Abstract
Background
The risks attributed to drug-herb interactions, even when known, are often ignored or underestimated, especially for those involving anti-clotting drugs and Chinese medicines. The aim of this study was to structurally search and evaluate the existing evidence-based data associated with potential drug interactions between anticoagulant/antiplatelet drugs and Chinese herbal medicines (CHMs) and evaluate the documented mechanisms, consequences, and/or severity of interactions.
Methodology and Findings
Information related to anticoagulant/antiplatelet drug-CHM interactions was retrieved from eight interaction-based textbooks, four web resources and available primary biomedical literature. The primary literature searches were conducted in English and/or Chinese from January 2000 through December 2011 using the secondary databases (e.g., PubMed, Airiti Library, China Journal full-text database). The search terms included the corresponding medical subject headings and key words. Herbs or natural products not used as a single entity CHM or in Chinese Medicinal Prescriptions were excluded from further review. The corresponding mechanisms and severity ratings of interactions were retrieved using MicroMedex®, Lexicomp® and Natural Medicines Comprehensive Database®. Finally, we found 90 single entity CHMs contributed to 306 documented drug-CHM interactions. A total of 194 (63.4%) interactions were verified for its evidence describing possible mechanisms and severity. Of them, 155 interactions (79.9%) were attributable to pharmacodynamic interactions, and almost all were rated as moderate to severe interactions. The major consequences of these interactions were increased bleeding risks due to the additive anticoagulant or antiplatelet effects of the CHMs, specifically danshen, dong quai, ginger, ginkgo, licorice, and turmeric.
Conclusions/Significance
Conventional anticoagulants and antiplatelet drugs were documented to have harmful interactions with some commonly used single entity CHMs. For those patients who are taking conventional anti-clotting medications with CHMs for cardiovascular or cerebrovascular diseases, the potential risks of increased bleeding due to drug-CHM interactions should not be ignored.
Introduction
Ischemic heart disease and stroke are the two primary causes of death worldwide, accounting for 24% of all deaths reported in 2008 [1]. Anticoagulants and antiplatelet drugs are important standard therapies used to prevent clot formation in the treatment and prevention of cardiovascular and cerebrovascular diseases [2]. However, most anticoagulant and antiplatelet agents have drug interactions with a variety of other medications, foods, and dietary supplements [3], [4]. A systematic review published in 2008 reported that medications with anticoagulant or antiplatelet activity are most likely to have interactions with herbal medicines [5]. Specifically, those patients given anticoagulant agents are at a higher risk of suffering from potentially harmful drug interactions, compared to other cardiovascular drugs, when co-administered with herbal medicines [6].
The use of complementary and alternative medicine (CAM), including herbal medicines, is widespread and has increased worldwide over the last decade [7], [8]. A systematic review of CAM found that the prevalence of CAM use varied widely from 9% to 65% [9]. Nearly 40% of patients with cardiovascular disease or stroke had used CAM therapies concomitantly with their prescribed medications [10], [11]. Traditional Chinese Medicine (TCM) is one of the dominant forms of CAM for many Asian populations [7], [12], [13], including those who migrated to western countries. A substantial percentage of Asian patients were likely to use TCM in combination with the conventional medicines [14]–[17]. For instance, 13% of patients using anticoagulant or antiplatelet agents were also prescribed concentrated CHMs concomitantly in a Taiwanese population-based study [17].
To date, there is very limited documented information about potential prescription drug interactions with CHMs. Considerable proportions of clinical practitioners or patients underreport their use of natural health products, including CHMs [18]. The majority of physicians and professional trainees have limited training on herbal adverse events, toxicities, and drug interactions [19]. The lack of training and education may be associated with a reduced recognition of potential drug-herb interactions, specifically their occurrence and associated adverse events. While patients taking anticoagulant/antiplatelet drugs are more likely to be exposed to potential drug-herb interactions (i.e., increased risks of bleeding [20]), the aim of this study was to systematically review the available published evidence-based data and biomedical reports associated with drug interactions between anticoagulant/antiplatelet drugs and CHMs, and further to evaluate the documented mechanisms, consequences, and/or severity of interactions.
Materials and Methods
Evidence retrieval and literature search
The evidence regarding “harmful” interactions between anticoagulant/antiplatelet drugs and herbal medications used in TCM (in terms of Chinese herbal medicine [CHM]) was the main focus and extracted from eight interaction-based textbooks [21]–[28], four web resources [29]–[32], and available primary biomedical literature sources. In this review, we defined CHM as the same definition derived from the web site of National Center for Complementary and Alternative Medicine and originally cited from Chinese Materia Medica [33]. The search of primary articles was conducted in four English and three Chinese secondary databases, including MEDLINE, PubMed, EMBASE, Cochrane Library, Airiti Library, Index to Taiwan Periodical Literature System, and the China Journal full-text database. The search terms in these databases included the corresponding medical subject headings (MeSH terms) and key words as follows: ‘herb drug interactions’; ‘anticoagulants’ OR ‘antiplatelet drugs’ OR ‘warfarin’ OR ‘heparin’ OR ‘aspirin’ OR ‘clopidogrel’ OR ‘dipyridamole’ OR ‘ticlopidine’ AND ‘Traditional Chinese Medicine’ OR ‘Chinese medication’ OR ‘herbal medicine’ OR ‘phytotherapy’ AND ‘interaction.’ The searches were restricted to either the English or Chinese language during 2000 to 2011 (from January 2000 through December 2011). The articles were selected based on the title and abstract. The selected articles were retrieved independently by two authors (YLC, YHL), and then validated by the other two authors (HHT, HWL). Specifically, all retrieved literature were selected regardless of types of study, i.e., animal studies, clinical trials, observational studies, or review articles. Those in vitro studies or literature without interaction reports, or corresponding studies about pure compounds (but not for single entity CHMs), were excluded.
Evidence extraction and review
Initially, any level of evidence-based interactions, as long as it was retrieved from the aforementioned books, web sites, or primary literature, between the anticoagulant/antiplatelet agents and any natural products or herbs (i.e., either being classified as CHMs or not) was first extracted and documented. However, animal-derived medications used in TCM were not included. Then, we used a standardized data abstraction checklist on Excel® spreadsheet to extract all relevant data, including common name, scientific name and/or binomial source, plant parts, dose, route of administration, drug name, consequence of interactions, and evidence resources, etc, if they were available. Data for all anticoagulant/antiplatelet agents was extracted regardless of belonging to a drug class or an individual drug. The anticoagulants were composed of heparin and warfarin while antiplatelet agents included aspirin, clopidogrel, dipyridamole and ticlopidine. All relevant data in the literature was extracted and compiled by two of the authors (YLC, YHL), and then validated by the other two authors (HHT, HWL). To cross-validate the retrieved and reviewed information, we conducted the focus group review and discussions. A focus group included five experienced pharmacists practicing in either Western medicine or Chinese medicine on a daily basis in Taiwanese hospitals. Any disagreements about classifications and information conflict were resolved by consensus. Specifically, all of the members in the focus group were trained in both Western and Chinese medicines in a School of Pharmacy and/or their master graduate programs. They all have more than 15 years of experience practicing in TCM pharmacy or clinical pharmacy services in hospitals that provide medical services with both Western and TCM medicines. None of these members were authors.
Data management and analysis
Next, the following review and analysis particularly focused on those single entity CHMs commonly listed in Chinese Medicine books, including the Taiwan Herbal Pharmacopoeia (THP) [34], Chinese Medicinal Herbs Preparation [35], and Chinese Materia Medica [36]. Thus, those natural products or herbs that were not documented in the aforementioned books and/or any combination of Chinese Medicinal Prescriptions, which were listed in some ancient TCM books, were excluded for further analyses. Moreover, those single entity CHMs included for further review were verified by two experienced pharmacists who were members of the focus group.
To obtain proof about clinical significance of interactions, we identified and verified the severity ratings and possible mechanisms of each interaction using three well-known interactions databases (MicroMedex® [37], Lexi-Interact in Lexicomp® [38], or “Natural Product/Drug Interaction Checker” in Natural Medicines Comprehensive Database® (NMCD®) [39]). Because of the various definitions of severity ratings among these three databases, we compiled the rating schemes and categorized the severity of each interaction into six types in this review: “contraindicated,” “major,” “moderate,” “minor,” “no interaction,” and “no available information for the item.” The comparisons of severity rating definitions in different databases with this review were listed in Table 1. Accordingly, the mechanisms for interactions were also categorized into pharmacokinetics, pharmacodynamics, pharmacokinetics plus pharmacodynamics, and unknown based upon the records from these three databases. All of the related data was compiled and managed using the Excel® spreadsheets. Last, the descriptive analyses were performed to explore the frequency and proportion of the retrieved evidence associated with the interaction numbers, the corresponding types of retrieved mechanisms and severity ratings of interactions.
Table 1. The comparisons of severity rating definitions in different databases with current review.
| MicroMedex® | Lexicomp® | NMCD® | Current review | |
| Contraindicated | Do not use combination; contraindicated. | - | - | Interactions with severity rated as “contraindicated” according to MicroMedex®. |
| Major | Combination may cause life-threatening damage and/or need medical intervention to prevent severe adverse effect. | Effects may result in death, hospitalization, permanent injury, or therapeutic failure. | Do not use combination; contraindicated; strongly discourage patients from using this combination; a serious adverse outcome could occur. | Interactions with severity rated as “major” in any of the three databases. |
| Moderate | Combination may cause worsen the patient’s condition and/or need to change the therapy. | Medical intervention needed to treat effects; effects do not meet criteria for Major. | Use cautiously or avoid combination; warn patients that a significant interaction or adverse outcome could occur. | Interactions rated as “moderate” and without “major” score in any of these databases. |
| Minor | Do not need to change the therapy. | Effects would be considered tolerable in most cases; no need for medical intervention. | Be aware that there is a chance of an interaction; advise patients to watch for warning signs of a potential interaction. | Interactions rated as “minor” and without “moderate” or “major” score in any of these databases. |
| No interaction | - | - | - | There was no documented interaction between the medication and the single entity CHM |
| No available documented information for the item | - | - | - | There was no available information about the single entity CHM in these databases. |
NMCD: Natural Medicines Comprehensive Database.
Result
Literature search and evidence retrieval
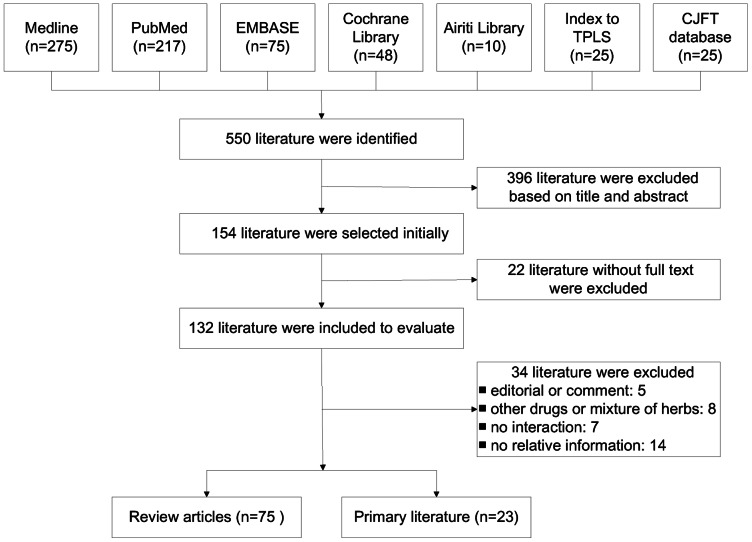
A total of 154 articles were selected from the initial 550 retrieved literature reports based on their relevance to the purpose of review. After thorough evaluation and analysis, 98 articles with full text (75 review articles [5], [6], [40]–[112], and 23 primary studies, i.e., 4 animal studies [113]–[116], 4 clinical trials [117]–[120], and 15 observational studies [20], [121]–[134]) were included for further evaluation (Figure 1). The summaries of retrieved primary literature are listed in Table 2, while the summaries of retrieved review articles are listed in Appendix S1. The retrieved data missing information concerning the common name, scientific name, plant source, plant parts, and dose for the natural products/herbs or CHM were excluded from Table 2 and Appendix S1, but may be accessed upon specific request to the authors.
Figure 1. Flow chart of primary literature search.
TPLS: Taiwan Periodical Literature System; CJFT: China Journals Full-text.
Table 2. Summary of the included primary literature and the retrieved relevant information about natural products or herbs (including CHMs).
| Clinical trials | |||||
| Reference | Natural products/ herbs (including CHMs) | Dose schedule of Natural products/herbs (including CHMs) | Medication | Study design | Observed/measured outcomes |
| Abdul 2010 [117] | Commercial products of echinacea (oral) | 14 days | Warfarin (oral) | Open-label, randomized, three-treatment, cross- over study | INR, platelet activity, Warfarin concentration |
| Jiang 2006 [118] | Commercial products of St John’s wort, Asian ginseng, ginkgo, or ginger (oral) | 7 or 14 days | Warfarin (oral) | Two randomized, open-label, controlled, crossover studies | INR, Warfarin concentration |
| Jiang 2004 [119 | Commercial products of St John's wort, ginseng (oral) | 7 days or 14 days | Warfarin (oral) | Open-label, three-way crossover randomized study | Platelet aggregation, INR, Warfarin protein binding, Warfarin concentration |
| Yuan 2004 [120] | Root of American ginseng (oral) | 3 weeks | Warfarin (oral) | Randomized, double-blind, placebo-controlled trial. | INR, Warfarin concentration |
CYP: cytochrome P450; VKORC1: vitamin K epoxide reductase complex subunit 1; INR: international normalized ratio.
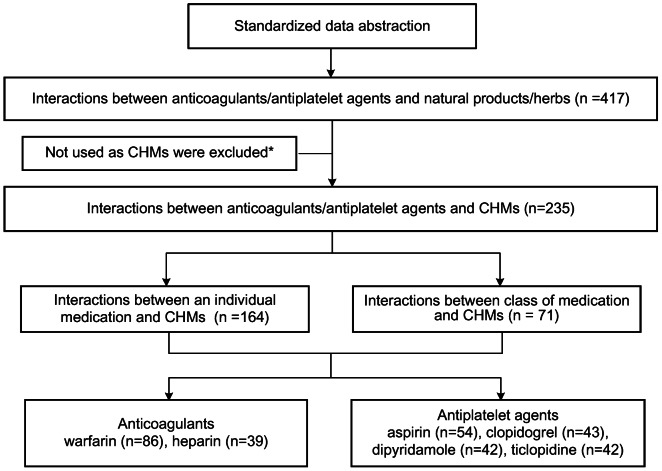
Initially, there were 417 interactions in total obtained from the aforementioned evidence-based resources. Of them, 235 interactions between anticoagulant/antiplatelet agents and specific single CHMs were extracted for further evaluation. After expanding the interactions to individual medications from the different drug classes, a total of 306 interactions between distinct anticoagulant/antiplatelet agents and single entity CHMs were identified. Warfarin and aspirin accounted for the majority of documented, evidenced-based interactions with single entity CHMs (Figure 2). Overall, 90 distinct single entity CHMs contributed to the 306 documented interactions.
Figure 2. Retrieved findings of interactions between anticoagulant/antiplatelet agents and single Chinese herbal medicines.
* Natural products or herbs which were not identified in the Taiwan Herbal Pharmacopoeia, Chinese Medicinal Herbs Preparation, or Chinese Materia Medica were excluded. CHMs: Chinese herbal medicines.
Severity rating and mechanisms upon availability in three databases
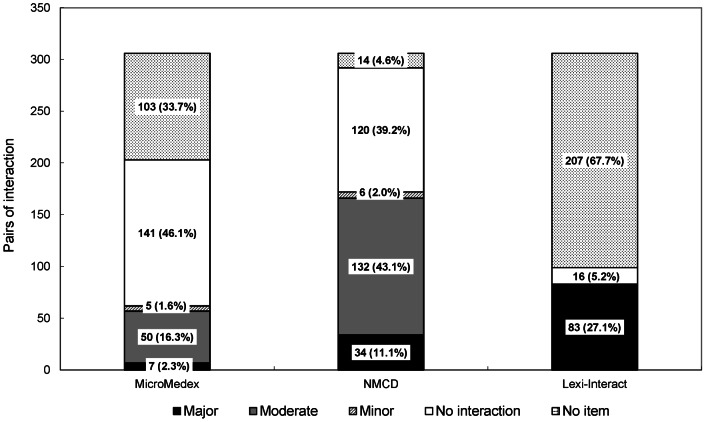
Of the 306 interactions between distinct anticoagulant/antiplatelet agents and single entity CHMs, 203 (66.3%), 292 (95.4%), and 99 (32.3%) drug-CHM interactions were retrieved with some relevant information about interactions from MicroMedex®, NMCD®, and Lexicomp®, respectively. However, only 62 (20.2%), 172 (56.2%), and 83 (27.1%) of these interactions in MicroMedex®, NMCD®, and Lexicomp®, respectively, contained identifiable severity ratings and interaction mechanisms (Figure 3). Overall, a total of 194 (63.4%) interactions were found to have severity ratings and mechanisms in at least one of these databases. While 50 interactions with information in MicroMedex® (80.6%) were categorized as the moderate interactions, 132 interactions documented in NMCD® (76.7%) were classified as moderate. However, all of the 83 documented interactions in Lexicomp® were found to have major drug-CHM interactions (Figure 3). Consequently, of all 194 interactions being identified with the severity rating in any of the three databases, only 29 (15.0%) were found in all of the three databases, 65 (33.5%) could be identified in two databases, while more than half (100, 51.5%) were documented in only one database.
Figure 3. Documented severity ratings of interactions between distinct anticoagulant/antiplatelet agents and single Chinese herbal medicines.
The total interactions between distinct anticoagulant/antiplatelet agents and single CHMs were 306. The classification of “No interaction” meant that there is no interaction between the medication and the single CHMs, while “No item” meant that there was no available information about the single CHMs in the database. NMCD: Natural Medicines Comprehensive Database.
Of those identifiable mechanisms for interactions, the majority (155, 79.9%) were attributable to pharmacodynamic-related mechanisms. 8.3% were attributed to pharmacokinetic-related mechanisms and 11.3% were the results of both pharmacodynamic and pharmacokinetic mechanisms. Most of the pharmacodynamic-related interactions were due to the additive anticoagulant/antiplatelet effect of the single entity CHM. For example, some CHMs, i.e., clove (Eugenia caryophyllata), cat's claw (Uncaria tomentosa), and ginger (Zingiber officinale), were reported to inhibit cyclooxygenase activity, platelet aggregation, and thromboxane synthetase activity, respectively [37], [39]. Most of these potential interactions have not been documented in clinical practice. In contrast, a few documented single entity CHMs with pharmacodynamic-related interactions causing decreased anticoagulant effects were reported in the literature. For example, alfalfa (Medicago sativa) and green tea (Camellia sinensis) may antagonize the anticoagulant effects of warfarin due to the presence of vitamin K in these products [37], [39]. As for the documented pharmacokinetic-related interactions, the majority were due to the inhibition of warfarin or clopidogrel metabolism via the Cytochrome P450 (CYP) pathway, including CYP 1A2, 3A4, and 2C9. For example, dandelion (Taraxacum officinale), bitter orange (Citrus aurantium), and milk thistle (Silybum marianum) were reported to inhibit CYP1A2, 3A4, and 2C9, respectively. Therefore, these herbal products may inhibit warfarin or clopidogrel catabolism, and result in an increase in their anticoagulation/antiplatelet activities [39]. Similarly, ginkgo (Ginkgo biloba), red clover (Trifolium pratense), and Siberian ginseng (Eleutherococcus senticosus) were documented to inhibit all of these three CYP enzymes, thus similar effects on the anticoagulation action of warfarin or clopidogrel may be expected [37], [39].
Consequences of drug–CHM Interactions
Of the 90 interactions that involve a single entity CHM, the majority would result in an increased risk of bleeding when used in combination with anticoagulants or antiplatelet agents (Table 3). For instance, commonly used CHMs including danshen (Salvia miltiorrhiza), dong quai (Angelica sinensis), ginger (Zingiber officinale), and licorice (Glycyrrhizae uralensis) were documented to have major interactions with anticoagulants or antiplatelet drugs. The combinations causing major interactions may cause life-threatening damage and/or severe adverse events. Thus, we strongly discourage patients and clinicians from using these combinations [37]–[39]. Specifically, celery, a functional food and classified as a CHM in the Chinese Materia Medica, is reported to increase bleeding in Philp's book and a few review articles and was then verified in the Lexicomp® record [26], [81], [82], [85], [94]. There were no published primary data that describe the rationale or potential mechanism of this interaction. Therefore, interpretation of the data related to celery should be cautious.
Table 3. Documented interactions between anticoagulant/antiplatelet drugs and Chinese herbal medicine, a which might increase the risks of bleeding.
| Severity rating b | CHM documented to increase bleeding risks of the six anticoagulant/antiplatelet drugs | CHM documented to increase bleeding risks of at least one of the six anticoagulant/antiplatelet drugs |
| Major | Celery, Chamomile, Danshen, Dong quai, Evening primrose, Fenugreek, Garlic, Ginger, Ginkgo, Horse chestnut, Licorice, Red clover, Reishi,c Turmeric, Willow | Cat's claw |
| Moderate | Andrographis, Bogbean, Cayenne, Clove, Flaxseed, Kudzu, Onion, Saw palmetto | Aloe vera, Asafetida, Bitter orange, Blackcurrant seed, Burdock, Cassia, Cinchona, Coltsfoot, Da huang, Eucalyptus, Lycium, Milk thistle, Nutmeg, Peony, Royal jelly, Safflower, Soybean, Valerian, Yarrow |
| No available information for the item | Artichoke, Astragalus, Dandelion,d Glehnia, Kelp, Lavender, Meadowsweet,e Red yeast rice,f Skullcap | American ginseng, Angelica,f Artemesia, Black cohosh,f Chrysanthemum flower, Corydalis yanhuso, Dang shen, Erigeron, Geum japonicum, Hawthorn,e Horseradish, Jamaica quassia, Japanese brome, Lovage, Magnolia bark, Motherwort, Plantain, Pubescent angelica, Rue, Shiitake, Sweet melilot, Tamarind,e Wood ear mushrooms |
Chinese herbal medicine were identified according to Taiwan Herbal Pharmacopoeia,[34] Chinese Medicinal Herbs Preparation,[35] or Chinese Materia Medica.[36]. b The severity rating was categorized according to MicroMedex®, Lexicomp® and Natural Medicines Comprehensive Database®. Interactions with severity rated as major in any of the three databases were included in the “major” type, while interactions rated as moderate and without major score in any database were included in the “moderate” type. “No available documented information for the item” meant that there was no available information about the single CHM in these databases. c Interaction with heparin is lack of information about severity rating. d Interaction with clopidogrel or warfarin is moderate. e Interaction with aspirin is moderate. f Interaction with warfarin is moderate.
Furthermore, a few reports showed that single entity CHMs may decrease the effectiveness of anticoagulant/antiplatelet agents. Agrimony (Agrimonia eupatoria), myrrh (Commiphora myrrha), and St. John's wort (Hypericum perforatum) were documented to result in a decreased effectiveness of warfarin [21], [22], [24], [25], [72], [135], while pepper (Piper nigrum) and cannabis (Cannabis sativa) were reported to reduce the antiplatelet efficacy of aspirin [28]. Nevertheless, we also noted the conflicting consequences of interactions reported in the different evidence resources. Some evidence suggested that alfalfa, Asian ginseng (Panax ginseng), green tea, and Siberian ginseng may reduce the anticoagulant effects of anticoagulant/antiplatelet agents [5], [37], [73], [74], while some published data suggested that these single entity CHMs might increase the risk of bleeding episodes if they were used concurrently with anticoagulants and antiplatelet drugs [38], [39], [73].
Discussion
In this review, the potential interactions between anticoagulant/antiplatelet drugs and single entity CHMs were analyzed and evaluated based on retrievable and published evidence. We found 90 commonly used single entity CHMs (such as danshen, dong quai, ginger, and licorice) were involved in 306 evidence-based drug interactions with anticoagulant and/or antiplatelet drugs. Warfarin and aspirin were the two drugs reported to have the largest numbers of documented interactions with the greatest number of single entity CHMs. Most of these interactions were moderate to severe and attributable to pharmacodynamic mechanisms. The majority of these interactions were found to increase the risks of bleeding.
In the United States and Singapore, 10–20% of patients used herbal therapies or CHMs when using prescribed anticoagulant or antiplatelet medications for cardiovascular diseases or stroke [10], [11]. A survey also showed that one in five patients in Malaysia who took anticoagulant or antiplatelet drugs, also concurrently used herbal therapies [20]. While the use of TCM is prevalent among Asian populations [12], [13], there is also an increasing trend of using TCM in Western countries. Thus, it is necessary that clinicians be aware of these drug-CHMs interactions in order to effectively manage those patients who are using anticoagulant/antiplatelet drugs with the listed CHMs.
In this review, the majority of documented interactions were rated as “major or severe” in scale. However, the actual consequences of concurrent use might be changed depending on the used medications, dose levels, binomial source, plant parts, and/or route of administration of CHMs, in addition to the patients' characteristics (such as genetic differences and dietary habits), their use patterns, and combinations of single CHMs and/or Chinese Medicinal Prescriptions (based on TCM theory). As for the safety and effectiveness of using anticoagulants and antiplatelets, health care providers need to be more judicious in counseling their patients regarding the possibility of interactions between these drugs and herbal remedies or CHMs. If concurrent use is unavoidable, then health care providers should closely monitor patients for potential adverse events associated with these possible interactions (e.g., increased international normalized ratio, bleeding).
In fact, the interactions between herbal remedies or CHMs and drugs are often more complex due to the multiple ingredients found in all single entity CHMs [87], [94] and their binomial source, plant parts, dose, quality, preparation and/or route of administration [54]. Again, the likelihood of drug-CHM interactions may be different when using combinations of several single entity CHMs or those Chinese Medicinal Prescriptions. In particular, Chinese Medicinal Prescriptions (combined TCM medicines based on the theory of TCM) are sometimes the first line of therapy in Asian countries [136], [137]. For instance, Jia Wei Hiaxo Yao San is commonly used for anxiety, irritability, and depression, and is one of the most frequently used Chinese Medicinal Prescriptions in Taiwan. This well known ancient Chinese herbal formula is generally used for liver Qi stagnation but contains at least ten distinct entity of CHMs [137], including dong quai, ginger, and licorice, which are documented to have major interactions with anticoagulant or antiplatelet drugs [37]–[39]. Therefore, those patients using Jia Wei Hiaxo Yao San might encounter higher bleeding risks due to the occurrence of significant major interactions. In this case, health care professionals could more aggressively take appropriate actions to prevent them from causing serious life threatening adverse events in their patients if they were aware of such potential risks.
In this review, we also found that the major documented mechanisms of interactions between anticoagulant/antiplatelet drugs and CHMs were attributable to their pharmacodynamic interactions, especially due to additive anticoagulant or antiplatelet effects. Many of the commonly used CHMs such as clove, danshen, ginger, and licorice are documented to have intrinsic anticoagulant or antiplatelet properties [85], [138]. However, these blood-activating or stasis-resolving CHMs (e.g., danshen, and dong quai) are widely used in TCM for the treatment of cardiovascular or cerebrovascular diseases. Although some clinical studies have been conducted to investigate the effects of these CHMs on stroke or coronary diseases [139], [140], further large scale, rigorous clinical trials are needed to confirm the benefits and risks of using these CHMs. With limited robust evidence and clinical trials supporting the use of CHMs, it is crucial for physicians and pharmacy practitioners to educate their patients on why they need to disclose their concurrent use of conventional medications and CAM, including CHMs, especially for those patients who are taking anticoagulant or antiplatelet drugs.
Currently, there is no single comprehensive database to identify the facts of drug-CHM interactions, levels of evidence sufficiency, corresponding mechanism, and/or clinical significance. Instead, we utilized three commonly used natural products or drug interaction-related databases to identify and verify the information needed and retrieved this information regarding their mechanism and severity rating. The results of this review show that more than half of documented drug interactions retrieved from books, web sites, or primary literature could be identified in NMCD®, and less of them could be retrieved in MicroMedex® or Lexicomp®. This finding is consistent with the results of a previous study which was conducted in 2008 to evaluate the commonly used dietary supplement databases [141]. Of these databases, NMCD® had been recognized as the most appropriate database used to answer questions (including drug interactions) about dietary supplements [141]. In addition, we also found that only a few interactions could be identified in all of the tertiary literature (MicroMedex®, Drug interaction facts) and there existed variations in the severity rating of interactions [142]. This finding is also in agreement with Abarca's evaluation, which concluded that only a few major drug-drug interactions were listed in the four available interaction compendium and with very little agreement among them [142]. Thus, further rigorous qualitative studies that include opinions from a variety of experts on CHMs and drug interactions are needed to provide more consistent and robust information about the levels of clinical significance and mechanisms and combinations to be avoided to prevent drug-CHM interactions from occurring.
Nevertheless, our review has a number of limitations. First, there exists discordance in the nomenclature of Chinese medicine and herbs, as well as in both of the English and Chinese resources that need to be addressed. In particular, some evidence was retrieved from the English resources showing that an inconsistency of homonyms, synonyms, or translation may exist. Furthermore, only a small portion of the literature listed the common names of the natural products or herbal remedies, and the majority of them lacked any information on the scientific names and/or binomial source. Therefore, we need to be cautious when interpreting the findings on the natural products, herbs and CHMs per se. Second, the majority of literature reports did not specify which parts of natural products/herbs and CHMs (e.g., roots, leaves, seeds, or flowers) were used, on what doses, or which routes of administration were taken. In fact, the active ingredients and doses of CHMs may vary by situation and person based on TCM theory. Usually, the kinds and parts of CHMs and their preparation procedures are chosen based upon the stages of patients' disease status or condition. Lastly, only those interactions with evidence-based data retrieved from MicroMedex®, NMCD®, or Lexicomp®, were further reviewed for the corresponding mechanisms and rating of severity in this study. Thus, the interpretations might be subjective to information obtained from the retrieved database(s), and validated by the reviewers and members in the focus group. However, we have tried our best to screen and highlight the interactions using the best available evidence and consistent approach, although not all reports or studies were published or relevant documented interactions analyzed.
Conclusions
Conventional anticoagulants and antiplatelet drugs were documented to have harmful interactions with some commonly used single entity CHMs. Concurrent use of some herbal remedies may increase or reduce the pharmacologic effects of anticoagulant and antiplatelet drugs with moderate or severe consequences. For those patients who are taking conventional anti-clotting medications with CHMs for cardiovascular or cerebrovascular diseases, the potential risks of increased bleeding due to drug-CHM interactions should not be ignored. This review provides some guidance to health care professionals as to how to recognize the potential risks of interactions between anticoagulants/antiplatelets and single entity CHMs. Moreover, it is critical for all health care professionals to be able to initiate and effectively communicate and discuss the proper use of CHM-related products with conventional medications (i.e., anticoagulant or antiplatelet agents for cardiovascular or cerebrovascular diseases) because many patients may not voluntarily disclose their CHM-related use to their physicians.
Supporting Information
Summary of review articles to retrieve relevant information about interactions between anticoagulants/antiplatelet drugs and natural products/herbs (including CMHs).
(DOCX)
PRISMA checklist.
(DOCX)
Acknowledgments
The authors would like to express their gratitude to Chiu-Lin Tsai for his insights and comments initially on the identification of CHMs, Po-Ming Huang and Shan-Chieh Wu for their assistance with the review and manuscript, Jun-Fon Wang and Po-Ming Huang for their help on data management, the other reviewers for their insights of drug-CHM interaction information, as well as Dan Lu, Matthias C. Lu and Jenna Beall for their comments on the manuscript.
Funding Statement
This study was fully supported by the Committee on Chinese medicine and Pharmacy, Department of Health, Executive Yuan, Taiwan, R.O.C. (grant number: CCMP99-RD-016) and partially supported by National Science Council (grant number: NSC 99-2320-B-039 -031 -MY3). Those founders have no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (WHO) (2008). The 10 leading causes of death by broad income group. http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed May 25, 2012.
- 2. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, et al. (2011) Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 42: 227–276. [DOI] [PubMed] [Google Scholar]
- 3. Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, et al. (2005) Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 165: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 4. Dunn SP, Macaulay TE (2011) Drug-Drug Interactions Associated with Antiplatelet Therapy. Cardiovasc Hematol Agents Med Chem 9: 231. [DOI] [PubMed] [Google Scholar]
- 5. Ulbricht C, Chao W, Costa D, Rusie-Seamon E, Weissner W, et al. (2008) Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab 9: 1063–1120. [DOI] [PubMed] [Google Scholar]
- 6. Izzo AA, Di Carlo G, Borrelli F, Ernst E (2005) Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol 98: 1–14. [DOI] [PubMed] [Google Scholar]
- 7. Su D, Li L (2011) Trends in the use of complementary and alternative medicine in the United States: 2002–2007. J Health Care Poor Underserved 22: 296–310. [DOI] [PubMed] [Google Scholar]
- 8. Rossler W, Lauber C, Angst J, Haker H, Gamma A, et al. (2007) The use of complementary and alternative medicine in the general population: Results from a longitudinal community study. Psychol Med 37: 73–84. [DOI] [PubMed] [Google Scholar]
- 9. Ernst E (2000) Prevalence of use of complementary/alternative medicine: a systematic review. Bull World Health Organ 78: 252–257. [PMC free article] [PubMed] [Google Scholar]
- 10. Yeh GY, Davis RB, Phillips RS (2006) Use of complementary therapies in patients with cardiovascular disease. Am J Cardiol 98: 673–680. [DOI] [PubMed] [Google Scholar]
- 11. Lee GBW, Charn TC, Chew ZH, Ng TP (2004) Complementary and alternative medicine use in patients with chronic diseases in primary care is associated with perceived quality of care and cultural beliefs. Fam Pract 21: 654–660. [DOI] [PubMed] [Google Scholar]
- 12. Chang LC, Huang N, Chou YJ, Lee CH, Kao FY, et al. (2008) Utilization patterns of Chinese medicine and Western medicine under the National Health Insurance Program in Taiwan, a population-based study from 1997 to 2003. BMC Health Serv Res 8: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim MK, Sadarangani P, Chan HL, Heng JY (2005) Complementary and alternative medicine use in multiracial Singapore. Complement Ther Med 13: 16–24. [DOI] [PubMed] [Google Scholar]
- 14. Lai D, Chappell N (2007) Use of Traditional Chinese Medicine by older Chinese immigrants in Canada. Fam Pract 24: 56–64. [DOI] [PubMed] [Google Scholar]
- 15. Chen FN, Lee CPD, Hsieh CL, Lin CC, Chiang HM, et al. (2008) Survey on Adult Behavior and Medication of Chinese and Western Medicine-Based on the Outpatients of a Medical Center in Mid-Taiwan. J Int Chin West Med 10: 25–34. [Google Scholar]
- 16. Rochelle TL, Marks DF (2010) Medical pluralism of the Chinese in London: an exploratory study. Br J Health Psychol 15: 715–728. [DOI] [PubMed] [Google Scholar]
- 17.Tsai HH, Lin HW, Chien CR, Li TC (2012) Concurrent use of antiplatelets, anticoagulants, or digoxin with Chinese medications: a population-based cohort study. Eur J Clin Pharmacol DOI 10.1007/s00228-012-1359-6. [DOI] [PubMed]
- 18. Alherbish A, Charrois TL, Ackman ML, Tsuyuki RT, Ezekowitz JA (2011) The prevalence of natural health product use in patients with acute cardiovascular disease. PLoS One 6: e19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suchard JR, Suchard MA, Steinfeldt JL (2004) Physician knowledge of herbal toxicities and adverse herb-drug interactions. Eur J Emerg Med 11: 193–197. [DOI] [PubMed] [Google Scholar]
- 20. Saw JT, Bahari MB, Ang HH, Lim YH (2006) Potential drug-herb interaction with antiplatelet/anticoagulant drugs. Complement Ther Clin Pract 12: 236–241. [DOI] [PubMed] [Google Scholar]
- 21.Mahady GB (2001) Botanical dietary supplements: Quality, safety and efficacy. Lisse, The Netherlands: Swets & Zeitlinger Publishers.
- 22.Cassileth BC, Yeung KS (2003) Herb-drug interactions in oncology. Hamilton: London BC Decker Inc. [Google Scholar]
- 23.Ulbricht CE (2005) Natural standard herbs & supplement reference: Evidence-based clinical review. St. Louis, Mo.: Mosby/Elsevier. [Google Scholar]
- 24.Tatro DS (2011) Drug interaction facts. Saint Louis: Wolters Kluwer Health/Facts & Comparisons. [Google Scholar]
- 25.Stargrove MB, Treasure J, McKee DL (2008) Herb, nutrient, and drug interactions: Clinical implications and therapeutic strategies. St. Louis: Mosby Elsevier. [Google Scholar]
- 26.Philp RB (2004) Herbal-drug interactions and adverse effects: An evidence-based quick reference guide. USA: McGraw-Hill Professional. [Google Scholar]
- 27.Jennes F, Flaws B (2007) Herb toxicities & drug interactions: A formula approach. Boulder: Blue Poppy Press. [Google Scholar]
- 28.Williamson E, Driver S, Baxter K (2009) Stockley's herbal medicines interactions. London: Pharmaceutical Press. [Google Scholar]
- 29.Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan. Abstract of yearbook. http://www.ccmp.gov.tw/en/research/result.asp?relno=51&level=C. Accessed March 10, 2012.
- 30.Department of Health, Executive Yuan, R.O.C. (TAIWAN). Government research bulletin. http://grbsearch.stpi.narl.org.tw/GRB/. Accessed March 15, 2012.
- 31.Office of Dietary Supplements. Dietary Supplement Fact Sheets. http://ods.od.nih.gov/factsheets/list-all/. Accessed March 19, 2012.
- 32.National Center for Complementary and Alternative Medicine. Herbs at a Glance. http://nccam.nih.gov/health/herbsataglance.htm. Accessed March 20, 2012.
- 33.National Center for Complementary and Alternative Medicine (2010) Traditional Chinese Medicine: An Introduction. http://nccam.nih.gov/health/whatiscam/chinesemed.htm. Accessed March 1, 2012.
- 34.Committee on Chinese Medicine and Pharmacy (2004) Taiwan Herbal Pharmacopoeia. Taipei: Department of Health, Executive Yuan. [Google Scholar]
- 35.Chang HC, Tsai KU (2003) Chinese Medicinal Herbs Preparation. Taichung: China Medical University. [Google Scholar]
- 36.State Administration of Traditional Chinese Medicine (1999) Chinese Materia Medica. Shanghai: Shanghai scientific and Technical Publishers. [Google Scholar]
- 37.MICROMEDEX® 1.0 (Healthcare Series). Interaction Database. http://www.thomsonhc.com/hcs/librarian/ND_T/HCS/ND_PR/Main/CS/A304ED/DUPLICATIONSHIELDSYNC/95C0EB/ND_PG/PRIH/ND_B/HCS/ND_P/Main/PFActionId/hcs.Interactions.FindDrugInteractions. Accessed April 15, 2012.
- 38.Lexicomp. Lexi-Interact Online (2012). http://www.uptodate.com/crlsql/interact/frameset.jsp.Accessed April 18, 2012.
- 39.Natural Medicines Comprehensive Database. Natural Product/Drug Interaction Checker http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?s=ND&cs=&pt=7&rli=1&sh=. Accessed April 20, 2012.
- 40. Awang DV, Fugh-Berman A (2002) Herbal interactions with cardiovascular drugs. J Cardiovasc Nurs 16: 64–70. [DOI] [PubMed] [Google Scholar]
- 41. Bone KM (2008) Potential interaction of Ginkgo biloba leaf with antiplatelet or anticoagulant drugs: what is the evidence? Mol Nutr Food Res 52: 764–771. [DOI] [PubMed] [Google Scholar]
- 42. Borrelli F, Izzo AA (2009) Herb-drug interactions with St John's wort (Hypericum perforatum): an update on clinical observations. AAPS J 11: 710–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brazier NC, Levine MA (2003) Drug-herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am J Ther 10: 163–169. [DOI] [PubMed] [Google Scholar]
- 44. Bressler R (2005) Herb-drug interactions. Interactions between saw palmetto and prescription medications. Geriatrics 60: 32. [PubMed] [Google Scholar]
- 45. Bressler R (2005) Herb-drug interactions: interactions between ginseng and prescription medications. Geriatrics 60: 16–17. [PubMed] [Google Scholar]
- 46. Bressler R (2005) Herb-drug interactions. St. John's wort and prescription medications. Geriatrics 60: 21–23. [PubMed] [Google Scholar]
- 47. Bressler R (2005) Herb-drug interactions: interactions between Ginkgo biloba and prescription medications. Geriatrics 60: 30–33. [PubMed] [Google Scholar]
- 48. Butterweck V, Derendorf H (2008) Potential of pharmacokinetic profiling for detecting herbal interactions with drugs. Clin Pharmacokinet 47: 383–397. [DOI] [PubMed] [Google Scholar]
- 49. Chan E, Tan M, Xin J, Sudarsanam S, Johnson DE (2010) Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Devel 13: 50–65. [PubMed] [Google Scholar]
- 50. Chavez ML, Jordan MA, Chavez PI (2006) Evidence-based drug-herbal interactions. Life Sci 78: 2146–2157. [DOI] [PubMed] [Google Scholar]
- 51. Choi YH, Chin YW, Kim YG (2011) Herb-drug interactions: focus on metabolic enzymes and transporters. Arch Pharm Res 34: 1843–1863. [DOI] [PubMed] [Google Scholar]
- 52. Cohen PA, Ernst E (2010) Safety of herbal supplements: a guide for cardiologists. Cardiovasc Ther 28: 246–253. [DOI] [PubMed] [Google Scholar]
- 53. Colalto C (2010) Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol Res 62: 207–227. [DOI] [PubMed] [Google Scholar]
- 54. Coxeter PD, McLachlan AJ, Duke CC, Roufogalis BD (2004) Herb-drug interactions: an evidence based approach. Curr Med Chem 11: 1513–1525. [DOI] [PubMed] [Google Scholar]
- 55. De Smet PAGM, Floor-Schreudering A, Bouvy ML, Wensing M (2008) Clinical risk management of interactions between natural products and drugs. Curr Drug Metab 9: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 56. Di Carlo G, Borrelli F, Ernst E, Izzo AA (2001) St John's wort: Prozac from the plant kingdom. Trends Pharmacol Sci 22: 292–297. [DOI] [PubMed] [Google Scholar]
- 57. Di YM, Li CG, Xue CC, Zhou S-F (2008) Clinical drugs that interact with St. John's wort and implication in drug development. Curr Pharm Des 14: 1723–1742. [DOI] [PubMed] [Google Scholar]
- 58. Ernst E (2000) Herb-drug interactions: potentially important but woefully under-researched. Eur J Clin Pharmacol 56: 523–524. [DOI] [PubMed] [Google Scholar]
- 59. Ernst E (2004) Risks of herbal medicinal products. Pharmacoepidemiol Drug Saf 13: 767–771. [DOI] [PubMed] [Google Scholar]
- 60. Fugh-Berman A (2000) Herb-drug interactions. Lancet 355: 134–138. [DOI] [PubMed] [Google Scholar]
- 61. Fugh-Berman A, Ernst E (2001) Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol 52: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gardiner P, Phillips R, Shaughnessy AF (2008) Herbal and dietary supplement – drug interactions in patients with chronic illnesses. Am Fam Physician 77: 73–78. [PubMed] [Google Scholar]
- 63. Greenblatt DJ, von Moltke LL (2005) Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol 45: 127–132. [DOI] [PubMed] [Google Scholar]
- 64. Greeson JM, Sanford B, Monti DA (2001) St. John's wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology (Berl) 153: 402–414. [DOI] [PubMed] [Google Scholar]
- 65. Haller CA (2006) Clinical approach to adverse events and interactions related to herbal and dietary supplements. Clin Toxicol (Phila) 44: 605–610. [DOI] [PubMed] [Google Scholar]
- 66. Holcomb SS (2009) Common herb-drug interactions: what you should know. Nurse Pract 34: 21–29. [DOI] [PubMed] [Google Scholar]
- 67. Hu Z, Yang X, Ho PCL, Chan SY, Heng PWS, et al. (2005) Herb-drug interactions: a literature review. Drugs 65: 1239–1282. [DOI] [PubMed] [Google Scholar]
- 68. Huang S-M, Lesko LJ (2004) Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J Clin Pharmacol 44: 559–569. [DOI] [PubMed] [Google Scholar]
- 69. Izzo AA (2005) Herb-drug interactions: an overview of the clinical evidence. Fundam Clin Pharmacol 19: 1–16. [DOI] [PubMed] [Google Scholar]
- 70. Izzo AA, Ernst E (2001) Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 61: 2163–2175. [DOI] [PubMed] [Google Scholar]
- 71. Izzo AA, Ernst E (2009) Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 69: 1777–1798. [DOI] [PubMed] [Google Scholar]
- 72. Javed F, Golagani A, Sharp H (2008) Potential effects of herbal medicines and nutritional supplements on coagulation in ENT practice. J Laryngol Otol 122: 116–119. [DOI] [PubMed] [Google Scholar]
- 73. Ko RJ (2004) A U.S. perspective on the adverse reactions from traditional Chinese medicines. J Chin Med Assoc 67: 109–116. [PubMed] [Google Scholar]
- 74.Kuhn MA (2002) Herbal remedies: drug-herb interactions. Crit Care Nurse 22: 22–28, 30, 32. [PubMed] [Google Scholar]
- 75. Madabushi R, Frank B, Drewelow B, Derendorf H, Butterweck V (2006) Hyperforin in St. John's wort drug interactions. Eur J Clin Pharmacol 62: 225–233. [DOI] [PubMed] [Google Scholar]
- 76. Markowitz JS, DeVane CL (2001) The emerging recognition of herb-drug interactions with a focus on St. John's wort (Hypericum perforatum). Psychopharmacol Bull 35: 53–64. [PubMed] [Google Scholar]
- 77. McFadden R, Peterson N (2011) Interactions between drugs and four common medicinal herbs. Nurs Stand 25: 65–68. [DOI] [PubMed] [Google Scholar]
- 78. Mills E, Wu P, Johnston BC, Gallicano K, Clarke M, et al. (2005) Natural health product-drug interactions: a systematic review of clinical trials. Ther Drug Monit 27: 549–557. [DOI] [PubMed] [Google Scholar]
- 79. Norred CL, Brinker F (2001) Potential coagulation effects of preoperative complementary and alternative medicines. Altern Ther Health Med 7: 58–67. [PubMed] [Google Scholar]
- 80. Nutescu E, Chuatrisorn I, Hellenbart E (2011) Drug and dietary interactions of warfarin and novel oral anticoagulants: An update. J Thromb Thrombolysis 31: 326–343. [DOI] [PubMed] [Google Scholar]
- 81. Nutescu EA, Shapiro NL, Ibrahim S, West P (2006) Warfarin and its interactions with foods, herbs and other dietary supplements. Expert Opin Drug Saf 5: 433–451. [DOI] [PubMed] [Google Scholar]
- 82. Ohnishi N, Yokoyama T (2004) Interactions between medicines and functional foods or dietary supplements. Keio J Med 53: 137–150. [DOI] [PubMed] [Google Scholar]
- 83. Pal D, Mitra AK (2006) MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci 78: 2131–2145. [DOI] [PubMed] [Google Scholar]
- 84. Poppenga RH (2002) Herbal medicine: potential for intoxication and interactions with conventional drugs. Clin Tech Small Anim Pract 17: 6–18. [DOI] [PubMed] [Google Scholar]
- 85. Samuels N (2005) Herbal remedies and anticoagulant therapy. Thromb Haemost 93: 3–7. [DOI] [PubMed] [Google Scholar]
- 86. Singh YN (2005) Potential for interaction of kava and St. John's wort with drugs. J Ethnopharmacol 100: 108–113. [DOI] [PubMed] [Google Scholar]
- 87. Skalli S, Zaid A, Soulaymani R (2007) Drug interactions with herbal medicines. Ther Drug Monit 29: 679–686. [DOI] [PubMed] [Google Scholar]
- 88. Tachjian A, Maria V, Jahangir A (2010) Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol 55: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tirona RG, Bailey DG (2006) Herbal product-drug interactions mediated by induction. Br J Clin Pharmacol 61: 677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tomlinson B, Hu M, Lee VWY (2008) In vivo assessment of herb-drug interactions: possible utility of a pharmacogenetic approach? Mol Nutr Food Res 52: 799–809. [DOI] [PubMed] [Google Scholar]
- 91. Ueng YF, Chen RM (2004) The role of cytochrome P450 in herb-drug interactions. Curr Pharmacogenomics Person Med 2: 209–218. [Google Scholar]
- 92. Venkataramanan R, Komoroski B, Strom S (2006) In vitro and in vivo assessment of herb drug interactions. Life Sci 78: 2105–2115. [DOI] [PubMed] [Google Scholar]
- 93. Whitten DL, Myers SP, Hawrelak JA, Wohlmuth H (2006) The effect of St John's wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol 62: 512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Williamson EM (2003) Drug interactions between herbal and prescription medicines. Drug Saf 26: 1075–1092. [DOI] [PubMed] [Google Scholar]
- 95. Williamson EM (2005) Interactions between herbal and conventional medicines. Expert Opin Drug Saf 4: 355–378. [DOI] [PubMed] [Google Scholar]
- 96. Wittkowsky AK (2008) Dietary supplements, herbs and oral anticoagulants: the nature of the evidence. J Thromb Thrombolysis 25: 72–77. [DOI] [PubMed] [Google Scholar]
- 97. Woodward KN (2005) The potential impact of the use of homeopathic and herbal remedies on monitoring the safety of prescription products. Hum Exp Toxicol 24: 219–233. [DOI] [PubMed] [Google Scholar]
- 98. Yang XX, Hu ZP, Duan W, Zhu YZ, Zhou SF (2006) Drug-herb interactions: Eliminating toxicity with hard drug design. Curr Pharm Des 12: 4649–4664. [DOI] [PubMed] [Google Scholar]
- 99. Zhou L, Zuo Z, Chow MS (2005) Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45: 1345–1359. [DOI] [PubMed] [Google Scholar]
- 100. Zhou S, Chan E, Pan S-Q, Huang M, Lee EJD (2004) Pharmacokinetic interactions of drugs with St John's wort. J Psychopharmacol 18: 262–276. [DOI] [PubMed] [Google Scholar]
- 101. Zhou S, Gao Y, Jiang W, Huang M, Xu A, et al. (2003) Interactions of herbs with cytochrome P450. Drug Metab Rev 35: 35–98. [DOI] [PubMed] [Google Scholar]
- 102. Zhou S-F, Zhou Z-W, Li C-G, Chen X, Yu X, et al. (2007) Identification of drugs that interact with herbs in drug development. Drug Discov Today 12: 664–673. [DOI] [PubMed] [Google Scholar]
- 103. Mao XQ, Xie HT, Zhou HH (2007) MDR-and CYP3A4-mediated drug-herbal Interactions. Chinese Journal of Clinical Pharmacology and Therapeutics 12: 728–734. [Google Scholar]
- 104. Wang TY (2011) Outcomes Influenced by Drug Interactions. The Journal of Long Term Care 15: 24–32. [Google Scholar]
- 105. Wang YY (2003) Interactions between warfarin and other anticoagulant agents or herbal medicine. The Journal of Taiwan Pharmacy 19: 5–10. [Google Scholar]
- 106. Li JY, Shih HJ, Tsai ML (2008) Interactions between warfarin and chinese herbal medicine. The Journal of Taiwan Pharmacy 24: 135–142. [Google Scholar]
- 107. Lin JJ (2003) Interactions between Herbal and Conventional Therapies: Focus on Ginseng and Ginkgo Biloba. Show Chwan Medical Journal 4: 1–4. [Google Scholar]
- 108. Chen FP, Zhong MX, Huang XZ (2011) The whole aspects of interaction between traditional Chinese medicine and western medicine. Family Medicine & Primary Medical Care 26: 168–176. [Google Scholar]
- 109. Chen JF, Hu YJ, Miao CY (2007) Advancement of pharmacokinetic studies on Chinese herbal medicine-synthetic drug interactions. Clin J Clin Pharmacol Ther 12: 1348–1353. [Google Scholar]
- 110.Peng B, Li JR, He R (2010) The research status of herb-drug interactions. World Science and Technology-Modernization of Traditional Chinese Medicine. doi: 10.3969/j.issn.1674–3849.2010.02.024.
- 111. Tsai CH, Lu CY (2009) Analysis on the interaction of warfarinand ginseng. Taipei Journal of Chinese Medicine 15: 98–109. [Google Scholar]
- 112. Lai YC, Chen YL, Lai JS (2007) Toxicity of Chinese Herbal Medicine and drug-herb Interactions. The Chung Shan Medical Journal 18: 343–357. [Google Scholar]
- 113. Kuo YH, Lin YL, Don MJ, Chen RM, Ueng YF (2006) Induction of cytochrome P450-dependent monooxygenase by extracts of the medicinal herb Salvia miltiorrhiza. J Pharm Pharmacol 58: 521–527. [DOI] [PubMed] [Google Scholar]
- 114. Li Y, Wang N (2010) Antithrombotic effects of Danggui, Honghua and potential drug interaction with clopidogrel. J Ethnopharmacol 128: 623–628. [DOI] [PubMed] [Google Scholar]
- 115. Makino T, Wakushima H, Okamoto T, Okukubo Y, Deguchi Y, et al. (2002) Pharmacokinetic interactions between warfarin and kangen-karyu, a Chinese traditional herbal medicine, and their synergistic action. J Ethnopharmacol 82: 35–40. [DOI] [PubMed] [Google Scholar]
- 116. Wu WWP, Yeung JHK (2010) Inhibition of warfarin hydroxylation by major tanshinones of Danshen (Salvia miltiorrhiza) in the rat in vitro and in vivo. Phytomedicine 17: 219–226. [DOI] [PubMed] [Google Scholar]
- 117. Abdul MI, Jiang X, Williams KM, Day RO, Roufogalis BD, et al. (2010) Pharmacokinetic and pharmacodynamic interactions of echinacea and policosanol with warfarin in healthy subjects. Br J Clin Pharmacol 69: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jiang X, Blair EYL, McLachlan AJ (2006) Investigation of the effects of herbal medicines on warfarin response in healthy subjects: a population pharmacokinetic-pharmacodynamic modeling approach. J Clin Pharmacol 46: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 119. Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, et al. (2004) Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol 57: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yuan CS, Wei G, Dey L, Karrison T, Nahlik L, et al. (2004) Brief communication: American ginseng reduces warfarin's effect in healthy patients: a randomized, controlled Trial. Ann Intern Med 141: 23–27. [DOI] [PubMed] [Google Scholar]
- 121. Blalock SJ, Gregory PJ, Patel RA, Norton LL, Callahan LF, et al. (2009) Factors associated with potential medication-herb/natural product interactions in a rural community. Altern Ther Health Med 15: 26–34. [PMC free article] [PubMed] [Google Scholar]
- 122. Bush TM, Rayburn KS, Holloway SW, Sanchez-Yamamoto DS, Allen BL, et al. (2007) Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med 13: 30–35. [PubMed] [Google Scholar]
- 123. Canter PH, Ernst E (2004) Herbal supplement use by persons aged over 50 years in Britain: frequently used herbs, concomitant use of herbs, nutritional supplements and prescription drugs, rate of informing doctors and potential for negative interactions. Drugs Aging 21: 597–605. [DOI] [PubMed] [Google Scholar]
- 124. Chan ALF, Leung HWC, Wu J-W, Chien T-W (2011) Risk of hemorrhage associated with co-prescriptions for Ginkgo biloba and antiplatelet or anticoagulant drugs. J Altern Complement Med 17: 513–517. [DOI] [PubMed] [Google Scholar]
- 125. Chan H-T, So L-T, Li S-W, Siu C-W, Lau C-P, et al. (2011) Effect of herbal consumption on time in therapeutic range of warfarin therapy in patients with atrial fibrillation. J Cardiovasc Pharmacol 58: 87–90. [DOI] [PubMed] [Google Scholar]
- 126. Charrois TL, Hill RL, Vu D, Foster BC, Boon HS, et al. (2007) Community identification of natural health product-drug interactions. Ann Pharmacother 41: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 127. Clauson KA, Santamarina ML, Rutledge JC (2008) Clinically relevant safety issues associated with St. John's wort product labels. BMC Complement Altern Med 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dergal JM, Gold JL, Laxer DA, Lee MSW, Binns MA, et al. (2002) Potential interactions between herbal medicines and conventional drug therapies used by older adults attending a memory clinic. Drugs Aging 19: 879–886. [DOI] [PubMed] [Google Scholar]
- 129. Goldman RD, Rogovik AL, Lai D, Vohra S (2008) Potential interactions of drug-natural health products and natural health products-natural health products among children. J Pediatr 152: 521–526. [DOI] [PubMed] [Google Scholar]
- 130. Rogers EA, Gough JE, Brewer KL (2001) Are emergency department patients at risk for herb-drug interactions? Acad Emerg Med 8: 932–934. [DOI] [PubMed] [Google Scholar]
- 131. Segal R, Pilote L (2006) Warfarin interaction with Matricaria chamomilla. CMAJ 174: 1281–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shalansky S, Lynd L, Richardson K, Ingaszewski A, Kerr C (2007) Risk of warfarin-related bleeding events and supratherapeutic international normalized ratios associated with complementary and alternative medicine: a longitudinal analysis. Pharmacotherapy 27: 1237–1247. [DOI] [PubMed] [Google Scholar]
- 133. Su Q, Li Y (2010) Interaction between warfarin and the herbal product shengmai-yin: A case report of intracerebral hematoma. Yonsei Med J 51: 793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lai JN (2010) Causal inference of bleeding complications in patients with warfarin treatment in Taiwan – a eleven year follow up study. Yearbook of Chinese Medicine and Pharmacy 28: 291–388. [Google Scholar]
- 135. Al Faraj S (2005) Antagonism of the anticoagulant effect of warfarin caused by the use of Commiphora molmol as a herbal medication: a case report. Ann Trop Med Parasitol 99: 219–220. [DOI] [PubMed] [Google Scholar]
- 136. Yamashita H, Tsukayama H, Sugishita C (2002) Popularity of complementary and alternative medicine in Japan: a telephone survey. Complement Ther Med 10: 84–93. [DOI] [PubMed] [Google Scholar]
- 137. Hsieh SC, Lai JN, Lee CF, Hu FC, Tseng WL, et al. (2008) The prescribing of Chinese herbal products in Taiwan: a cross-sectional analysis of the national health insurance reimbursement database. Pharmacoepidemiol Drug Saf 17: 609–619. [DOI] [PubMed] [Google Scholar]
- 138. Mousa SA (2010) Antithrombotic effects of naturally derived products on coagulation and platelet function. Methods Mol Biol 663: 229–240. [DOI] [PubMed] [Google Scholar]
- 139. Xu G, Zhao W, Zhou Z, Zhang R, Zhu W, et al. (2009) Danshen extracts decrease blood C reactive protein and prevent ischemic stroke recurrence: A controlled pilot study. Phytother Res 23: 1721–1725. [DOI] [PubMed] [Google Scholar]
- 140. Zhu YF, Luo HM, Deng ZL, Fu DY, Yao W, et al. (2012) Effects of the Chinese patent medicine, Honghua injection, on platelet glycoprotein Tib/Ufa receptors in patients with acute coronary syndrome: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 10: 318–323. [DOI] [PubMed] [Google Scholar]
- 141. Clauson KA, Peak AS, Marsh WA, DiScala S, Bellinger RR (2008) Clinical decision support tools: focus on dietary supplement databases. Altern Ther Health Med 14: 36–40. [PubMed] [Google Scholar]
- 142. Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, et al. (2004) Concordance of severity ratings provided in four drug interaction compendia. J Am Pharm Assoc 44: 136–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of review articles to retrieve relevant information about interactions between anticoagulants/antiplatelet drugs and natural products/herbs (including CMHs).
(DOCX)
PRISMA checklist.
(DOCX)