Abstract
Rats with combined lesions of the perirhinal and postrhinal cortices were trained on a complex discrimination task, the simultaneous feature-positive and feature-negative discrimination task. In this task, a panel light (L) paired with an auditory stimulus determined whether a tone (T) or white noise (N) would be rewarded (+) or not rewarded (−). Thus, the light feature determined whether the target auditory stimuli were rewarded or not. In each session, trial types were LT+, T−, N+, and LN−. We had hypothesized that damage to the target regions would impair performance on this task. Acquisition was altered in the lesioned rats, but not in the predicted direction. Instead, lesioned rats exhibited significantly enhanced acquisition of the discrimination. Manipulation of intertrial intervals indicated that reduction of proactive interference did not explain the enhancement. Lesioned rats were not different from controls on a multiple cued-interval timing task, providing evidence that the enhancement does not extend to all types of discriminations and is not due to a deficit in timing. Other research shows that rats with perirhinal lesions are impaired on similar tasks, thus the enhancement is likely due to the effects of postrhinal damage. Normally in this task, context is thought to accrue inhibitory control over other cues. Without this inhibitory control, animals might be expected to learn the task more efficiently. Our conclusion is that deficits in processing contextual information underlie the enhanced acquisition observed in rats with combined perirhinal and postrhinal damage on this complex discrimination task.
Keywords: parahippocampal, configural, timing, excitatory conditioning, inhibitory conditioning
Introduction
Sensory information acquired by experience is conveyed from neocortex to the hippocampus through medial temporal lobe pathways that include the perirhinal (PER) and postrhinal (POR) cortices. The PER and POR, which are strongly interconnected with each other and with the entorhinal cortex, provide the predominant polymodal sensory input to the hippocampus, both directly and through entorhinal connections (Burwell and Amaral, 1998; Dolorfo and Amaral, 1998; Kerr et al., 2007). The hippocampus then binds the information acquired by experience into episodes for storage in memory (Eichenbaum, 2000; Squire et al., 2004; Sutherland and Rudy, 1990).
In the late 1980s, configural association theory was proposed as an account of hippocampal function (Rudy and Sutherland, 1989; Sutherland and Rudy, 1990). Configural learning is required when an association cannot be learned on the basis of individual or elemental cues, alone. Tasks designed to test configural learning are often executed in conditioning chambers such that simple cues, e.g. lights and tones are rewarded or not depending on the pattern of cue presentation. Such tasks include, for example, negative patterning (A+, B+, AB−), positive patterning (A−, B−, AB+), and biconditional discriminations (AC+, AD−, BC−, BD+). Theoretically, configural tasks require processing of compound cues such that individual elements maintain their identity.
Another example of a configural task is the feature-positive, feature-negative discrimination task (FPFN) in which cues are presented in four trial types (CA+, A−, CB−, B+). When the so-called feature, C, is paired with the target, A, the compound is positive (reinforced with food reward), but when C is paired with the target, B, the compound is negative (no reward is presented). This task is powerful as an assessment of configural learning because each individual cue, whether presented as an individual element or part of a compound, is rewarded on some trials, but not on other trials. This combination of both compound and elemental cues biases the animal to maintain the identity of the individual cues. Indeed, considerable behavioral evidence indicates that rats must extract the identity of the individual elements in the compounds in order to acquire this discrimination (Holland and Reeve, 1991; Holland, 1991).
Rats with kainic acid-cholchicine lesions of the hippocampus were severely impaired on the FPFN task, but more selective ibotenic acid lesions resulted in no deficit (Alvarado and Rudy, 1995; Gallagher and Holland, 1992). Jarrard and Meldrum (1993) found that kainate acid injections in the hippocampus resulted in extensive extrahippocampal damage. One possibility is that distal damage resulting from hippocampal kainic acid-cholchicine lesions in the cortical regions that surround the hippocampus is responsible for these deficits. Indeed, the PER expresses high densities of kainate receptors in layers II–VI (Wisden and Seeburg, 1993). Also, recent work in humans suggests that the PER plays a role in identifying familiar configurations of partial stimuli (Peterson et al., this issue). Although there is no information available about kainate receptors in the POR, the similarities to PER in other markers (Burwell, 2001) suggests that POR may express a similar distribution of kainate receptors. Based on this evidence, we hypothesized that the PER and POR cortices are important for acquisition of this complex discrimination task.
The present study addressed the question of whether PER and POR are involved in the acquisition of a discrimination task in which compound cues have overlapping stimulus elements, the identity of which must be extracted to acquire the task. In this case, individual stimuli were overlapping in time. Rats with electrolytic PER plus POR (PER-POR) lesions were trained on the FPFN task using lights, tones, and white noise (trial types LT+, T−, LN−, N+) at short intertrial intervals (ITIs). Surprisingly, PER-POR lesioned rats exhibited significantly enhanced acquisition of both the feature positive (excitatory, LT+, T−) and the feature negative (inhibitory, LN−, N+) components of the task. To examine the possibility that enhanced performance was due to reduction of proactive interference, rats were also tested at longer ITIs. The same subjects were unimpaired on the Multiple Cued Interval (MCI) task, a concurrent discrimination task, which requires association of a time interval and a sensory cue, but does not require processing of overlapping sensory stimulus elements. The MCI task verified that timing and the ability to process complex associations were not impaired or enhanced by combined PER/POR damage.
Methods
Subjects
Subjects were 18 male Long-Evans rats (Charles River Laboratories, Portage, MI) weighing 177 – 220 g at the time of surgery. Rats were housed in wire mesh cages with free access to water. Food was scheduled to maintain body weight at 85–90% ad libitum body weight. Rats were housed in a 12:12 hr light:dark cycle with all testing performed during the light phase. All procedures were approved by the Brown University animal care and use committee.
Surgery
Ten rats received bilateral electrolytic PER-POR lesions using coordinates adapted from prior studies (Bucci and Burwell, 2004; Wiig and Burwell, 1998). A lesion was made at each of 6 sites by passing 2 mA current for 10 seconds with a tungsten microelectrode (Frederick Haer & Co., Bowdoinham, ME, Table 1). Four rats received sham lesions, undergoing the same procedure minus the passing of current. Four unoperated controls were also included in the study.
Table 1.
Lesion Coordinates
| Site | AP (mm) | ML (mm) | DV (mm) | Angle (°) |
|---|---|---|---|---|
| 1 | 3.3 | 5.2 | 6.7 | 12 |
| 2 | 4.3 | 5.2 | 6.7 | 12 |
| 3 | 5.3 | 5.2 | 6.7 | 12 |
| 4 | 6.3 | 5.2 | 6.7 | 12 |
| 5 | 7.3 | 5.2 | 6.0 | 12 |
| 6 | 8.3 | 4.9 | 5.5 | 12 |
| 7 | 9.3 | 3.9 | 4.7 | 12 |
The coordinates used for the lesions of both hemispheres (14 lesion sites total for each animal). The electrode entered the skull at 12° from vertical. These coordinates were adapted to Long-Evans rats from Wiig & Burwell (1998). AP – anterior-posterior; ML – medial-lateral; DV – dorsal-ventral. The DV coordinate was taken in reference to skull.
Procedure for FPFN Task
Rats received training in six sound-attenuating chambers (Med Associates, Inc., St. Albans, VT) interfaced with personal computer and controlled by MED-PC V 2.1 software (MED Associates). A Med Associates computer-interfaced audio generator provided the 74 dB, 1.5 kHz tone and the 73 dB white noise. A panel light located centrally below the house light provided the light stimulus.
The behavioral procedure for the FPFN task is shown in Table 2. Initially, rats were taught to lever press (Shaping). For the next two sessions, rats were pretrained on a 60-minute program that presented either the light with the tone or the white noise (LT+, N+). Lever pressing was rewarded during the entire 15-second duration of stimulus presentation. In addition, no lever pressing was rewarded during the inter-trial intervals (ITIs) that varied between 5, 10, 15, and 20 s. Rats had three sessions of a final pretraining program in which LT or N stimuli were presented and lever pressing was rewarded on a fixed interval of 1 food pellet per lever press during the last 5 s of the 15 s stimulus presentation.
Table 2.
Shaping and Training Schedule for FPFN task, Experiment 1.
| Task | Sessions | Cues Presented | Unrewarded: Rewarded | ITIs | Reward Period |
|---|---|---|---|---|---|
| Shaping | 10–14 | (none) | n/a | 1 s | Entire trial |
| Pretraining I | 2 | LT+, N+ | n/a | 5, 10, 15, 20 s | Final 15 s |
| Pretraining II | 3 | LT+, N+ | n/a | 5, 10, 15, 20 s | Final 5 s |
| Phase I | 12 | LT+, N+, LN−, T− | 1:1 | 5, 10, 15, 20 s | Final 5 s |
| Phase II | 12 | LT+, N+, LN−, T− | 2:1 | 5, 10, 15, 20 s | Final 5 s |
| Phase III | 16 | LT+, N+, LN−, T− | 2:1 | 15, 22.5, 30, 37.5, 45, 60, 75, 90, 105 s | Final 5 s |
| Phase IV | 16 | LT+, N+, LN−, T− | 2:1 | 30, 45, 60, 75, 90, 120, 150, 180, 210 s | Final 5 s |
| Light Only Test | 2 | LT+, N+, LN−, T−, L− | 3:2 | 15, 22.5, 30, 37.5, 45, 60, 75, 90, 105 s | Final 5 s |
Summary of data from pretraining and testing schedule for the FPFN task. Only data from the testing trials was analyzed. L – light; T – tone; N – white noise.
Training consisted of three phases. Phase I began with 60-minute sessions that presented 100 trials, randomly intermixed, of all four stimuli (LT+, N+, LN−, T−). Once again, each stimulus presentation was 15 seconds in duration and reinforcement on a fixed interval of 1 was available only in the final 5 seconds. ITIs were randomly varied between 5, 10, 15, and 20 s. After 12 sessions of phase I, the number of negatively reinforced stimuli (LN− and T−) presented was doubled for the next 12 sessions. During phase III animals were presented with 16 sessions nearly identical to the phase II; however, ITIs were increased such that they randomly varied between 15, 22.5, 30, 37.5, 45, 60, 75, 90, and 105 s. In addition, the total number of trials for this task was 60. In Phase IV (16 sessions) the duration of the ITIs was increased to 30, 45, 60, 75, 90, 120, 150, 180, and 210 s.
At the conclusion of Phase IV, animals were presented with two sessions in which, in addition to the other cues, the panel light (L) was presented as an individual cue for some trials, but was not reinforced. These sessions were designed to examine whether lesioned and control animals used different strategies to process the compound cues. For example, if an animal fused or compressed the compound cues into a single, elemental cue, the light presented alone would be processed as a novel cue and that animal would show increased responding to the light only cue.
For data analysis of the FPFN task, training sessions were averaged into blocks of four sessions. To avoid competition with food consumption during the last 5 seconds of each trial, rate of lever press responses during the first 10 seconds was analyzed. For these analyses, the dependent variable was the mean lever response rate during the 10 seconds of stimulus presentation that was not reinforced. We also analyzed response rates during the ITI. Data were analyzed by repeated measures analysis of variance (rANOVA). The within subject independent variable was Block and the between subject independent variables were Group (lesion vs. control) and Stimulus (reinforced vs. nonreinforced). Because there is some evidence that the feature positive and feature negative components of the task are learned differently (Holland and Gallagher, 1993; Holland et al., 1999), the LT+, T− discrimination was first analyzed separately from the LN−, N+ discrimination. This analysis approach is consistent with prior literature (Alvarado and Rudy, 1995; Gallagher and Holland, 1992). For these analyses, a main effect of Stimulus indicated successful discrimination, i.e. a difference in response rates to reinforced vs nonreinforced stimuli. A Group × Stimulus interaction indicated group differences in discrimination. A Group × Stimulus × Block interaction indicated group differences in the rate of learning the discrimination. A main effect of Group indicated a difference in overall responding regardless of whether the stimulus was reinforced or not.
Naïve and sham controls were not different on any analyses described above. Analysis of response rates for the excitatory component of the task yielded p values for main effects of Group and interactions with Group ranging from p>0.20 to p>0.99. For the inhibitory component of the task p values for main effects of Group and interactions with Group ranged from p>0.17 to p>0.59. Naïve and sham controls were combined into a single control group for the remaining analyses.
In order to compare acquisition of both components of the task in the same analysis, we calculated a simple discrimination index by subtracting the mean response rate during the non-reinforced stimulus presentation from the mean response rate during the reinforced stimulus presentation per session (Han et al., 1998). This index was also analyzed by rANOVA. The within subject independent variable was Block and the between subject independent variables were Group (lesion vs. control) and Component (positive vs. negative).
The effects of PER/POR damage on the light only cue in the last two test sessions was assessed by univariate ANOVA. This were also analyzed by rANOVA. The within subject independent variable was Block and the between subject independent variable was Group.
All analyses were conducted using SAS (SAS Institutes, Inc., Cary, NC). The level of significance for all analyses was set at 0.05.
Procedure for MCI Task
The same rats tested on the FPFN task were given 9 consecutive sessions of the Multiple Cued Interval Task (MCI), excluding weekends (Guilhardi and Church, 2005). The experimental sessions ended at 100 trials or 160 min. Each trial consisted of a 20-s period in which the discriminative stimulus was off, followed by one of three fixed intervals during which the discriminative stimulus was on. Food was available at the end of each fixed interval. Immediately after the next head entry into the food port (measured as the breaking of a photo beam across the food port opening), food was delivered, the discriminative stimulus was turned off, and the next trial began. The relevant variable was the rate of head entries, or nose pokes, during the fixed intervals during which the stimulus was on.
Fixed intervals were 30, 60, and 120 seconds. The three cues in this experiment were 72 dB white noise, the house light, and clicks. The clicker (ENV-135M, Med Associates), was mounted on the outside of the chamber and produced single auditory clicks at 4 Hz. Cue-duration pairings were counterbalanced. Rats were counterbalanced across six cue-interval conditions (Table 3). For example, a particular rat might have a 30-s interval signaled by white noise, a 60-s interval signaled by light, and a 120-s interval signaled by the clicker.
Table 3.
Timing Design for MCI
| Condition (R variable) | Stimulus cues
|
||
|---|---|---|---|
| White Noise | House Light | Clicker | |
| 1 | FI 30 | FI 60 | FI 120 |
| 2 | FI 30 | FI 120 | FI 60 |
| 3 | FI 60 | FI 30 | FI 120 |
| 4 | FI 120 | FI 30 | FI 60 |
| 5 | FI 60 | FI 120 | FI 30 |
| 6 | FI 120 | FI 60 | FI 30 |
The three cues for the multiple cue interval task (MCI) were white noise, house light, and clicker. Each cue was distributed across 30 s, 60 s, and 120 s intervals. Rats received 9 sessions of the task.
For analysis of the MCI task, the last 5 sessions were analyzed for group differences. Three measures of temporal discrimination were based on temporal gradients of responding. The mean response rate (in responses per minute) as a function of time since stimulus onset (in seconds) was calculated. The best-fitting ogive to this response gradient was calculated based on Equation 1.
| Equation 1 |
A nonlinear search algorithm that minimized the sum of squares was used for the estimation of the parameters a, b, and c, which served as measures of temporal discrimination. This was done with the NLINFIT function of Matlab (The MathWorks, Inc., Natick, MA).
The parameter a is an estimate of the center (the time at which response rate reached half of the way to its estimated maximum response rate)
The parameter b is an estimate of the slope of the function, and
The parameter c is an estimate of the maximum response rate of the function.
The parameters for center, slope, and maximum response rate were analyzed using rANOVA. Data were first analyzed for differences between the control groups followed by overall analyses of lesion effects. Interval was the within subject variable.
Histology
Subjects were deeply anesthetized with Beuthanasia-D© Special solution (100mg/kg, i.p.) and transcardially perfused with 0.9 % saline and 10% phosphate buffered formalin at a rate of 35/40 mL/min. Coronal sections were prepared and Nissl-stained with thionin. Lesions were evaluated for damage consisting of missing tissue, necrosis, or thinning of cortex. Subjects were retained in the study if lesions accomplished significant bilateral PER and POR damage and did not cause substantial bilateral damage outside of the target area.
Results
One rat was removed from the study due to extensive tissue loss outside the target regions, likely due to congenital defect, in the right hemisphere, leaving a total of 9 subjects in the lesion group and 8 in the control group. Other lesioned brains had minor unilateral cortical damage outside the target region, and one brain exhibited minor unilateral amygdala damage. None of the cases, however, exhibited hippocampal damage or bilateral damage outside the target regions. Figure 1 shows the extent of the largest and smallest lesions. The same subjects were tested on the FPFN task and the MCI task.
Figure 1.
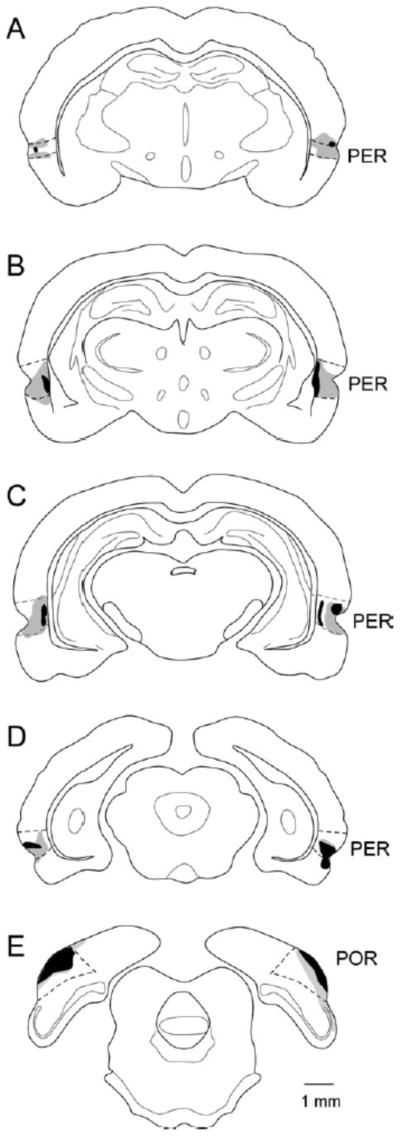
Combined perirhinal (PER) and postrhinal (POR) lesions. Schematics of coronal sections through the rat brain are shown with the largest lesion in gray and the smallest in black. Lesions were relatively small but distributed along the rostrocaudal extent of the PER and POR.
Response rate during the ITI decreased in both groups across four-session blocks as confirmed by rANOVA in a significant main effect of Block [F(1,13)= 22.05, p<0.0001]. There was no main effect of Group and no Group × Block interaction (p>0.44) suggesting no differences in overall activity levels.
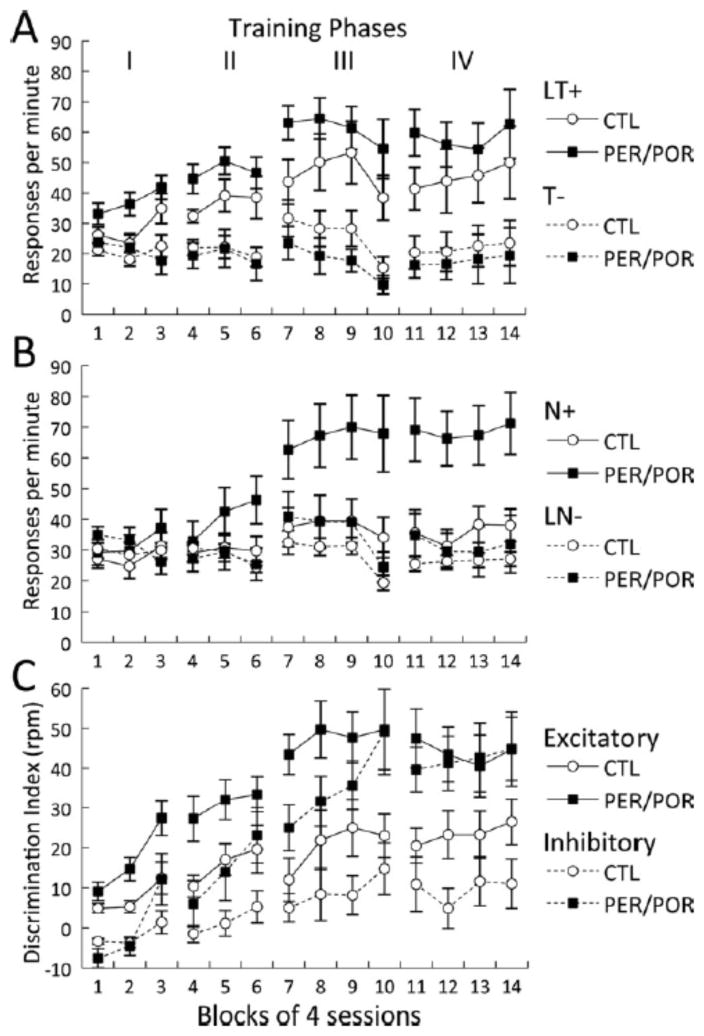
Analysis of the excitatory component of the task (Figure 2A) suggested that all rats learned to discriminate between the light-tone compound (LT+) and the tone alone (T−), but that the lesioned rats showed enhanced learning. There was no main effect of Group (p>0.80), as both groups of animals learned to respond more to the reinforced cue and less to the nonreinforced cue. There was, however, a main effect of Stimulus [F(1,15)= 61.84, p<0.0001] and a Stimulus × Group interaction [F(1,15)= 7.66, p<0.01]. Thus, although both control and lesion groups learned the excitatory component of the task, the significant interaction confirms that the lesion group showed enhanced learning. There was also a trend toward a Stimulus × Block × Group interaction [F(13,195)= 2.32, p<0.06] indicating that not only did lesion subjects learn the excitatory component of the task better, they exhibited a trend to learn this component faster.
Figure 2.
FPFN task results. A. Feature-positive (excitatory) component of the discrimination. B. Feature-negative (inhibitory) component of the discrimination. C. Discrimination index (reinforced response rate – nonreinforced response rate). Data are shown in responses per minute (rpm). Control and lesioned rats learned both the excitatory and the inhibitory components of the discrimination, but the lesioned rats showed significantly enhanced learning of both components. Moreover, by the end of testing the lesioned rats had learned the more difficult inhibitory component as well as they learned the excitatory component.
Analysis of the inhibitory component of the task (Figure 2B) also suggested that all rats learned to discriminate between the noise alone (N+) and the light-noise compound (LN−). Again, lesioned rats showed enhanced learning. The results of the rANOVA revealed a main effect of Stimulus [F(1,15)=22.18, p<0.0003]. There was no main effect of Group (p>0.15), but there was a significant Group × Stimulus interaction [F(13.195)= 9.55, p<0.007], as well as a significant Stimulus × Block × Group interaction [F(13,195)= 5.63, p<0.001]. Thus, similar to the excitatory component, in the inhibitory component of the task the lesioned group appeared to show better and faster learning, as compared with the control group.
To confirm that both groups did, indeed, learn to discriminate the stimuli in each component of the task, we conducted followup analyses for each group. For the excitatory component, the control group exhibited a significant main effect of Stimulus (p<0.005) and a significant Stimulus × Block interaction (p<0.0001) indicating successful discrimination. For the inhibitory component, the control group showed no main effect for Stimulus (p>0.22), but there was a Stimulus × Block interaction (p<0.0001). For the excitatory component, the lesioned group showed a significant effect of Stimulus (p<0.0001) and a Stimulus × Block interaction (p<0.0001). For the inhibitory component, the lesioned group also showed both a significant main effect of Stimulus (p<0.0009) and a significant Stimulus × Block interaction (p<0.0001). Thus, both groups successfully discriminated between the reinforced and non reinforced stimuli of each component.
We next wondered whether control and lesioned rats showed relative differences in acquisition of the two components, i.e. was enhanced acquisition apparent in the lesion group similar for the excitatory and inhibitory components of the task. In order to directly compare group differences in relative acquisition of both the excitatory and inhibitory components of the discrimination, we constructed simple discrimination indexes for both the excitatory and inhibitory components by subtracting the response rate to the nonreinforced stimulus from that of the reinforced stimulus (Figure 2C). The lesioned animals showed enhanced acquisition of the task as confirmed by a main effect of Group [F=9.13, p<0.0086]. The excitatory component was easier to learn as confirmed by a main effect of Component [F=52.15, p<0.0001]. A significant Component × Block × Group interaction indicated that acquisition of the two components differed for the two groups. Examination of Figure 2C suggests that the control animals showed better performance on the excitatory component than the inhibitory component for all phases. In contrast, by phase IV in which the ITIs were the longest, the lesioned animals appeared to perform equally well on both components.
To further examine differences in excitatory and inhibitory acquisition and performance across phases, we conducted follow up analyses of the discrimination indexes by task Phase. Response rates in each phase were analyzed by rANOVA with Group as the between subject variable and Component as the within subject variable. In Phase I in which ITIs were low and equal numbers of reinforced and nonreinforced trials were presented, there was a trend toward a main effect of Group (p<0.08), a significant main effect of Component (p<0.0001), and a significant Group × Component interaction (p<0.004). In Phases II, III, and IV, there were significant main effects of Group (p<0.04) and Component (p<0.002). The Group × Component interaction was not significant for Phases II and III (p>0.50). There was, however, a significant Group × Component interaction in Phase IV (p<0.04). Taken together, these results are consistent with the view that the lesioned rats showed enhanced performance and that the inhibitory component is more difficult to learn, but not as difficult for the lesioned rats, at least in Phases I when there were equal numbers of reinforced and nonreinforced trials and in Phase IV when ITIs were longest.
After completion of Phase IV, the effect of the panel light alone was analyzed in two final test sessions to determine if there were group differences in conditioning to the panel light, perhaps indicating a difference in how the two groups learned the task. As demonstrated by Figure 3, no significant difference was found between the response rate of the lesion and control groups to the light alone (p>0.23).
Figure 3.
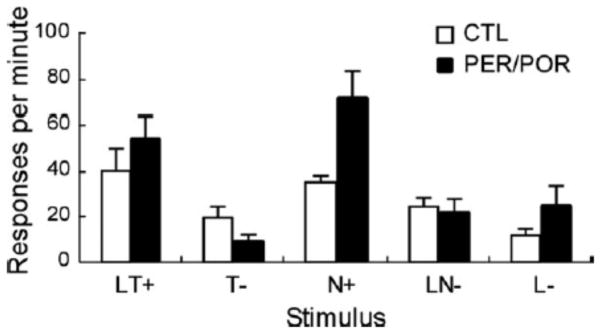
FPFN task results of light only trials. Responses to the unreinforced light only trials did not differ between control and lesioned groups, suggesting that the light component of the compound cues was processed similarly in both groups.
It is possible that the lesioned animals had a deficit in representing intervals or durations. If rats were unable to encode duration, this might have resulted in lever pressing during the entire 15 seconds of cue presentation while controls were able to withhold pressing until the final 5 seconds in which reward was available. If this were the case, the enhanced learning in the FPFN task might have resulted from a timing deficit rather than stronger learning in the lesion subjects. Training on the MCI task tested the hypothesis that PER and POR damage causes a deficit in representing a timed interval. In this experiment, a multiple cued interval task was designed in which animals were required to pair a cue with duration.
Figure 4 shows the rate of responding plotted against time for each of the cued intervals. No significant difference was found between control groups for Center (p<0.14), Slope (p<0.11), or Max Response (p<0.44). Thus, the control groups were combined for all further analyses. Visual inspection of plots in Figure 4 for each animal suggests that all subjects were able to represent the fixed intervals and to associate the appropriate interval with its signaling cue. As expected, there were also no differences between lesion and control rats in the curve fitting parameters (Table 4). Analysis by rANOVA indicated a trend toward a group difference in Center (p>0.07), but no Group × Interval interaction (p>0.15). Likewise there was no group difference or interaction for Slope (p>0.28) or Maximum Response (p>0.86).
Figure 4.
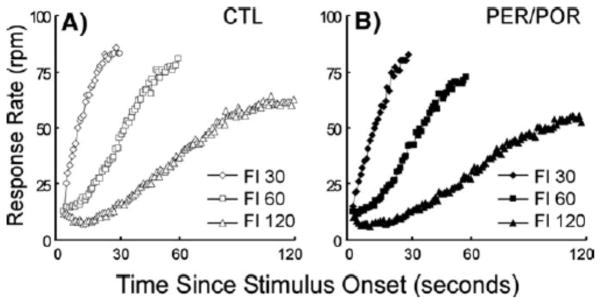
Response rates for the MCI task plotted against time. Rates are shown for the control (A) and lesioned (B) groups. There were no differences between groups in the ability to associate a discrete cue with a duration in this concurrent discrimination task using cues similar to those in the FPFN task except that there were no compound cues.
Discussion
We were expecting that PER/POR damage would result in impaired acquisition of the FPFN task; however, acquisition was enhanced in animals with bilateral, electrolytic PER/POR lesions as compared to control animals. This effect was even more pronounced for the inhibitory component of the discrimination. This was not a general enhancement in acquisition or performance, as there were no differences in the MCI task in which rats were trained on concurrent cue-interval associations using similar, but non-overlapping, sensory stimuli. Our conclusion is that combined PER/POR damage alters acquisition on the FPFN task and not the simple concurrent discrimination task, because the FPFN task requires processing of stimulus elements that are overlapping in time, whereas the MCI task does not.
There is substantial evidence that the PER is necessary for resolving ambiguity in discrimination tasks similar to the FPFN task, that is, tasks conducted in operant chambers and using simple stimuli with overlapping stimulus elements. Rats with PER damage were impaired on a feature-negative task (LT+, T−) when the compound stimuli were presented simultaneously, but not when the compound was presented serially (L—>T+, T−, Campolattaro and Freeman, 2006a ). Rats with PER damage were also shown to be impaired on positive patterning (LT+, L−, T−) when the compounds were presented simultaneously (Campolattaro and Freeman, 2006b). Again, when the same stimuli were presented serially in the positive patterning task, PER damage did not cause deficits (Campolattaro and Freeman, 2006b). Similar results were obtained with a purely visual task. Rats with selective PER lesions were impaired on a two-dimensional (2D) visual biconditional discrimination presented in a computer controlled Y maze (Eacott et al., 2001), but the same rats were not impaired on acquisition of simple two-dimensional discriminations. In yet another type of task, one using three-dimensional (3D) objects, rats with PER damage were impaired in discriminating an array of familiar objects from an array in which one or more familiar objects were replaced with a novel object (Gilbert and Kesner, 2003). The authors suggested that the PER is necessary for resolving stimulus interference, an interpretation similar to the notion that PER resolves feature ambiguity in visual discrimination tasks. These studies provide substantial evidence that the PER is necessary for resolving stimulus ambiguity whether stimuli are simple lights and tones overlapping in time, complex visual stimuli with overlapping features, or arrays of objects with overlapping members.
Although there are no prior studies of the effects of combined PER/POR lesions on the FPFN task, there are studies using paradigms that are formally the same, but employ more complex visual stimuli. Rats with combined PER and POR lesions were impaired on tasks involving discrimination of 2D visual stimuli when perceptual similarity was high due to overlapping features (Bartko et al., 2007). In another study rats with combined PER and POR damage showed no deficits on simple discriminations of 2D visual stimuli presented in a water maze choice apparatus, but were impaired when an additional cue was added to make the discrimination biconditional (Davies et al., 2007). The interpretation was that the additional cue resulted in the increase of overlapping features and thus increased perceptual similarity.
Taken together, prior findings suggest that PER or combined PER/POR damage might have resulted in impaired acquisition in the FPFN task, and not the observed enhancement. Hippocampal damage sometimes results in better performance on similar tasks and this outcome is often attributed to a reduction of interference (Han et al., 1998; Saksida et al., 2007; Sanderson et al., 2011). In the present study, however, performance on the FPFN task was enhanced even when inter-trial intervals were substantially increased. In addition, our lesions were small and in no case was hippocampal damage observed. Thus, reduction of proactive interference does not appear to underlie the observed enhancement.
Another possibility is that the POR lesion alters the associative strategy in a manner that results in enhanced acquisition. If there were differences in the processing of the compound cues, then it might be expected that lesioned and control animals would respond differently to the light cue presented alone (for discussion of strategy selection, see also Peterson et al., this issue). The control and lesion groups, however, did not differ in response rates to the panel light when presented as an individual cue on the last two days of training. This suggests that both groups were using the same associative strategy to solve the task, at least with respect to the light cue.
Although the processing of the light stimulus did not differ, associative processing of context may have been altered as a consequence of combined damage to PER and POR. The rodent POR (Bucci et al., 2000; Bucci et al., 2002; Norman and Eacott, 2005) and the primate parahippocampal cortex (Aminoff et al., 2007; Bar and Aminoff, 2003; Mullally and Maguire, 2011) have an identified role in processing contextual information, and there is a substantial amount of evidence in rats and primates that the PER also processes contextual information (Bucci et al., 2000; Bucci et al., 2002; Burwell et al., 2004; Corodimas and LeDoux, 1995; Kholodar-Smith et al., 2008a; Kholodar-Smith et al., 2008b; Moyer and Brown, 2006; Preston and Gabrieli, 2008; Watson et al., 2012). Thus, the combined PER/POR lesion likely resulted in an impairment in contextual processing. Such an impairment may have led to enhanced acquisition of the FPFN task in the following way. Context, itself, has associative properties (Holland and Bouton, 1999). Although context was not manipulated in the FPFN task, it may have interacted with the explicit cues such that context (represented by X) and the explicit cues in the task (represented by LT+, T−, N+, LN−) become LTX+, TX−, NX+, LNX−, and X−. Context is present during the controlled cues, but is present, alone and unrewarded (X−), during the long ITIs. It may be that context accrues a strong inhibitory association throughout the task that exerts inhibitory control over the other cues. This overall inhibition would need to be overcome in animals that were processing context. In the lesioned animals, however, the ability to process context is weakened, lessening the inhibitory control over the stimuli, and possibly enabling faster and better learning. It might be expected that the ambiguous (overlapping) stimuli in the FPFN task would be more sensitive to contextual control than the simple associations required by the MCI task. Bouton and Nelson (1994) showed in a feature-negative discrimination task that performance to the negative compound increased when the context was switched. This result provides some insight into why the binary associations in the MCI task were not affected by PER/POR damage. This result is also consistent with our findings showing that acquisition of the inhibitory of the FPFN task was more robustly enhanced.
Conclusions
Rats with PER/POR damage showed alterations in the acquisition of a discrimination in which simple stimulus elements overlapping in time must be disambiguated. In contrast, no alteration was observed in learning a control task, a simple concurrent discrimination task in which elemental stimuli were paired with durations in a binary fashion. The control task verified that subjects could learn a complex discrimination, and that there were no deficits in time perception. Our findings are consistent with prior studies reporting that PER and combined PER/POR damage cause deficits in tasks that require disambiguation of overlapping features of associative stimuli. The alteration in acquisition and performance, however, was an enhancement rather than a deficit. The lesioned subjects showed enhanced performance even when ITIs were relatively long, providing evidence against an interference hypothesis. Previous studies have indicated that PER or POR damage results in impairment in contextual learning. Thus, impairments in processing context may have resulted in decreased inhibitory control over other cues in the lesioned group, resulting in enhanced acquisition of the FPFN task. Future experiments could examine this hypothesis by exploiting learning paradigms that involve contextual associations, for example, contextual sensory pre-conditioning and a form of second-order conditioning in which a context-US is the first-order association. We would predict that rats with damage to perirhinal and postrhinal cortices would be impaired in these paradigms.
Acknowledgments
This research was supported by an NSF Award (IOB-0522220) to RDB. PG is currently at the New England Center for Children. EDG is currently at Columbia University.
References
- Alvarado MC, Rudy JW. A comparison of kainic acid plus chochicine and ibotenic acid-induced hippocampal formation damage on four configural tasks in rats. Behavioral Neuroscience. 1995;109(6):1052–1062. doi: 10.1037//0735-7044.109.6.1052. [DOI] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17(7):1493–503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38(2):347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(10):2548–59. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. Journal of experimental psychology Animal behavior processes. 1994;20(1):51–65. [PubMed] [Google Scholar]
- Bucci DJ, Burwell RD. Deficits in Attentional Orienting Following Damage to the Perirhinal or Postrhinal Cortices. Behav Neurosci. 2004;118(5):1117–1122. doi: 10.1037/0735-7044.118.5.1117. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114(5):882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to postrhinal or perirhinal cortex. Behav Neurosci. 2002;116(3):479–488. [PubMed] [Google Scholar]
- Burwell RD. The perirhinal and postrhinal cortices of the rat: Borders and cytoarchitecture. J Comp Neurol. 2001;437(1):17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: Interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998;391(3):293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Postrhinal and perirhinal contributions to remote memory for context. J Neurosci. 2004;24(49):11023–11028. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Perirhinal cortex lesions impair feature-negative discrimination. Neurobiology of learning and memory. 2006a;86(2):205–13. doi: 10.1016/j.nlm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Perirhinal cortex lesions impair simultaneous but not serial feature-positive discrimination learning. Behavioral neuroscience. 2006b;120(4):970–5. doi: 10.1037/0735-7044.120.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: Contributions of contextual cues. Behav Neurosci. 1995;109(4):613–619. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- Davies M, Machin PE, Sanderson DJ, Pearce JM, Aggleton JP. Neurotoxic lesions of the rat perirhinal and postrhinal cortices and their impact on biconditional visual discrimination tasks. Behavioural brain research. 2007;176(2):274–83. doi: 10.1016/j.bbr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. The entorhinal cortex of the rat: Topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398(1):25–48. [PubMed] [Google Scholar]
- Eacott MJ, Machin PE, Gaffan EA. Elemental and configural visual discrimination learning following lesions to perirhinal cortex in the rat. Behav Brain Res. 2001;124(1):55–70. doi: 10.1016/s0166-4328(01)00234-0. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. Preserved configural learning and spatial learning impairment in rats with hippocampal damage. Hippocampus. 1992;2(1):81–88. doi: 10.1002/hipo.450020111. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learning & memory. 2003;10(6):525–30. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhardi P, Church RM. Dynamics of temporal discrimination. Learning & Behavior. 2005;33(4):399–416. doi: 10.3758/bf03193179. [DOI] [PubMed] [Google Scholar]
- Han J-S, Holland P, Gallagher M. Hippocampal lesions enhance configural learning by reducing proactive interference. Behav Neurosci. 1998;113(1):143–151. doi: 10.1002/(SICI)1098-1063(1998)8:2<138::AID-HIPO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Holland P, Reeve C. Acquisition and transfer of control by an ambiguous cue. Learning & Behavior. 1991;19(2):113–124. [Google Scholar]
- Holland PC. Transfer of control in ambiguous discriminations. J Exp Psychol Anim Behav Process. 1991;17(3):231–48. doi: 10.1037//0097-7403.17.3.231. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Current opinion in neurobiology. 1999;9(2):195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav Neurosci. 1993;107(2):246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Lamoureux JA, Han J-S, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9(2):143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Meldrum BS. Selective excitotoxic pathology in the rat hippocampus. Neuropathology and Applied Neurobiology. 1993;19(5):381–389. doi: 10.1111/j.1365-2990.1993.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17(9):697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Allen TA, Brown TH. Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behavioral neuroscience. 2008a;122(5):1178–85. doi: 10.1037/a0012902. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Boguszewski P, Brown TH. Auditory trace fear conditioning requires perirhinal cortex. Neurobiology of learning and memory. 2008b;90(3):537–43. doi: 10.1016/j.nlm.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Brown TH. Impaired trace and contextual fear conditioning in aged rats. Behavioral neuroscience. 2006;120(3):612–24. doi: 10.1037/0735-7044.120.3.612. [DOI] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(20):7441–9. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119(2):557–66. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Peterson MA, Cacciamani L, Barense MD, Scalf PE. The perirhinal cortex modulates V2 activity in response to the agreement between part familiarity and configuration familiarity. Hippocampus. doi: 10.1002/hipo.22065. this issue. [DOI] [PubMed] [Google Scholar]
- Preston AR, Gabrieli JD. Dissociation between explicit memory and configural memory in the human medial temporal lobe. Cerebral cortex. 2008;18(9):2192–207. doi: 10.1093/cercor/bhm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav Brain Res. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ, Buckmaster CA, Murray EA. Impairment and facilitation of transverse patterning after lesions of the perirhinal cortex and hippocampus, respectively. Cerebral cortex. 2007;17(1):108–15. doi: 10.1093/cercor/bhj128. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Rawlins JN, Deacon RM, Cunningham C, Barkus C, Bannerman DM. Hippocampal lesions can enhance discrimination learning despite normal sensitivity to interference from incidental information. Hippocampus. 2011 doi: 10.1002/hipo.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiol. 1990;17(2):129–144. [Google Scholar]
- Watson HC, Wilding EL, Graham KS. A role for perirhinal cortex in memory for novel object-context associations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(13):4473–81. doi: 10.1523/JNEUROSCI.5751-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig KA, Burwell RD. Memory impairment on a delayed-non-matching-to-position task following lesions of the perirhinal cortex in the rat. Behav Neurosci. 1998;112(4):828–838. doi: 10.1037//0735-7044.112.4.827. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(8):3582–98. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]