preoperative risk factors. A 4-point preoperative scoring system based on a patient's body mass index and the presence or absence of diabetes is one practical way to determine the risk of mediastinitis, and other risk-estimate methods are being refined. Intraoperative risk factors include prolonged perfusion time, the use of one or more internal mammary arteries as grafts, blood transfusion, and mechanical circulatory assistance. The chief postoperative risk factor is reoperation, usually for bleeding. Unresolved issues include the optimal approach to Staphylococcus aureus nasal colonization and the choice of a prophylactic antibiotic regimen. We recommend that cardiac surgery programs supplement their audit processes and ongoing vigilance for infections with periodic, multidisciplinary reviews of best-practice standards for preoperative, intraoperative, and postoperative patient care.
Key words: Anti-bacterial agents/administration & dosage/therapeutic use, antibiotic prophylaxis/methods/standards, bacterial infections/prevention & control, clinical trials as topic, coronary artery bypass/adverse effects, mediastinitis/prevention & control, postoperative complications/prevention & control, quality assurance/health care/methods, risk factors, surgical wound infection/prevention & control
Deep infections complicate between 0.25% and 4% of major cardiac surgical procedures, cause death or substantial morbidity, concern healthcare administrators as indices of hospital quality, and challenge surgeons, other healthcare workers, and hospitals to keep infection rates at an irreducible minimum.1–4 The principles discussed in this review apply broadly to cardiac surgery, with a focus on coronary artery bypass grafting (CABG). (Of note, superficial infections complicating CABG,5 and also infections complicating heart transplantation, device implantation, and pediatric cardiac surgery, have somewhat different risk factors than do CABG-related deep infections.)
Pathogenesis, Risk Factors, and Preoperative Evaluation
Deep infection is defined here as infection below the level of the subcutaneous tissue with involvement of the muscle, fascia, bone (particularly the sternum), and body spaces (particularly the mediastinum). These infections typically result from contamination during surgery. The inevitability of wound contamination was shown in a study in which human albumin microspheres labeled with technetium-99m pertechnetate were applied preoperatively to patients' skin (outside the area of incision, and often to remote sites covered with a plastic drape) and to the surgeon's forehead, temples, and mask before clean orthopedic surgery. Considerable wound contamination from both the patient and the surgeon was invariable,6 confirming the adage that every surgical procedure is an experiment in applied microbiology. Less often, deep infection results from the postoperative tracking of organisms along the surgical wound or from hematogenous seeding (blood-borne infection from another site, such as a vascular-access catheter) to the surgically wounded tissue, which becomes a “place of least resistance” or locus minoris resistentiae.
One formula approximates the risk of infection by means of the following quotient: (number of organisms in the inoculum × virulence of the organisms) divided by host resistance to infection. Low-virulence skin-flora organisms such as coagulase-negative staphylococci require high inocula to cause infection, except in the case of implanted devices such as prosthetic heart valves. More virulent organisms such as Staphylococcus aureus and Pseudomonas aeruginosa require fewer organisms to cause infection. The likelihood that infection will complicate an operation depends on the outcome of a 6-hour “grace period” during which contaminative organisms battle the body's defense mechanisms, independent of preventive antibiotics. In patients undergoing CABG, the impairment of host defenses is the rule, and it includes conditions that are not usually mentioned in discussions about an immune-compromised host. Prominent among these conditions is obesity, which predisposes the patient to wounds that are highly contaminated, poorly perfused, and lacking in adequate antibiotic concentrations.7
A MEDLINE search yielded 42 studies in which multivariate regression analysis was used to identify risk factors for CABG-related deep infections (Table I).8–59 Fifteen of these studies were performed in the United States, and 27 were conducted in 16 other nations. Diabetes mellitus and obesity— this last of which is often defined in terms of body mass index (BMI)—were the preoperative risk factors identified most often. Intraoperative risk factors included prolonged perfusion time, multiple grafts or the use of one or more internal mammary arteries, and mechanical circulatory assistance. The chief postoperative risk factor was reoperation, usually for bleeding.
TABLE I. Independent Risk Factors for Deep Infectious Complications of Coronary Artery Bypass Grafting (CABG)*
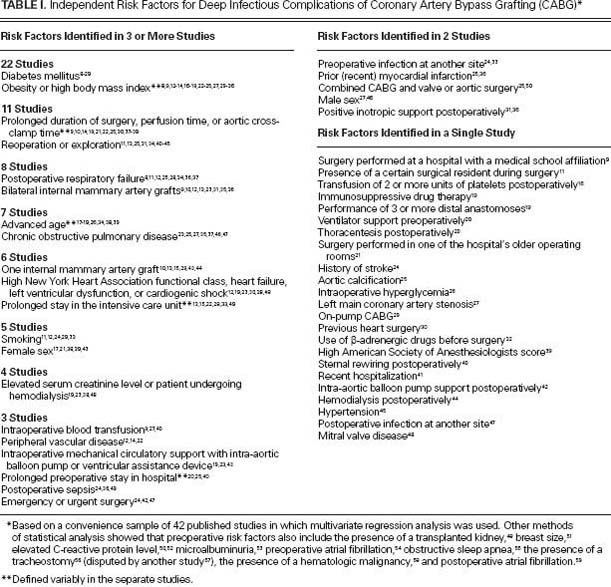
Preoperative risk evaluation improves the informed-consent process and occasionally identifies modifiable factors, such as smoking. Various frameworks for evaluating an individual patient's risks have been developed. The National Nosocomial Infections Surveillance (NNIS) risk index for surgical infection, developed by the Centers for Disease Control and Prevention (CDC),60 is less well suited for CABG than for certain other types of surgery, in part because CABG is always “clean” surgery and the American Society of Anesthesiologists scores are by definition greater than 2.61 Australian investigators found that the NNIS risk index performed less well in CABG than in 6 other types of surgery.62,63 The most elaborate and specific system for estimating CABG-related major infection was developed by Fowler and colleagues.19 They analyzed 331,429 CABG operations that were performed from 2002 through 2003 and were recorded in the Society of Thoracic Surgeons (STS) National Cardiac Database. A risk score based on 12 variables enabled the investigators to estimate the probability of infection, which ranged from 0.9% (risk score, 0) to 16% (risk score, ≥26). Patients with a major infection had a higher mortality rate than did patients without such infection (17.3% vs 3%) and were more likely to have a postoperative hospital stay exceeding 14 days (47% vs 5.9%).19 Investigators subsequently found that combining the use of the system developed by Fowler and colleagues (based on the STS database) and the EuroSCORE system (originally designed to predict mortality rates64) facilitated risk stratification.64,65 A simpler system, the Australian Clinical Risk Index, uses a 4-point score based on just 2 variables: the presence or absence of diabetes mellitus, and the patient's BMI.62 On the basis of 1 point for the presence of diabetes mellitus, 1 point for a BMI from 30 through 34.9, and 2 points for a BMI ≥35, a patient's risk score can vary from 0 to 3. Investigators in the United States validated this prediction method and determined that each additional point was associated with a 2-fold increase in the risk of surgical-site infection; however, the definition of infection was not limited to deep infection.66 In view of the limitations of the NNIS risk index for CABG, the CDC proposed a new risk model in 2012. This model is being considered for endorsement as a measure for public reporting, an act that is required in 28 U.S. states and the District of Columbia.39
Minimizing Preoperative Risk Factors
Most preoperative risk factors for CABG-related deep infections, or at least those identified by multivariate regression analysis (Table I), lend themselves poorly to preoperative intervention. The control of diabetes, as evaluated in accordance with hemoglobin A1C levels, ideally should be optimized, although preoperative glucose levels probably matter less than do intraoperative and postoperative levels. The appropriate management of antiplatelet drugs such as aspirin and clopidogrel, and also of anticoagulative drugs such as warfarin, is important, complicated, and controversial.67–71 Here, we will review 3 interventions pertaining to preoperative care: identification of nasal staphylococcal carriage and the use of decolonization therapy with mupirocin nasal ointment; preoperative bathing; and prophylactic antimicrobial therapy. We will not discuss familiar standard-of-care measures such as preoperative scrubbing or the use of clippers instead of razors for hair removal (although policies for these measures need to be rigorously implemented72), or potentially useful but unproved therapy with preoperative statins73,74 or nutritional supplements that are beneficial to the immune system.75,76 It could be preferable to schedule patients who run a high risk of infection and other complications as first-in-the-morning cases, because the time of operation (morning vs afternoon) may make a difference.77
Preoperative Screening for Staphylococcus aureus Nasal Carriage and the Use of Mupirocin Nasal Ointment
Many regulatory and public-reporting issues center on healthcare-associated infections from S. aureus, especially infections caused by methicillin-resistant strains (MRSA). A large body of literature, including some comprehensive reviews,78,79 focuses on preoperative screening for S. aureus nasal carriage and the use of mupirocin nasal ointment to reduce or eliminate that bacterium. However, determining a best-practices standard is not straightforward. Reservations about universal screening for staphylococcal nasal carriage and using mupirocin in all patients include questions about efficacy, cumbersome follow-up of culture results, the potential for widespread high-level mupirocin resistance, and cost. A cardiac surgery program can adopt any of several approaches (Table II).
TABLE II. Approaches to Preoperative Nasal Cultures for Staphylococcus aureus and Decolonization Therapy with Mupirocin in CABG

Three reasons underlie the present rationale for preoperative screening and treatment with mupirocin nasal ointment. First, from 40% to more than 80% of CABG-complicating infections are due to S. aureus, increasingly including MRSA strains.80,81 Deep surgical infections caused by MRSA possibly carry higher risks of morbidity and death than do infections caused by methicillin-susceptible S. aureus (MSSA); however, patients who contract MRSA infections tend to be older and have more comorbidities.82,83 Second, most surgical infections arise from the patient's own flora, and data suggest that nasal colonization with S. aureus often precedes deep infection. In a multicenter study,84 1,640 S. aureus isolates were collected off nasal swabs from 1,278 patients over 5 years. Fourteen of the 1,278 patients subsequently developed S. aureus bacteremia, and in 12 of those 14 patients the blood isolates were clonally identical to the previous nasal isolates. Third, some studies suggest that identifying S. aureus carriage and treating the carriers with 2% mupirocin ointment lowers infection rates.85
In 2007, the STS issued practice guidelines that included this Class IA recommendation: “Routine mupirocin administration is recommended for all patients undergoing cardiac surgical procedures in the absence of a documented negative testing for staphylococcal colonization.”86 However, the results of individual studies81,87 and meta-analyses of the published literature88,89 have been conflicting. In 2008, one meta-analysis determined that mupirocin is useful88; however, another revealed that no blanket recommendation could be made for mupirocin use in cardiac surgery patients.89 The authors of the second analysis89 noted that the only prospective, randomized, double-blinded trial of mupirocin in cardiac-surgery patients showed no benefit. In contrast with MSSA-related mediastinitis, none of 8 patients with post-sternotomy mediastinitis caused by MRSA had identical isolates (as tested by means of pulsed-field gel electrophoresis) in preoperative and surgical-site cultures.90 The same clone of MRSA was found in all 8 instances, suggesting that hospital infection-control measures might be more important in MRSA infections than in MSSA infections.90 Neither MRSA nasal carriage on admission nor topical decolonization treatment predicted MRSA surgical-site infections.91 In an evaluation of MRSA cultured from nasal and inguinal swabs, preoperative MRSA carriers undergoing elective heart surgery did not have a higher incidence of MRSA wound infections than did non-carriers.92 This study was one of the few to have examined extra-nasal carriage sites, which are especially important in the transmission of community-acquired MRSA strains.
The recommendation that all patients be screened preoperatively for S. aureus nasal carriage (the “screen all” approach) or, alternatively, treated empirically with mupirocin nasal ointment (the “treat all” approach), is strongly endorsed during outbreaks of MRSA or MSSA.93 Screen-all or treat-all approaches also make sense in hospitals that have a high incidence of deep S. aureus infections after CABG. A hospital in which MRSA caused 56% of postoperative infections adopted the following practice: giving intranasal mupirocin to all patients (regardless of colonization status) for 5 days before surgery, giving combined mupirocin and vancomycin prophylaxis to all MRSA-colonized patients, and applying mupirocin to chest-tube sites at the time of tube removal. These steps yielded a near-complete and sustained reduction of MRSA wound infections after cardiac surgery.85
The resistance of S. aureus to mupirocin, a natural antibiotic produced by Pseudomonas fluorescens, was recognized shortly after mupirocin was introduced into clinical practice during the 1980s.94 High-level resistance to mupirocin (minimum inhibitory concentration [MIC], ≥512 µg/mL) is currently less than 5% among MRSA isolates in the United States.95 However, new mechanisms of resistance continue to appear.96,97 Few clinical laboratories currently screen S. aureus isolates for resistance to mupirocin. Although an empiric treat-all approach has been endorsed for use in cardiac and other types of surgery,98 and although it is unclear whether short-term mupirocin for nasal colonization promotes high-level resistance, it seems reasonable to ask whether a treat-all approach is consistent with long-term social responsibility (that is, the desirability of holding down the emergence of mupirocin-resistant S. aureus strains). For this reason alone, screen-all is better for hospitals with an appreciable incidence of S. aureus-related deep infections after CABG. Screening, if performed at all, should probably be universal. A patient's medical history is a poor predictor of MRSA colonization.99 Selective screening—that is, screening only those patients who are considered to be at high risk of infection—raises the issue of how to decide who is screened and who is not.
The major drawbacks of a screen-all approach are logistics and cost. The recommended duration of mupirocin therapy for suppressing S. aureus nasal carriage is 5 days, so therapy should ideally begin several days before surgery. However, patients who have been scheduled for elective CABG are commonly admitted to the hospital the previous afternoon or evening, because a longer preoperative stay seems to be a risk factor for infection.20,25,40 Culture-based screening therefore necessitates outpatient procurement of the culture, someone to follow up on the culture result, and someone to prescribe timely therapy if the culture is positive. Rapid screening by means of the polymerase chain reaction (PCR), which largely overcomes these logistical problems, has been studied in cardiac-surgery patients for more than a decade.80 As a basis for mupirocin therapy, PCR-based screening has been shown to reduce the overall usage of mupirocin while also lowering the rate of MRSA infections that complicate cardiac surgery.101,102 Rapid PCR-based screening followed by the treatment of S. aureus nasal carriers with mupirocin ointment and chlorhexidine soap reduced the risk of postoperative infections by nearly 60% in various types of surgery.102 In an economic analysis, routine preoperative screening for MRSA was financially feasible over a wide range of MRSA-colonization prevalence levels: the incremental cost-effectiveness ratio was well under U.S. $15,000 per quality-adjusted life-year gained from hospital and third-party-payer perspectives.103 Therefore, it behooves cardiac surgery programs that do not currently use this methodology to review its potential applicability.
Another decolonization approach is to combine a nasal ointment and an oral rinse, both containing 0.12% chlorhexidine gluconate.104 The oral rinse, Peridex™ (3M ESPE; St. Paul, Minn), is approved for use in the United States.
Preoperative Bathing
When patients shower or bathe preoperatively with antiseptic agents, it reduces bacterial colonization. This approach is widely used before cardiac and other surgery. Chlorhexidine reduces skin bacterial-colony counts to a greater extent than does povidone-iodine or other agents that have been studied. However, in a comprehensive, systematic literature review published in 2012,105 the authors concluded only that preoperative antiseptic showers may be effective in preventing postoperative infections. Three randomized, controlled trials yielded no difference in postoperative infection rates between 3 groups of patients who showered preoperatively (with chlorhexidine or povidone-iodine, with soap and water, or with a placebo) and a group that was given no showering instructions. The authors reported no clear advantage of one agent over another and noted the difficulty in drawing conclusions about an active ingredient, because disinfectants are often mixed with alcohol or water.105
One explanation for the inability to show benefit from preoperative antiseptic showering comes from a study of quantitative cultures obtained during cardiac surgery from subcutaneous sternal tissue and skin surrounding the wound. Bacteria—predominantly coagulase-negative staphylococci and Propionibacterium acnes—were isolated from the subcutaneous sternal tissue in 89% of cases and from the skin surrounding the wound in 98%. In nearly half of these instances, the density exceeded 10,000 colony-forming units per culture pad. It was concluded that preoperative skin preparation with ethanol and chlorhexidine cannot prevent skin-flora organisms from contaminating wounds and the surrounding tissue for the duration of the operation.106 Reasons include the large numbers of organisms in skin appendages such as sweat glands, and the constant turnover of surface epithelial cells.
Prophylactic Antibiotic Therapy
The benefits of appropriately administered prophylactic antibiotic therapy in patients undergoing cardiac surgery are so beyond dispute that placebo-controlled trials would no longer be permissible. Of note, however, is one small early trial in which antibiotics did not lower the incidence of infection but instead appeared to influence which organisms were causative.107 Current guidelines are that a cephalosporin—usually cefazolin or cefuroxime—should be given within 60 minutes of the skin incision and be continued for no longer than 48 hours.2,86 Vancomycin is reserved mainly for patients with a history of type I allergic reaction (anaphylaxis, urticaria, angioedema, or bronchospasm) to β-lactam agents or when MRSA is of special concern, as discussed below.
Proper timing of the preoperative antibiotic dose is now widely used as a quality-of-care indicator.108–110 Investigators continue to study how to maintain adequate antibiotic levels in serum and tissue throughout surgery and the immediate postoperative period. Consider, for example, the following studies of cefazolin. In 2001, it was reported that intraoperative re-dosing of cefazolin reduced infection after cardiac surgery by 16%, including procedures lasting less than 4 hours; as a result, an automated reminder system was introduced.111,112 In 2006, the need was confirmed for an intraoperative dose of cefazolin after 120 minutes of cardiopulmonary bypass time.113 In 2008, it was reported that a 24-hour, multiple-dose regimen of cefazolin more than halved the infection rate (from 8.3% to 3.6%) compared with single-dose cefazolin.114 In 2010, investigators reported that a cefazolin bolus followed by continuous infusion improved pharmacokinetic and pharmacodynamic values, including concentrations in the heart muscle.115 However, even a 2-g dose of cefazolin failed to provide adequate tissue levels in morbidly obese patients,116 which suggested the need for additional studies in this important subgroup. In another study, continuing cefazolin beyond 24 hours did not reduce the incidence of deep sternal wound infections,117 supporting the general consensus that 24-hour prophylaxis suffices in most major surgical procedures.118 However, a systematic review and meta-analysis of the literature led to the conclusion that perioperative antibiotic prophylaxis beyond 24 hours might be more effective than shorter regimens for preventing sternal wound infections, with the caveat that heterogeneous regimens in various studies and possible investigator bias might preclude definite conclusions.119
The frequent identification of MRSA as a cause of deep sternal wound infection calls into question whether older cephalosporins should still be the prophylactic drugs of choice. Specifically, should vancomycin become the preferred agent?120 Vancomycin is often used during outbreaks of MRSA infection, in concert with other measures such as the screen-all or treat-all approaches to MRSA nasal colonization.85,93 However, vancomycin falls short of “blockbuster” drug status. Unlike the β-lactam antibiotics (the penicillins and cephalosporins), vancomycin is only slowly bactericidal; indeed, some authorities classify vancomycin as bacteriostatic. Vancomycin is considerably less active than nafcillin and oxacillin against mutually susceptible strains of S. aureus. In a tertiary-care center with a high prevalence of MRSA infection, patients undergoing cardiac surgery were randomized to receive vancomycin or cefazolin; the overall infection rates were similar, but infections caused by MSSA occurred more often in the patients who received vancomycin.121 The activity of vancomycin is essentially limited to gram-positive bacteria. Although MRSA strains with high-level resistance to vancomycin (MIC, ≥16 µg/mL) remain rare, strains with reduced susceptibility are increasingly prevalent. This phenomenon, “MIC creep,” is of wide concern, because strains with an MIC of 2 µg/mL or more respond less well to vancomycin therapy. Because the rapid infusion of vancomycin can trigger a histamine-release phenomenon characterized by extensive flushing in the upper chest (“red man's syndrome”),122 many guidelines indicate that infusion should begin about 120 minutes before the skin incision. However, as with other antibiotics, the incidence of infection is lower when the drug is given within 60 minutes of incision.123 In the meantime, careful attention should be given to vancomycin dosage. The preoperative dose should be 15 mg/kg (rather than the commonly used 1-g dose for all adult patients)124; a postoperative dose (10 mg/kg) is also recommended125; and findings in the literature should be heeded with respect to vancomycin dosing in morbidly obese patients, because the optimal dose has not been determined.126
The current consensus is that vancomycin should not be the routine or default drug of choice for non–penicillin-allergic patients who undergo cardiac and other surgery.86,120 Vancomycin can be an important component of an “MRSA-prevention bundle” in selected circumstances.85,93,127 Studies performed a decade or more ago indicated that β-lactam antibiotics (in particular, the cephalosporins) surpassed vancomycin in overall performance; however, vancomycin was superior in preventing infections due to methicillin-resistant gram-positive bacteria (in particular, MRSA and MRSE—methicillin-resistant coagulase-negative staphylococci such as S. epidermidis).128 The MIC creep of S. aureus in relation to vancomycin is disquieting to those who formulate guidelines for prophylaxis.129,130 Should randomized controlled trials be conducted to compare the now-traditional cephalosporins (such as cefazolin and cefuroxime) against combination therapy with vancomycin and a drug active against gram-negative pathogens—for example, single-dose ceftriaxone, which has favorable pharmacokinetics?131,132 Should daptomycin be tried, because it is rapidly bactericidal against staphylococci? (Many infectious-disease specialists might prefer daptomycin for themselves in this situation but would question routine prophylactic daptomycin use: daptomycin is currently a drug of last resort for life-threatening MRSA infection, and daptomycin-nonsusceptible S. aureus strains are becoming more prevalent. A MEDLINE search revealed no studies of daptomycin for prophylaxis in cardiac and thoracic surgery.) In summary, the optimal choices of agents, doses, and dosage schedules for prophylactic antibiotic therapy necessitate ongoing scrutiny.
Minimizing Intraoperative Risk Factors
In minimizing intraoperative risk factors, considerations include a sterile operating room with adequate ventilation, because airborne pathogens such as Aspergillus and Legionella species can cause outbreaks in cardiac surgery patients21,133–136; hygienic operating-room practices, including limited traffic flow; and adherence to basic surgical principles, as expressed in particular by William Stewart Halsted. These last include control of bleeding, accurate anatomic dissection, the use of completely sterile equipment, strict adherence to aseptic operative technique, exact approximation of tissue in wound closures without excessive tightness, and gentle handling of tissues. The control of bleeding is especially important. Excessive bleeding and hematoma formation creates a culture medium or locus minoris resistentiae that is a major risk factor for mediastinitis. Intraoperative risk factors predisposing patients to hemorrhage include prolonged perfusion time, the use of a ventricular assist device or intra-aortic balloon pump, and aortic dissection.23 In one study, 71 of 136 patients (52%) who had been supported with an intra-aortic balloon pump during cardiac surgery developed a postoperative infection.137 Blood transfusion also seems to increase the risk of infection138–141 and could be the major preventable intraoperative risk factor for mediastinitis.27 The benefits of transfusing leukocyte-reduced blood are unclear.142,143
The use of internal mammary artery grafts in high-risk patients, specifically bilateral grafts in patients with diabetes mellitus,10,144,145 continues to be controversial. The effects on infection rates of off-pump CABG and of minimally invasive surgical techniques such as mini-sternotomy are not yet clear.146,147 Inadvertent paramedian sternotomy, which reduces sternal stability, might increase the risk of infection.148 In a multicenter study of 815 patients at high risk of sternal instability and infection, external reinforcement with use of the method described by Robicsek did not reduce the complication rates.149 A reinforced sternal-closure system in elderly patients with osteoporosis yielded no benefit150; however, rigid-plate sternal fixation in high-risk patients reduced the incidence of postoperative infection.151 Studies examining techniques of sternal wiring (figure-of-8 vs interrupted wires, and number of wires) and skin closure (intracutaneous vs transcutaneous) have yielded somewhat conflicting results.36,152–154 A multicenter trial of the Posthorax® support vest (Posthorax GmbH; Vienna, Austria) showed a significant lowering of sternal complications, including the need for reoperation155; however, further experience is needed before this device can be endorsed as standard-of-care. Levels of concern have varied in regard to the risks of mediastinitis and sternal osteomyelitis from the liberal application of bone wax. Animal studies showed that lower numbers of S. aureus organisms (inocula size) were needed to cause infection,156 but a prospective, randomized study of 400 patients157 showed no detrimental effect; the authors concluded that bone wax is “obviously safe but not particularly beneficial.”157
Control of Hyperglycemia
Hyperglycemia promotes pathogen proliferation, impairs neutrophil function, and possibly has other effects on host defenses. Most but not all retrospective studies indicate that poor glucose control promotes CABG-related complications and increases mortality rates.158,159 Investigators at the Mayo Clinic concluded that intraoperative hyperglycemia is an independent risk factor for complications: a 20-mg/dL increase in the mean intraoperative glucose level correlated with an increase of more than 30% in adverse outcomes (a composite of death, infections, or major organ-system complications).160 Investigators with the Portland Diabetic Project, a large prospective study of diabetic patients undergoing cardiac surgery, confirmed that hyperglycemia was an independent risk factor for death, length of hospital stay, and infection rates, and showed that continuous insulin infusions eliminated these risks.161,162 The danger of tight glucose control is inadvertent hypoglycemia. Literature from the late 2000s emphasizes continuous infusion protocols with frequent blood-glucose monitoring during surgery and the immediate postoperative period, supplemented by a multidisciplinary approach that incorporates nursing education, feedback, and ongoing audits of procedures.163–167
Novel Approaches to Infection Control
In regard to deep sternal infections, efforts continue worldwide to examine variables and try novel approaches. In a prospective study of more than a thousand patients, Spanish investigators could not show a relationship between infection rates and the inspired oxygen fraction during surgery.168 Italian investigators reviewed randomized, double-blinded trials of intraoperative steroids that have been used in cardiac surgery, with the rationale that the acute inflammatory response might contribute to postoperative morbidity. Steroid prophylaxis had no effect on mortality rates, the duration of mechanical ventilation, re-exploration for bleeding, or postoperative infection.169 Japanese investigators claimed to reduce infection rates by spraying an antibiotic solution containing cefazolin and gentamicin into the operative field.170 In a Swedish study, carbon dioxide insufflation into the cardiothoracic wound cavity—a technique for preventing arterial air embolism—reduced the risk of airborne contamination and postoperative infection when the insufflation was performed with a gas-diffuser. Conversely, insufflation with an open-ended tube substantially increased the risk of airborne contamination and wound infection.171
There is no consensus about the effectiveness of topical agents with antimicrobial activity. Sutures coated with triclosan, a phenolic compound used in toothpaste, attracted initial interest172 that subsided after results of a large observational study and a randomized trial showed no benefit.173,174 In a single randomized trial, applying topical vancomycin to the cut sternal edges reduced infection rates.175 Swedish and Finnish investigators generated enthusiasm for leaving a gentamicin-collagen sponge in the sternotomy wound176–179; however, a large multicenter trial conducted in the United States in patients with diabetes, high BMI, or both failed to show a benefit from implanting 2 gentamicin-collagen sponges during cardiac surgery.180 In 2012, it was reported that the routine use of a gentamicin-collagen sponge reduced the incidence of infection from 3.5% to 0.6%,181 but 2 systematic reviews showed no clear benefit.182,183 Applying topical bacitracin to the sternotomy incision after closure seemed to be effective.184 Applying a platelet gel reportedly promoted wound-healing and reduced the incidence of superficial and deep sternal wound infections; however, the mechanisms of action were unclear.185,186 Also unresolved is the use of the skin adhesive InteguSeal® (Kimberly-Clark Worldwide, Inc.; Roswell, Ga), which contains a cyanoacrylate-based antimicrobial skin sealant. Observations in Brazil, Turkey, Germany, the United Kingdom, and Chile suggested clinical and experimental benefit187–193; conversely, a subsequent large, nonrandomized study from Germany revealed no reduction in the incidence of postoperative mediastinitis.194
Minimizing Postoperative Risk Factors
Aggressive environmental cleaning of the intensive care unit (ICU) and cardiovascular recovery room is important, because patients admitted to a room previously occupied by a carrier of MRSA or a vancomycin-resistant Enterococcus have as much as a 40% increased risk of acquisition.195 Early extubation is desirable.26 Daily attention should be given to whether patients' indwelling urinary and central venous catheters continue to be necessary. In a large case-control study, central venous catheter-related infection was found to increase wound infection by 5-fold.196 Peripherally inserted vascular-access devices, including radial artery catheters for pressure-monitoring, can also cause sternal wound infections.197 Postoperative S. aureus bacteremia can be a cause or a consequence of deep sternal wound infection. In one study, a positive blood culture for S. aureus within 60 days of surgery had a 68% sensitivity, 98% specificity, 87% positive predictive value, and 95% negative predictive value for S. aureus mediastinitis.198 Protocols for insulin administration and glucose monitoring should be implemented for patients with diabetes. A negative study199 has partially allayed concerns that infection rates are increased by intravenous iron that is used to promote red blood cell production as part of blood-conservation programs.
Clinical evaluations of sternal and vein-harvest wounds should be documented daily.200 Sound scientific evidence is scanty in regard to wound-dressing choices. Australian investigators found no differences in rates of post-sternotomy healing or rates of infection among 3 types of dressing: PRIMAPORE®, a dry absorbent dressing (Smith & Nephew, Inc.; St. Petersburg, Fla); DuoDERM® Extra Thin, a hydrocolloid dressing (ConvaTec Professional Services; Skillman, NJ); and Opsite®, a hydroactive dressing (Smith & Nephew). PRIMAPORE was the most comfortable for patients.201 An incision-care program that involved a sterile, impermeable adhesive drape performed no better than an absorbent dressing.202 After conducting a prospective study that compared a silver nylon dressing to a standard gauze dressing, the investigators suggested that a large randomized trial might settle the issue.203
The chief postoperative contributors to deep surgical wound infection after CABG are prolonged treatment in the ICU and reoperation for bleeding (Table I). Although the causes of postoperative bleeding remain poorly understood, related deaths might be declining because of more aggressive management.204 Early reoperation for bleeding might substantially reduce risks of infection and other complications, such as renal failure and prolonged mechanical ventilation.205,206 Re-exploring the chest in the ICU for bleeding or tamponade might be a safe alternative to returning to the operating room.207 Few systematic studies have dealt with the choice and duration of prophylactic antibiotic therapy for repeat operations, which are important but unresolved issues. Because standard preoperative antibiotics substantially alter the patient's flora, there is a high likelihood of wound contamination by drug-resistant organisms: gram-positive bacteria that are resistant to the β-lactam antibiotics, gram-negative bacilli, and even yeasts. It was concluded from a best-evidence topic review that, although it is common practice to administer additional antibiotics, no well-conducted studies appear to support the practice.208
Vigilance, Audits, and Periodic Policy Reviews
Reducing the risk of CABG-related infection requires constant vigilance and attention to detail, both in caring for individual patients and in ensuring that policies conform with up-to-date knowledge and experience. Every case of life-threatening infection, such as mediastinitis, should be reviewed and the root cause considered. Clusters or outbreaks of infection should prompt an epidemiologic investigation. These investigations occasionally pinpoint a specific source, such as chemical solutions, equipment, or an individual involved in the patient's care. More often, these efforts foster better adherence to standard practices, with the result that infection rates decline with no clear explanation other than the Hawthorne effect (behavioral change that occurs when subjects know that they are being watched). Ideally, cardiac surgery programs should supplement ongoing vigilance with process audits and periodic, multidisciplinary reviews of best-practice standards (Table III).26,209–213
TABLE III. Proposed Checklist for Scheduled Institutional Reviews of Experiences, Policies, and Procedures
Deep infections will continue to complicate CABG procedures, chiefly because so many patients have severe comorbidities. However, to paraphrase football coach Vince Lombardi (“in chasing perfection we catch excellence”), scrupulous attention to the details of preoperative, intraoperative, and postoperative care should enable all programs to keep rates at an irreducible minimum.
Footnotes
Address for reprints: Charles S. Bryan, MD, Providence Hospitals, 2435 Forest Dr., Columbia, SC 29204
E-mail: charles.bryan@providencehospitals.com
References
- 1.Hollenbeak CS, Murphy DM, Koenig S, Woodward RS, Dunagan WC, Fraser VJ. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest 2000;118(2):397–402. [DOI] [PubMed]
- 2.Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR; Society of Thoracic Surgeons. The Society of Thoracic Surgeons Practice Guideline Series: antibiotic prophylaxis in cardiac surgery, part I: duration. Ann Thorac Surg 2006;81 (1):397–404. [DOI] [PubMed]
- 3.Ferris TG, Torchiana DF. Public release of clinical outcomes data-online CABG report cards. N Engl J Med 2010;363(17): 1593–5. [DOI] [PubMed]
- 4.Masud F, Vykoukal D. Preventing healthcare-associated infections in cardiac surgical patients as a hallmark of excellence. Methodist DeBakey Cardiovasc J 2011;7(2):48–50. [DOI] [PubMed]
- 5.Fernandez-Ayala M, Nan DN, Farinas-Alvarez C, Revuelta JM, Gonzalez-Macias J, Farinas MC. Surgical site infection during hospitalization and after discharge in patients who have undergone cardiac surgery. Infect Control Hosp Epidemiol 2006;27(1):85–8. [DOI] [PubMed]
- 6.Wiley AM, Ha'eri GB. Routes of infection. A study of using “tracer particles” in the orthopedic operating room. Clin Orthop Relat Res 1979(139):150–5. [PubMed]
- 7.Yap CH, Mohajeri M, Yii M. Obesity and early complications after cardiac surgery. Med J Aust 2007;186(7):350–4. [DOI] [PubMed]
- 8.McDonald WS, Brame M, Sharp C, Eggerstedt J. Risk factors for median sternotomy dehiscence in cardiac surgery. South Med J 1989;82(11):1361–4. [DOI] [PubMed]
- 9.Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, et al. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49(2):179–87. [DOI] [PubMed]
- 10.Grossi EA, Esposito R, Harris LJ, Crooke GA, Galloway AC, Colvin SB, et al. Sternal wound infections and use of internal mammary artery grafts. J Thorac Cardiovasc Surg 1991;102 (3):342–7. [PubMed]
- 11.Wouters R, Wellens F, Vanermen H, De Geest R, Degrieck I, De Meerleer F. Sternitis and mediastinitis after coronary artery bypass grafting. Analysis of risk factors. Tex Heart Inst J 1994;21(3):183–8. [PMC free article] [PubMed]
- 12.Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg 2001;20 (6):1168–75. [DOI] [PubMed]
- 13.Gummert JF, Barten MJ, Hans C, Kluge M, Doll N, Walther T, et al. Mediastinitis and cardiac surgery–an updated risk factor analysis in 10,373 consecutive adult patients. Thorac Cardiovasc Surg 2002;50(2):87–91. [DOI] [PubMed]
- 14.Russo PL, Spelman DW. A new surgical-site infection risk index using risk factors identified by multivariate analysis for patients undergoing coronary artery bypass graft surgery. Infect Control Hosp Epidemiol 2002;23(7):372–6. [DOI] [PubMed]
- 15.Kohli M, Yuan L, Escobar M, David T, Gillis G, Comm B, et al. A risk index for sternal surgical wound infection after cardiovascular surgery. Infect Control Hosp Epidemiol 2003;24 (1):17–25. [DOI] [PubMed]
- 16.Crabtree TD, Codd JE, Fraser VJ, Bailey MS, Olsen MA, Damiano RJ Jr. Multivariate analysis of risk factors for deep and superficial sternal infection after coronary artery bypass grafting at a tertiary care medical center. Semin Thorac Cardiovasc Surg 2004;16(1):53–61. [DOI] [PubMed]
- 17.Dodds Ashley ES, Carroll DN, Engemann JJ, Harris AD, Fowler VG Jr, Sexton DJ, Kaye KS. Risk factors for postoperative mediastinitis due to methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2004;38(11):1555–60. [DOI] [PubMed]
- 18.Harrington G, Russo P, Spelman D, Borrell S, Watson K, Barr W, et al. Surgical-site infection rates and risk factor analysis in coronary artery bypass graft surgery. Infect Control Hosp Epidemiol 2004;25(6):472–6. [DOI] [PubMed]
- 19.Fowler VG Jr, O'Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation 2005;112(9 Suppl):I358–65. [DOI] [PubMed]
- 20.Garey KW, Kumar N, Dao T, Tam VH, Gentry LO. Risk factors for postoperative chest wound infections due to gram-negative bacteria in cardiac surgery patients. J Chemother 2006;18(4):402–8. [DOI] [PubMed]
- 21.Simsek Yavuz S, Bicer Y, Yapici N, Kalaca S, Aydin OO, Camur G, et al. Analysis of risk factors for sternal surgical site infection: emphasizing the appropriate ventilation of the operating theaters. Infect Control Hosp Epidemiol 2006;27(9): 958–63. [DOI] [PubMed]
- 22.Fakih MG, Sharma M, Khatib R, Berriel-Cass D, Meisner S, Harrington S, Saravolatz L. Increase in the rate of sternal surgical site infection after coronary artery bypass graft: a marker of higher severity of illness. Infect Control Hosp Epidemiol 2007;28(6):655–60. [DOI] [PubMed]
- 23.Robinson PJ, Billah B, Leder K, Reid CM; ASCTS Database Committee. Factors associated with deep sternal wound infection and haemorrhage following cardiac surgery in Victoria. Interact Cardiovasc Thorac Surg 2007;6(2):167–71. [DOI] [PubMed]
- 24.Cayci C, Russo M, Cheema FH, Martens T, Ozcan V, Argenziano M, et al. Risk analysis of deep sternal wound infections and their impact on long-term survival: a propensity analysis. Ann Plast Surg 2008;61(3):294–301. [DOI] [PubMed]
- 25.Filsoufi F, Castillo JG, Rahmanian PB, Broumand SR, Silvay G, Carpentier A, Adams DH. Epidemiology of deep sternal wound infection in cardiac surgery. J Cardiothorac Vasc Anesth 2009;23(4):488–94. [DOI] [PubMed]
- 26.Graf K, Sohr D, Haverich A, Kuhn C, Gastmeier P, Chaberny IF. Decrease of deep sternal surgical site infection rates after cardiac surgery by a comprehensive infection control program. Interact Cardiovasc Thorac Surg 2009;9(2):282–6. [DOI] [PubMed]
- 27.Risnes I, Abdelnoor M, Almdahl SM, Svennevig JL. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg 2010;89(5):1502–9. [DOI] [PubMed]
- 28.Lola I, Levidiotou S, Petrou A, Arnaoutoglou H, Apostolakis E, Papadopoulos GS. Are there independent predisposing factors for postoperative infections following open heart surgery? J Cardiothorac Surg 2011;6:151. [DOI] [PMC free article] [PubMed]
- 29.Sa MP, Soares EF, Santos CA, Figueiredo OJ, Lima RO, Escobar RR, et al. Risk factors for mediastinitis after coronary artery bypass grafting surgery. Rev Bras Cir Cardiovasc 2011; 26(1):27–35. [DOI] [PubMed]
- 30.Milano CA, Kesler K, Archibald N, Sexton DJ, Jones RH. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long-term survival. Circulation 1995;92(8):2245–51. [DOI] [PubMed]
- 31.The Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective, multicenter study. J Thorac Cardiovasc Surg 1996;111(6):1200–7. [DOI] [PubMed]
- 32.Bitkover CY, Gardlund B. Mediastinitis after cardiovascular operations: a case-control study of risk factors. Ann Thorac Surg 1998;65(1):36–40. [DOI] [PubMed]
- 33.Abboud CS, Wey SB, Baltar VT. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg 2004;77(2):676–83. [DOI] [PubMed]
- 34.Lucet JC; Parisian Mediastinitis Study Group. Surgical site infection after cardiac surgery: a simplified surveillance method. Infect Control Hosp Epidemiol 2006;27(12):1393–6. [DOI] [PubMed]
- 35.Diez C, Koch D, Kuss O, Silber RE, Friedrich I, Boergermann J. Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg 2007;2:23. [DOI] [PMC free article] [PubMed]
- 36.Shaikhrezai K, Robertson FL, Anderson SE, Slight RD, Brackenbury ET. Does the number of wires used to close a sternotomy have an impact on deep sternal wound infection? Interact Cardiovasc Thorac Surg 2012;15(2):219–22. [DOI] [PMC free article] [PubMed]
- 37.Newman LS, Szczukowski LC, Bain RP, Perlino CA. Suppurative mediastinitis after open heart surgery. A case control study of risk factors. Chest 1988;94(3):546–53. [DOI] [PubMed]
- 38.Ku CH, Ku SL, Yin JC, Lee AJ. Risk factors for sternal and leg surgical site infections after cardiac surgery in Taiwan. Am J Epidemiol 2005;161(7):661–71. [DOI] [PubMed]
- 39.Berrios-Torres SI, Mu Y, Edwards JR, Horan TC, Fridkin SK. Improved risk adjustment in public reporting: coronary artery bypass graft surgical site infections. Infect Control Hosp Epidemiol 2012;33(5):463–9. [DOI] [PubMed]
- 40.Ottino G, De Paulis R, Pansini S, Rocca G, Tallone MV, Comoglio C, et al. Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg 1987;44 (2):173–9. [DOI] [PubMed]
- 41.Lin CH, Hsu RB, Chang SC, Lin FY, Chu SH. Poststernotomy mediastinitis due to methicillin-resistant Staphylococcus aureus endemic in a hospital. Clin Infect Dis 2003;37(5):679–84. [DOI] [PubMed]
- 42.Sakamoto H, Fukuda I, Oosaka M, Nakata H. Risk factors and treatment of deep sternal wound infection after cardiac operation. Ann Thorac Cardiovasc Surg 2003;9(4):226–32. [PubMed]
- 43.Lepelletier D, Perron S, Bizouarn P, Caillon J, Drugeon H, Michaud JL, Duveau D. Surgical-site infection after cardiac surgery: incidence, microbiology, and risk factors. Infect Control Hosp Epidemiol 2005;26(5):466–72. [DOI] [PubMed]
- 44.Salehi Omran A, Karimi A, Ahmadi SH, Davoodi S, Marzban M, Movahedi N, et al. Superficial and deep sternal wound infection after more than 9000 coronary artery bypass graft (CABG): incidence, risk factors and mortality. BMC Infect Dis 2007;7:112. [DOI] [PMC free article] [PubMed]
- 45.Centofanti P, Savia F, La Torre M, Ceresa F, Sansone F, Veglio V, et al. A prospective study of prevalence of 60-days postoperative wound infections after cardiac surgery. An updated risk factor analysis. J Cardiovasc Surg (Torino) 2007; 48(5): 641–6. [PubMed]
- 46.Demmy TL, Park SB, Liebler GA, Burkholder JA, Maher TD, Benckart DH, et al. Recent experience with major sternal wound complications. Ann Thorac Surg 1990;49(3):458–62. [DOI] [PubMed]
- 47.Lee YP, Feng MC, Wu LC, Chen SH, Chen YH, Chiu CC, et al. Outcome and risk factors associated with surgical site infections after cardiac surgery in a Taiwan medical center. J Microbiol Immunol Infect 2010;43(5):378–85. [DOI] [PubMed]
- 48.Zhang L, Garcia JM, Hill PC, Haile E, Light JA, Corso PJ. Cardiac surgery in renal transplant recipients: experience from Washington Hospital Center. Ann Thorac Surg 2006;81(4): 1379–84. [DOI] [PubMed]
- 49.Gualis J, Florez S, Tamayo E, Alvarez FJ, Castrodeza J, Castano M. Risk factors for mediastinitis and endocarditis after cardiac surgery. Asian Cardiovasc Thorac Ann 2009;17(6): 612–6. [DOI] [PubMed]
- 50.Kim DH, Shim JK, Hong SW, Cho KR, Kang SY, Kwak YL. Predictive value of C-reactive protein for major postoperative complications following off-pump coronary artery bypass surgery: prospective and observational trial. Circ J 2009;73(5): 872–7. [DOI] [PubMed]
- 51.Copeland M, Senkowski C, Ulcickas M, Mendelson M, Griepp RB. Breast size as a risk factor for sternal wound complications following cardiac surgery. Arch Surg 1994;129(7): 757–9. [DOI] [PubMed]
- 52.Cappabianca G, Paparella D, Visicchio G, Capone G, Lionetti G, Numis F, et al. Preoperative C-reactive protein predicts mid-term outcome after cardiac surgery. Ann Thorac Surg 2006;82(6):2170–8. [DOI] [PubMed]
- 53.Mikkelsen MM, Andersen NH, Christensen TD, Hansen TK, Eiskjaer H, Gjedsted J, et al. Microalbuminuria is associated with high adverse event rate following cardiac surgery. Eur J Cardiothorac Surg 2011;39(6):932–8. [DOI] [PubMed]
- 54.Attaran S, Shaw M, Bond L, Pullan MD, Fabri BM. A comparison of outcome in patients with preoperative atrial fibrillation and patients in sinus rhythm. Ann Thorac Surg 2011;92 (4):1391–5. [DOI] [PubMed]
- 55.Kaw R, Golish J, Ghamande S, Burgess R, Foldvary N, Walker E. Incremental risk of obstructive sleep apnea on cardiac surgical outcomes. J Cardiovasc Surg (Torino) 2006;47(6):683–9. [PubMed]
- 56.Force SD, Miller DL, Petersen R, Mansour KA, Craver J, Guyton RA, Miller JI Jr. Incidence of deep sternal wound infections after tracheostomy in cardiac surgery patients. Ann Thorac Surg 2005;80(2):618–22. [DOI] [PubMed]
- 57.Rahmanian PB, Adams DH, Castillo JG, Chikwe J, Filsoufi F. Tracheostomy is not a risk factor for deep sternal wound infection after cardiac surgery. Ann Thorac Surg 2007;84(6): 1984–91. [DOI] [PubMed]
- 58.Sommer SP, Lange V, Yildirim C, Schimmer C, Aleksic I, Wagner C, et al. Cardiac surgery and hematologic malignancies: a retrospective single-center analysis of 56 consecutive patients. Eur J Cardiothorac Surg 2011;40(1):173–8. [DOI] [PubMed]
- 59.Attaran S, Shaw M, Bond L, Pullan MD, Fabri BM. Atrial fibrillation postcardiac surgery: a common but a morbid complication. Interact Cardiovasc Thorac Surg 2011;12(5):772–7. [DOI] [PubMed]
- 60.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32(8):470–85. [DOI] [PubMed]
- 61.Haley VB, Van Antwerpen C, Tsivitis M, Doughty D, Gase KA, Hazamy P, et al. Risk factors for coronary artery bypass graft chest surgical site infections in New York State, 2008. Am J Infect Control 2012;40(1):22–8. [DOI] [PubMed]
- 62.Friedman ND, Bull AL, Russo PL, Gurrin L, Richards M. Performance of the National Nosocomial Infections Surveillance risk index in predicting surgical site infection in Australia. Infect Control Hosp Epidemiol 2007;28(1):55–9. [DOI] [PubMed]
- 63.Friedman ND, Russo PL, Bull AL, Richards MJ, Kelly H. Validation of coronary artery bypass graft surgical site infection surveillance data from a statewide surveillance system in Australia. Infect Control Hosp Epidemiol 2007;28(7):812–7. [DOI] [PubMed]
- 64.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16(1):9–13. [DOI] [PubMed]
- 65.Paul M, Raz A, Leibovici L, Madar H, Holinger R, Rubinovitch B. Sternal wound infection after coronary artery bypass graft surgery: validation of existing risk scores. J Thorac Cardiovasc Surg 2007;133(2):397–403. [DOI] [PubMed]
- 66.Chen LF, Anderson DJ, Kaye KS, Sexton DJ. Validating a 3-point prediction rule for surgical site infection after coronary artery bypass surgery. Infect Control Hosp Epidemiol 2010;31(1):64–8. [DOI] [PubMed]
- 67.Ferraris VA, Ferraris SP, Moliterno DJ, Camp P, Walenga JM, Messmore HL, et al. The Society of Thoracic Surgeons practice guideline series: aspirin and other antiplatelet agents during operative coronary revascularization (executive summary). Ann Thorac Surg 2005;79(4):1454–61. [DOI] [PubMed]
- 68.Ferrandis R, Llau JV, Mugarra A. Perioperative management of antiplatelet-drugs in cardiac surgery. Curr Cardiol Rev 2009;5(2):125–32. [DOI] [PMC free article] [PubMed]
- 69.Fitchett D, Eikelboom J, Fremes S, Mazer D, Singh S, Bittira B, et al. Dual antiplatelet therapy in patients requiring urgent coronary artery bypass grafting surgery: a position statement of the Canadian Cardiovascular Society. Can J Cardiol 2009;25(12):683–9. [DOI] [PMC free article] [PubMed]
- 70.Gurbel PA, Mahla E, Tantry US. Peri-operative platelet function testing: the potential for reducing ischaemic and bleeding risks. Thromb Haemost 2011;106(2):248–52. [DOI] [PubMed]
- 71.Biancari F, Airaksinen KE, Lip GY. Benefits and risks of using clopidogrel before coronary artery bypass surgery: systematic review and meta-analysis of randomized trials and observational studies. J Thorac Cardiovasc Surg 2012;143(3): 665–75.e4. [DOI] [PubMed]
- 72.McBride T, Beamer J. Pre-operative patient preparation in the prevention of surgical site infections. Can Oper Room Nurs J 2007;25(4):26–7, 29–32, 4. [PubMed]
- 73.Mohamed R, McAlister FA, Pretorius V, Kapoor AS, Majumdar SR, Ross DB, et al. Preoperative statin use and infection after cardiac surgery: a cohort study. Clin Infect Dis 2009;48 (7):e66–72. [DOI] [PubMed]
- 74.Tleyjeh IM, Alasmari FA, Bin Abdulhak AA, Riaz M, Garbati MA, Erwin PJ, et al. Association between preoperative statin therapy and postoperative infectious complications in patients undergoing cardiac surgery: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2012;33(11): 1143–51. [DOI] [PubMed]
- 75.Tepaske R, Velthuis H, Oudemans-van Straaten HM, Heisterkamp SH, van Deventer SJ, Ince C, et al. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: a randomised placebo-controlled trial. Lancet 2001;358(9283):696–701. [DOI] [PubMed]
- 76.Tepaske R, te Velthuis H, Oudemans-van Straaten HM, Bossuyt PM, Schultz MJ, Eijsman L, Vroom M. Glycine does not add to the beneficial effects of perioperative oral immune-enhancing nutrition supplements in high-risk cardiac surgery patients. JPEN J Parenter Enteral Nutr 2007;31(3): 173–80. [DOI] [PubMed]
- 77.Strecker T, Rosch J, Horch RE, Weyand M, Kneser U. Sternal wound infections following cardiac surgery: risk factor analysis and interdisciplinary treatment. Heart Surg Forum 2007;10(5):E366–71. [DOI] [PubMed]
- 78.Tom TS, Kruse MW, Reichman RT. Update: methicillin-resistant Staphylococcus aureus screening and decolonization in cardiac surgery. Ann Thorac Surg 2009;88(2):695–702. [DOI] [PubMed]
- 79.Butterly A, Schmidt U, Wiener-Kronish J. Methicillin-resistant Staphylococcus aureus colonization, its relationship to nosocomial infection, and efficacy of control methods. Anesthesiology 2010;113(6):1453–9. [DOI] [PubMed]
- 80.Banbury MK. Experience in prevention of sternal wound infections in nasal carriers of Staphylococcus aureus. Surgery 2003;134(5 Suppl):S18–25. [DOI] [PubMed]
- 81.Nicholson MR, Huesman LA. Controlling the usage of intranasal mupirocin does impact the rate of Staphylococcus aureus deep sternal wound infections in cardiac surgery patients. Am J Infect Control 2006;34(1):44–8. [DOI] [PubMed]
- 82.Combes A, Trouillet JL, Joly-Guillou ML, Chastre J, Gibert C. The impact of methicillin resistance on the outcome of poststernotomy mediastinitis due to Staphylococcus aureus. Clin Infect Dis 2004;38(6):822–9. [DOI] [PubMed]
- 83.Reddy SL, Grayson AD, Smith G, Warwick R, Chalmers JA. Methicillin resistant Staphylococcus aureus infections following cardiac surgery: incidence, impact and identifying adverse outcome traits. Eur J Cardiothorac Surg 2007;32(1): 113–7. [DOI] [PubMed]
- 84.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001;344(1):11–6. [DOI] [PubMed]
- 85.Walsh EE, Greene L, Kirshner R. Sustained reduction in methicillin-resistant Staphylococcus aureus wound infections after cardiothoracic surgery. Arch Intern Med 2011;171(1): 68–73. [DOI] [PubMed]
- 86.Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, et al. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part II: antibiotic choice. Ann Thorac Surg 2007;83(4):1569–76. [DOI] [PubMed]
- 87.Konvalinka A, Errett L, Fong IW. Impact of treating Staphylococcus aureus nasal carriers on wound infections in cardiac surgery. J Hosp Infect 2006;64(2):162–8. [DOI] [PMC free article] [PubMed]
- 88.van Rijen MM, Bonten M, Wenzel RP, Kluytmans JA. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. J Antimicrob Chemother 2008;61(2):254–61. [DOI] [PubMed]
- 89.Trautmann M, Stecher J, Hemmer W, Luz K, Panknin HT. Intranasal mupirocin prophylaxis in elective surgery. A review of published studies. Chemotherapy 2008;54(1):9–16. [DOI] [PubMed]
- 90.San Juan R, Chaves F, Lopez Gude MJ, Diaz-Pedroche C, Otero J, Cortina Romero JM, et al. Staphylococcus aureus poststernotomy mediastinitis: description of two distinct acquisition pathways with different potential preventive approaches [published erratum appears in J Thorac Cardiovasc Surg 2008;136(2):542]. J Thorac Cardiovasc Surg 2007;134 (3):670–6. [DOI] [PubMed]
- 91.Harbarth S, Huttner B, Gervaz P, Fankhauser C, Chraiti MN, Schrenzel J, et al. Risk factors for methicillin-resistant Staphylococcus aureus surgical site infection. Infect Control Hosp Epidemiol 2008;29(9):890–3. [DOI] [PubMed]
- 92.Mohammad ZU, Yasin K, Niaz A, Aurangzeb D, Mohammad KA, Mohammad R. Incidence and outcome of nasal and groin methicillin-resistant Staphylococcus aureus carrier state in patients admitted for routine cardiac surgery. JPMI 2008; 22(1):21–6.
- 93.Carrier M, Marchand R, Auger P, Hebert Y, Pellerin M, Perrault LP, et al. Methicillin-resistant Staphylococcus aureus infection in a cardiac surgical unit. J Thorac Cardiovasc Surg 2002;123(1):40–4. [DOI] [PubMed]
- 94.Cookson BD. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother 1998;41(1):11–8. [DOI] [PubMed]
- 95.Tenover FC, Tickler IA, Goering RV, Kreiswirth BN, Mediavilla JR, Persing DH; MRSA Consortium. Characterization of nasal and blood culture isolates of methicillin-resistant Staphylococcus aureus from patients in United States hospitals. Antimicrob Agents Chemother 2012;56(3):1324–30. [DOI] [PMC free article] [PubMed]
- 96.Reiss S, Pane-Farre J, Fuchs S, Francois P, Liebeke M, Schrenzel J, et al. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob Agents Chemother 2012;56 (2):787–804. [DOI] [PMC free article] [PubMed]
- 97.Seah C, Alexander DC, Louie L, Simor A, Low DE, Longtin J, Melano RG. MupB, a new high-level mupirocin resistance mechanism in Staphylococcus aureus. Antimicrob Agents Chemother 2012;56(4):1916–20. [DOI] [PMC free article] [PubMed]
- 98.Courville XF, Tomek IM, Kirkland KB, Birhle M, Kantor SR, Finlayson SR. Cost-effectiveness of preoperative nasal mupirocin treatment in preventing surgical site infection in patients undergoing total hip and knee arthroplasty: a cost-effectiveness analysis. Infect Control Hosp Epidemiol 2012;33 (2):152–9. [DOI] [PubMed]
- 99.Strymish J, Branch-Elliman W, Itani KM, Williams S, Gupta K. A clinical history of methicillin-resistant Staphylococcus aureus is a poor predictor of preoperative colonization status and postoperative infections. Infect Control Hosp Epidemiol 2012;33(11):1113–7. [DOI] [PubMed]
- 100.Shrestha NK, Banbury MK, Weber M, Cwynar RE, Lober C, Procop GW, et al. Safety of targeted perioperative mupirocin treatment for preventing infections after cardiac surgery. Ann Thorac Surg 2006;81(6):2183–8. [DOI] [PubMed]
- 101.Jog S, Cunningham R, Cooper S, Wallis M, Marchbank A, Vasco-Knight P, Jenks PJ. Impact of preoperative screening for methicillin-resistant Staphylococcus aureus by real-time polymerase chain reaction in patients undergoing cardiac surgery. J Hosp Infect 2008;69(2):124–30. [DOI] [PubMed]
- 102.Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362(1):9–17. [DOI] [PubMed]
- 103.Lee BY, Wiringa AE, Bailey RR, Goyal V, Lewis GJ, Tsui BY, et al. Screening cardiac surgery patients for MRSA: an economic computer model. Am J Manag Care 2010;16(7):e163–73. [PMC free article] [PubMed]
- 104.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 2006;296(20):2460–6. [DOI] [PubMed]
- 105.Kamel C, McGahan L, Polisena J, Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review. Infect Control Hosp Epidemiol 2012;33(6):608–17. [DOI] [PubMed]
- 106.Kuhme T, Isaksson B, Dahlin LG. Wound contamination in cardiac surgery. A systematic quantitative and qualitative study of the bacterial growth in sternal wounds in cardiac surgery patients. APMIS 2007;115(9):1001–7. [DOI] [PubMed]
- 107.Goodman JS, Schaffner W, Collins HA, Battersby EJ, Koenig MG. Infection after cardiovascular surgery. Clinical study including examination of antimicrobial prophylaxis. N Engl J Med 1968;278(3):117–23. [DOI] [PubMed]
- 108.Bratzler DW, Houck PM, Richards C, Steele L, Dellinger EP, Fry DE, et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg 2005;140(2):174–82. [DOI] [PubMed]
- 109.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis 2006;43(3):322–30. [DOI] [PubMed]
- 110.Alexiou VG, Ierodiakonou V, Peppas G, Falagas ME. Antimicrobial prophylaxis in surgery: an international survey. Surg Infect (Larchmt) 2010;11(4):343–8. [DOI] [PubMed]
- 111.Zanetti G, Giardina R, Platt R. Intraoperative redosing of cefazolin and risk for surgical site infection in cardiac surgery. Emerg Infect Dis 2001;7(5):828–31. [DOI] [PMC free article] [PubMed]
- 112.Zanetti G, Flanagan HL Jr, Cohn LH, Giardina R, Platt R. Improvement of intraoperative antibiotic prophylaxis in prolonged cardiac surgery by automated alerts in the operating room. Infect Control Hosp Epidemiol 2003;24(1):13–6. [DOI] [PubMed]
- 113.Caffarelli AD, Holden JP, Baron EJ, Lemmens HJ, D'Souza H, Yau V, et al. Plasma cefazolin levels during cardiovascular surgery: effects of cardiopulmonary bypass and profound hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2006; 131(6):1338–43. [DOI] [PubMed]
- 114.Tamayo E, Gualis J, Florez S, Castrodeza J, Eiros Bouza JM, Alvarez FJ. Comparative study of single-dose and 24-hour multiple-dose antibiotic prophylaxis for cardiac surgery. J Thorac Cardiovasc Surg 2008;136(6):1522–7. [DOI] [PubMed]
- 115.Adembri C, Ristori R, Chelazzi C, Arrigucci S, Cassetta MI, De Gaudio AR, Novelli A. Cefazolin bolus and continuous administration for elective cardiac surgery: improved pharmacokinetic and pharmacodynamic parameters. J Thorac Cardiovasc Surg 2010;140(2):471–5. [DOI] [PubMed]
- 116.Edmiston CE, Krepel C, Kelly H, Larson J, Andris D, Hennen C, et al. Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery 2004;136(4):738–47. [DOI] [PubMed]
- 117.Paul M, Porat E, Raz A, Madar H, Fein S, Bishara J, et al. Duration of antibiotic prophylaxis for cardiac surgery: prospective observational study. J Infect 2009;58(4):291–8. [DOI] [PubMed]
- 118.Kallman J, Friberg O. Antibiotic prophylaxis in cardiac surgery–general principles. APMIS 2007;115(9):1012–5. [DOI] [PubMed]
- 119.Mertz D, Johnstone J, Loeb M. Does duration of perioperative antibiotic prophylaxis matter in cardiac surgery? A systematic review and meta-analysis. Ann Surg 2011;254(1):48–54. [DOI] [PubMed]
- 120.Crawford T, Rodvold KA, Solomkin JS. Vancomycin for surgical prophylaxis? Clin Infect Dis 2012;54(10):1474–9. [DOI] [PubMed]
- 121.Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, et al. Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J Thorac Cardiovasc Surg 2002;123(2):326–32. [DOI] [PubMed]
- 122.Kachroo S, Dao T, Zabaneh F, Reiter M, Larocco MT, Gentry LO, Garey KW. Tolerance of vancomycin for surgical prophylaxis in patients undergoing cardiac surgery and incidence of vancomycin-resistant enterococcus colonization. Ann Pharmacother 2006;40(3):381–5. [DOI] [PubMed]
- 123.Garey KW, Dao T, Chen H, Amrutkar P, Kumar N, Reiter M, Gentry LO. Timing of vancomycin prophylaxis for cardiac surgery patients and the risk of surgical site infections. J Antimicrob Chemother 2006;58(3):645–50. [DOI] [PubMed]
- 124.Movahed MR, Kasravi B, Bryan CS. Prophylactic use of vancomycin in adult cardiology and cardiac surgery. J Cardiovasc Pharmacol Ther 2004;9(1):13–20. [DOI] [PubMed]
- 125.Raikhelkar JK, Reich DL, Schure R, Varghese R, Bodian C, Scurlock C. The efficacy of post-cardiopulmonary bypass dosing of vancomycin in cardiac surgery. Semin Cardiothorac Vasc Anesth 2010;14(4):301–4. [DOI] [PubMed]
- 126.Grace E. Altered vancomycin pharmacokinetics in obese and morbidly obese patients: what we have learned over the past 30 years. J Antimicrob Chemother 2012;67(6):1305–10. [DOI] [PubMed]
- 127.Garey KW, Lai D, Dao-Tran TK, Gentry LO, Hwang LY, Davis BR. Interrupted time series analysis of vancomycin compared to cefuroxime for surgical prophylaxis in patients undergoing cardiac surgery. Antimicrob Agents Chemother 2008;52(2):446–51. [DOI] [PMC free article] [PubMed]
- 128.Bolon MK, Morlote M, Weber SG, Koplan B, Carmeli Y, Wright SB. Glycopeptides are no more effective than beta-lactam agents for prevention of surgical site infection after cardiac surgery: a meta-analysis. Clin Infect Dis 2004;38(10):1357–63. [DOI] [PubMed]
- 129.Kappeler R, Gillham M, Brown NM. Antibiotic prophylaxis for cardiac surgery. J Antimicrob Chemother 2012;67(3):521–2. [DOI] [PubMed]
- 130.Lador A, Nasir H, Mansur N, Sharoni E, Biderman P, Leibovici L, Paul M. Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. J Antimicrob Chemother 2012;67(3):541–50. [DOI] [PubMed]
- 131.Bryan CS, Morgan SL, Jordan AB, Smith CW, Sutton JP, Gangemi JD. Ceftriaxone levels in blood and tissue during cardiopulmonary bypass surgery. Antimicrob Agents Chemother 1984;25(1):37–9. [DOI] [PMC free article] [PubMed]
- 132.Salminen US, Viljanen TU, Valtonen VV, Ikonen TE, Sahlman AE, Harjula AL. Ceftriaxone versus vancomycin prophylaxis in cardiovascular surgery. J Antimicrob Chemother 1999;44(2):287–90. [DOI] [PubMed]
- 133.Vandecasteele SJ, Boelaert JR, Verrelst P, Graulus E, Gordts BZ. Diagnosis and treatment of Aspergillus flavus sternal wound infections after cardiac surgery. Clin Infect Dis 2002; 35(7):887–90. [DOI] [PubMed]
- 134.Heinemann S, Symoens F, Gordts B, Jannes H, Nolard N. Environmental investigations and molecular typing of Aspergillus flavus during an outbreak of postoperative infections. J Hosp Infect 2004;57(2):149–55. [DOI] [PubMed]
- 135.Triassi M, Di Popolo A, Ribera D'Alcala G, Albanese Z, Cuccurullo S, Montegrosso S, et al. Clinical and environmental distribution of Legionella pneumophila in a university hospital in Italy: efficacy of ultraviolet disinfection. J Hosp Infect 2006;62(4):494–501. [DOI] [PubMed]
- 136.Kronman MP, Baden HP, Jeffries HE, Heath J, Cohen GA, Zerr DM. An investigation of Aspergillus cardiac surgical site infections in 3 pediatric patients. Am J Infect Control 2007; 35(5):332–7. [DOI] [PubMed]
- 137.Pawar M, Mehta Y, Ansari A, Nair R, Trehan N. Nosocomial infections and balloon counterpulsation: risk factors and outcome. Asian Cardiovasc Thorac Ann 2005;13(4):316–20. [DOI] [PubMed]
- 138.Putney LJ. Bloodless cardiac surgery: not just possible, but preferable. Crit Care Nurs Q 2007;30(3):263–70. [DOI] [PubMed]
- 139.Rogers MA, Blumberg N, Heal JM, Hicks GL Jr. Increased risk of infection and mortality in women after cardiac surgery related to allogeneic blood transfusion. J Womens Health (Larchmt) 2007;16(10):1412–20. [DOI] [PubMed]
- 140.Shander A, Spence RK, Adams D, Shore-Lesserson L, Walawander CA. Timing and incidence of postoperative infections associated with blood transfusion: analysis of 1,489 orthopedic and cardiac surgery patients. Surg Infect (Larchmt) 2009; 10(3):277–83. [DOI] [PubMed]
- 141.Andreasen JJ, Dethlefsen C, Modrau IS, Baech J, Schonheyder HC, Moeller JK, et al. Storage time of allogeneic red blood cells is associated with risk of severe postoperative infection after coronary artery bypass grafting. Eur J Cardiothorac Surg 2011;39(3):329–34. [DOI] [PubMed]
- 142.Blumberg N, Heal JM, Cowles JW, Hicks GL Jr, Risher WH, Samuel PK, Kirkley SA. Leukocyte-reduced transfusions in cardiac surgery: results of an implementation trial. Am J Clin Pathol 2002;118(3):376–81. [DOI] [PubMed]
- 143.Capraro L, Kuitunen A, Vento AE, Suojaranta-Ylinen R, Kolho E, Pettila V. Universal leukocyte reduction of transfused red cells does not provide benefit to patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2007;21(2):232–6. [DOI] [PubMed]
- 144.Tavolacci MP, Merle V, Josset V, Bouchart F, Litzler PY, Tabley A, et al. Mediastinitis after coronary artery bypass graft surgery: influence of the mammary grafting for diabetic patients. J Hosp Infect 2003;55(1):21–5. [DOI] [PubMed]
- 145.Momin AU, Deshpande R, Potts J, El-Gamel A, Marrinan MT, Omigie J, Desai JB. Incidence of sternal infection in diabetic patients undergoing bilateral internal thoracic artery grafting. Ann Thorac Surg 2005;80(5):1765–72. [DOI] [PubMed]
- 146.Farhat F, Metton O, Jegaden O. Benefits and complications of total sternotomy and ministernotomy in cardiac surgery. Surg Technol Int 2004;13:199–205. [PubMed]
- 147.Aburumman AM. Tilted T ministernotomy: a new approach in cardiac surgery. JRMS 2010;17(2):61–9.
- 148.Zeitani J, Penta de Peppo A, Moscarelli M, Guerrieri Wolf L, Scafuri A, Nardi P, et al. Influence of sternal size and inadvertent paramedian sternotomy on stability of the closure site: a clinical and mechanical study. J Thorac Cardiovasc Surg 2006;132(1):38–42. [DOI] [PubMed]
- 149.Schimmer C, Reents W, Berneder S, Eigel P, Sezer O, Scheld H, et al. Prevention of sternal dehiscence and infection in high-risk patients: a prospective randomized multicenter trial. Ann Thorac Surg 2008;86(6):1897–904. [DOI] [PubMed]
- 150.Okutan H, Tenekeci C, Kutsal A. The reinforced sternal closure system is reliable to use in elderly patients. J Card Surg 2005;20(3):271–3. [DOI] [PubMed]
- 151.Song DH, Lohman RF, Renucci JD, Jeevanandam V, Raman J. Primary sternal plating in high-risk patients prevents mediastinitis. Eur J Cardiothorac Surg 2004;26(2):367–72. [DOI] [PubMed]
- 152.Risnes I, Abdelnoor M, Baksaas ST, Lundblad R, Svennevig JL. Sternal wound infections in patients undergoing open heart surgery: randomized study comparing intracutaneous and transcutaneous suture techniques. Ann Thorac Surg 2001;72(5):1587–91. [DOI] [PubMed]
- 153.Karabay O, Fermanci E, Silistreli E, Aykut K, Yurekli I, Catalyurek H, Acikel U. Intracutaneous versus transcutaneous suture techniques: comparison of sternal wound infection rates in open-heart surgery patients. Tex Heart Inst J 2005;32 (3):277–82. [PMC free article] [PubMed]
- 154.Ramzisham AR, Raflis AR, Khairulasri MG, Ooi Su Min J, Fikri AM, Zamrin MD. Figure-of-eight vs. interrupted sternal wire closure of median sternotomy. Asian Cardiovasc Thorac Ann 2009;17(6):587–91. [DOI] [PubMed]
- 155.Gorlitzer M, Wagner F, Pfeiffer S, Folkmann S, Meinhart J, Fischlein T, et al. A prospective randomized multicenter trial shows improvement of sternum related complications in cardiac surgery with the Posthorax support vest. Interact Cardiovasc Thorac Surg 2010;10(5):714–8. [DOI] [PubMed]
- 156.Bhatti F, Dunning J. Does liberal use of bone wax increase the risk of mediastinitis? Interact Cardiovasc Thorac Surg 2003;2 (4):410–2. [DOI] [PubMed]
- 157.Prziborowski J, Hartrumpf M, Stock UA, Kuehnel RU, Albes JM. Is bonewax safe and does it help? Ann Thorac Surg 2008; 85(3):1002–6. [DOI] [PubMed]
- 158.Cohen O, Dankner R, Chetrit A, Luxenburg O, Langenauer C, Shinfeld A, Smolinsky AK. Multidisciplinary intervention for control of diabetes in patients undergoing coronary artery bypass graft (CABG). Cardiovasc Surg 2003;11(3):195–200. [DOI] [PubMed]
- 159.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004;109(12): 1497–502. [DOI] [PubMed]
- 160.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc 2005;80(7):862–6. [DOI] [PubMed]
- 161.Brown JR, Edwards FH, O'Connor GT, Ross CS, Furnary AP. The diabetic disadvantage: historical outcomes measures in diabetic patients undergoing cardiac surgery – the pre-intravenous insulin era. Semin Thorac Cardiovasc Surg 2006; 18(4):281–8. [DOI] [PubMed]
- 162.Furnary AP, Wu Y. Eliminating the diabetic disadvantage: the Portland Diabetic Project. Semin Thorac Cardiovasc Surg 2006;18(4):302–8. [DOI] [PubMed]
- 163.Schmeltz LR, DeSantis AJ, Thiyagarajan V, Schmidt K, O'Shea-Mahler E, Johnson D, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care 2007;30 (4):823–8. [DOI] [PubMed]
- 164.Patel KL. Impact of tight glucose control on postoperative infection rates and wound healing in cardiac surgery patients. J Wound Ostomy Continence Nurs 2008;35(4):397–406. [DOI] [PubMed]
- 165.Kramer R, Groom R, Weldner D, Gallant P, Heyl B, Knapp R, Arnold A. Glycemic control and reduction of deep sternal wound infection rates: a multidisciplinary approach. Arch Surg 2008;143(5):451–6. [DOI] [PubMed]
- 166.Lehwaldt D, Kingston M, O'Connor S. Postoperative hyperglycaemia of diabetic patients undergoing cardiac surgery - a clinical audit. Nurs Crit Care 2009;14(5):241–53. [DOI] [PubMed]
- 167.Rogers SO Jr, Zinner MJ. The role of perioperative hyperglycemia in postoperative infections. Adv Surg 2009;43:103–9. [DOI] [PubMed]
- 168.Bustamante J, Tamayo E, Alvarez FJ, Garcia-Cuenca I, Florez S, Fierro I, Gomez-Herreras JI. Intraoperative PaO2 is not related to the development of surgical site infections after major cardiac surgery. J Cardiothorac Surg 2011;6:4. [DOI] [PMC free article] [PubMed]
- 169.Cappabianca G, Rotunno C, de Luca Tupputi Schinosa L, Ranieri VM, Paparella D. Protective effects of steroids in cardiac surgery: a meta-analysis of randomized double-blind trials. J Cardiothorac Vasc Anesth 2011;25(1):156–65. [DOI] [PubMed]
- 170.Yoshii S, Hosaka S, Suzuki S, Takahashi W, Okuwaki H, Osawa H, et al. Prevention of surgical site infection by antibiotic spraying in the operative field during cardiac surgery. Jpn J Thorac Cardiovasc Surg 2001;49(5):279–81. [DOI] [PubMed]
- 171.Persson M, van der Linden J. Wound ventilation with carbon dioxide: a simple method to prevent direct airborne contamination during cardiac surgery? J Hosp Infect 2004;56(2):131–6. [DOI] [PubMed]
- 172.Fleck T, Moidl R, Blacky A, Fleck M, Wolner E, Grabenwoger M, Wisser W. Triclosan-coated sutures for the reduction of sternal wound infections: economic considerations [published erratum appears in Ann Thorac Surg 2007;84(6): 2139]. Ann Thorac Surg 2007;84(1):232–6. [DOI] [PubMed]
- 173.Stadler S, Fleck T. Triclosan-coated sutures for the reduction of sternal wound infections? A retrospective observational analysis. Interact Cardiovasc Thorac Surg 2011;13(3):296–9. [DOI] [PubMed]
- 174.Isik I, Selimen D, Senay S, Alhan C. Efficiency of antibacterial suture material in cardiac surgery: a double-blind randomized prospective study. Heart Surg Forum 2012;15(1):E40–5. [DOI] [PubMed]
- 175.Vander Salm TJ, Okike ON, Pasque MK, Pezzella AT, Lew R, Traina V, Mathieu R. Reduction of sternal infection by application of topical vancomycin. J Thorac Cardiovasc Surg 1989;98(4):618–22. [PubMed]
- 176.Friberg O, Svedjeholm R, Soderquist B, Granfeldt H, Vikerfors T, Kallman J. Local gentamicin reduces sternal wound infections after cardiac surgery: a randomized controlled trial. Ann Thorac Surg 2005;79(1):153–62. [DOI] [PubMed]
- 177.Eklund AM, Valtonen M, Werkkala KA. Prophylaxis of sternal wound infections with gentamicin-collagen implant: randomized controlled study in cardiac surgery. J Hosp Infect 2005;59(2):108–12. [DOI] [PubMed]
- 178.Friberg O, Svedjeholm R, Kallman J, Soderquist B. Incidence, microbiological findings, and clinical presentation of sternal wound infections after cardiac surgery with and without local gentamicin prophylaxis. Eur J Clin Microbiol Infect Dis 2007;26(2):91–7. [DOI] [PubMed]
- 179.Friberg O. Local collagen-gentamicin for prevention of sternal wound infections: the LOGIP trial. APMIS 2007;115(9): 1016–21. [DOI] [PubMed]
- 180.Bennett-Guerrero E, Ferguson TB Jr, Lin M, Garg J, Mark DB, Scavo VA Jr, et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. JAMA 2010;304(7):755–62. [DOI] [PubMed]
- 181.Schimmer C, Ozkur M, Sinha B, Hain J, Gorski A, Hager B, Leyh R. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: a controlled, prospectively randomized, double-blind study. J Thorac Cardiovasc Surg 2012;143(1):194–200. [DOI] [PubMed]
- 182.Creanor S, Barton A, Marchbank A. Effectiveness of a gentamicin impregnated collagen sponge on reducing sternal wound infections following cardiac surgery: a meta-analysis of randomised controlled trials. Ann R Coll Surg Engl 2012;94 (4):227–31. [DOI] [PMC free article] [PubMed]
- 183.Godbole G, Pai V, Kolvekar S, Wilson AP. Use of gentamicin-collagen sponges in closure of sternal wounds in cardiothoracic surgery to reduce wound infections. Interact Cardiovasc Thorac Surg 2012;14(4):390–4. [DOI] [PMC free article] [PubMed]
- 184.MacIver RH, Stewart R, Frederiksen JW, Fullerton DA, Horvath KA. Topical application of bacitracin ointment is associated with decreased risk of mediastinitis after median sternotomy. Heart Surg Forum 2006;9(5):E750–3. [DOI] [PubMed]
- 185.Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M, Gilbert C. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol 2005;37(4):381–6. [PMC free article] [PubMed]
- 186.Englert SJ, Estep TH, Ellis-Stoll CC. Postoperative surgical chest and leg incision sites using platelet gel: a retrospective study. J Extra Corpor Technol 2008;40(4):225–8. [PMC free article] [PubMed]
- 187.Souza EC, Fitaroni RB, Januzelli DM, Macruz HM, Camacho JC, Souza MR. Use of 2-octyl cyanoacrylate for skin closure of sternal incisions in cardiac surgery: observations of microbial barrier effects. Curr Med Res Opin 2008;24(1): 151–5. [DOI] [PubMed]
- 188.Basaran M, Kafali E, Ugurlucan M, Kalko Y, Selimoglu O, Us MH, Ogus TN. Cyanoacrylate gluing increases the effectiveness of systemic antimicrobial treatment in sternal infection: experimental study in a rodent model. Thorac Cardiovasc Surg 2008;56(1):28–31. [DOI] [PubMed]
- 189.Dohmen PM, Gabbieri D, Weymann A, Linneweber J, Konertz W. Reduction in surgical site infection in patients treated with microbial sealant prior to coronary artery bypass graft surgery: a case-control study. J Hosp Infect 2009;72(2):119–26. [DOI] [PubMed]
- 190.Chambers A, Scarci M. Is skin closure with cyanoacrylate glue effective for the prevention of sternal wound infections? Interact Cardiovasc Thorac Surg 2010;10(5):793–6. [DOI] [PubMed]
- 191.Dohmen PM, Gabbieri D, Weymann A, Linneweber J, Geyer T, Konertz W. A retrospective non-randomized study on the impact of INTEGUSEAL, a preoperative microbial skin sealant, on the rate of surgical site infections after cardiac surgery. Int J Infect Dis 2011;15(6):e395–400. [DOI] [PubMed]
- 192.von Eckardstein AS, Lim CH, Dohmen PM, Pego-Fernandes PM, Cooper WA, Oslund SG, Kelley EL. A randomized trial of a skin sealant to reduce the risk of incision contamination in cardiac surgery. Ann Thorac Surg 2011;92(2):632–7. [DOI] [PubMed]
- 193.Dohmen PM, Weymann A, Holinski S, Linneweber J, Geyer T, Konertz W. Use of an antimicrobial skin sealant reduces surgical site infection in patients undergoing routine cardiac surgery. Surg Infect (Larchmt) 2011;12(6):475–81. [DOI] [PubMed]
- 194.Waldow T, Szlapka M, Hensel J, Plotze K, Matschke K, Jatzwauk L. Skin sealant InteguSeal(R) has no impact on prevention of postoperative mediastinitis after cardiac surgery. J Hosp Infect 2012;81(4):278–82. [DOI] [PubMed]
- 195.Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med 2011; 171(6):491–4. [DOI] [PubMed]
- 196.Le Guillou V, Tavolacci MP, Baste JM, Hubscher C, Bedoit E, Bessou JP, Litzler PY. Surgical site infection after central venous catheter-related infection in cardiac surgery. Analysis of a cohort of 7557 patients. J Hosp Infect 2011;79(3):236–41. [DOI] [PubMed]
- 197.El-Hamamsy I, Durrleman N, Stevens LM, Leung TK, Theoret S, Carrier M, Perrault LP. Incidence and outcome of radial artery infections following cardiac surgery. Ann Thorac Surg 2003;76(3):801–4. [DOI] [PubMed]
- 198.San Juan R, Aguado JM, Lopez MJ, Lumbreras C, Enriquez F, Sanz F, et al. Accuracy of blood culture for early diagnosis of mediastinitis in febrile patients after cardiac surgery. Eur J Clin Microbiol Infect Dis 2005;24(3):182–9. [DOI] [PubMed]
- 199.Torres S, Kuo YH, Morris K, Neibart R, Holtz JB, Davis JM. Intravenous iron following cardiac surgery does not increase the infection rate. Surg Infect (Larchmt) 2006;7(4):361–6. [DOI] [PubMed]
- 200.Elahi MM, Haesey AM, Graham KC, Battula NR, Manketlow B, Dhannapuneni RR, Hickey MS. Leg wound infections following cardiac surgery: a scoring system for assessment and management. J Wound Care 2005;14(7):337–40. [PubMed]
- 201.Wynne R, Botti M, Stedman H, Holsworth L, Harinos M, Flavell O, Manterfield C. Effect of three wound dressings on infection, healing comfort, and cost in patients with sternotomy wounds: a randomized trial. Chest 2004;125(1):43–9. [DOI] [PubMed]
- 202.Segers P, de Jong AP, Spanjaard L, Ubbink DT, de Mol BA. Randomized clinical trial comparing two options for postoperative incisional care to prevent poststernotomy surgical site infections [published erratum appears in Wound Repair Regen 2007;15(3):430]. Wound Repair Regen 2007;15(2): 192–6. [DOI] [PubMed]
- 203.Huckfeldt R, Redmond C, Mikkelson D, Finley PJ, Lowe C, Robertson J. A clinical trial to investigate the effect of silver nylon dressings on mediastinitis rates in postoperative cardiac sternotomy incisions. Ostomy Wound Manage 2008;54(10): 36–41. [PubMed]
- 204.Mehta RH, Sheng S, O'Brien SM, Grover FL, Gammie JS, Ferguson TB, et al. Reoperation for bleeding in patients undergoing coronary artery bypass surgery: incidence, risk factors, time trends, and outcomes. Circ Cardiovasc Qual Outcomes 2009;2(6):583–90. [DOI] [PubMed]
- 205.Boeken U, Eisner J, Feindt P, Petzold TH, Schulte HD, Gams E. Does the time of resternotomy for bleeding have any influence on the incidence of sternal infections, septic courses or further complications? Thorac Cardiovasc Surg 2001;49(1): 45–8. [DOI] [PubMed]
- 206.Karthik S, Grayson AD, McCarron EE, Pullan DM, Desmond MJ. Reexploration for bleeding after coronary artery bypass surgery: risk factors, outcomes, and the effect of time delay. Ann Thorac Surg 2004;78(2):527–34. [DOI] [PubMed]
- 207.Charalambous CP, Zipitis CS, Keenan DJ. Chest reexploration in the intensive care unit after cardiac surgery: a safe alternative to returning to the operating theater. Ann Thorac Surg 2006;81(1):191–4. [DOI] [PubMed]
- 208.Yap EL, Levine A, Strang T, Dunning J. Should additional antibiotics or an iodine washout be given to all patients who suffer an emergency re-sternotomy on the cardiothoracic intensive care unit? Interact Cardiovasc Thorac Surg 2008;7(3): 464–9. [DOI] [PubMed]
- 209.Lutarewych M, Morgan SP, Hall MM. Improving outcomes of coronary artery bypass graft infections with multiple interventions: putting science and data to the test. Infect Control Hosp Epidemiol 2004;25(6):517–9. [DOI] [PubMed]
- 210.Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, et al. Surgical site infection rates following cardiac surgery: the impact of a 6-year infection control program. Am J Infect Control 2005;33(8):450–4. [DOI] [PubMed]
- 211.Gardlund B. Postoperative surgical site infections in cardiac surgery–an overview of preventive measures. APMIS 2007; 115(9):989–95. [DOI] [PubMed]
- 212.Beckmann A, Doebler K, Schaefer E, Koetting J, Gastmeier P, Graf K. Sternal surgical site infection prevention - is there any room for improvement? Eur J Cardiothorac Surg 2011;40 (2):347–51. [DOI] [PubMed]
- 213.Rosenberger LH, Politano AD, Sawyer RG. The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect (Larchmt) 2011;12(3):163–8. [DOI] [PMC free article] [PubMed]


