Abstract
Adiponectin has well-established insulin-sensitizing effects in non-pregnant individuals. Pregnant women who are obese or have gestational diabetes typically have low circulating levels of adiponectin, which is associated with increased fetal growth. Lean women, on the other hand, have high circulating levels of adiponectin. As a result, maternal serum adiponectin is inversely correlated to fetal growth across the full range of birth weights, suggesting that maternal adiponectin may limit fetal growth. In the mother, adiponectin is predicted to promote insulin sensitivity and stimulate glucose uptake in maternal skeletal muscle thereby reducing nutrient availability for placental transfer. Adiponectin prevents insulin-stimulated amino acid uptake in cultured primary human trophoblast cells by modulating insulin receptor substrate phosphorylation. Furthermore, chronic administration of adiponectin to pregnant mice inhibits placental insulin and mammalian target of rapamycin complex 1 (mTORC1) signaling, down-regulates the activity and expression of key placental nutrient transporters and decreases fetal growth. Preliminary findings indicate that adiponectin binds to the adiponectin receptor-2 on the trophoblast cell and activates p38 MAPK and PPAR-α, which inhibits the insulin/IGF-1 signaling pathway. In contrast to maternal adiponectin, recent reports suggest that fetal adiponectin may promote expansion of adipose tissue and stimulate fetal growth. Regulation of placental function by adiponectin constitutes a novel physiological mechanism by which the endocrine functions of maternal adipose tissue influence fetal growth. These findings may help us better understand the factors determining birth weight in normal pregnancies and in pregnancy complications associated with altered maternal adiponectin levels such as obesity and gestational diabetes.
1. Introduction
It is well established that birth weight is positively correlated with maternal pre-pregnancy body mass index (BMI) [1]. Moreover, both small and large size at birth is associated with obesity later in life, indicating a generational transmission of risk for metabolic diseases [2]. Fetal growth is largely dependent on the delivery of oxygen and nutrients across the placenta. The ability of the placenta to supply nutrients to the fetus is determined by a multitude of factors, including the nutritional state of the mother, utero-placental blood flow, and the expression and function of trophoblast nutrient transporters [3]. However, the placenta is not merely a passive conduit for nutrients; growing evidence indicates that the placenta acts as a nutrient sensor, integrating signals from mother and fetus to balance fetal demand with maternal substrate supply by modulating placental growth and function [4–6]. To better understand the mechanisms governing maternal-fetal resource allocation it is important to identify the maternal and fetal factors that regulate placental function.
In recent years, adipocyte-derived signaling molecules referred to as ‘adipokines’, including leptin, resistin, adiponectin and pro-inflammatory cytokines, have been implicated in various pregnancy disorders [7]. Of these, only adiponectin is produced exclusively in adipose tissue in non-pregnant subjects and may therefore be considered a ‘true’ adipokine [8, 9]. In pregnancy, the placenta secretes an array of adipokines. Although some investigators have reported that adiponectin is produced by the placenta [10, 11], other studies have not confirmed these findings [12–14]. It is therefore likely that the adiponectin influencing placental function predominantly originates from maternal adipose tissue.
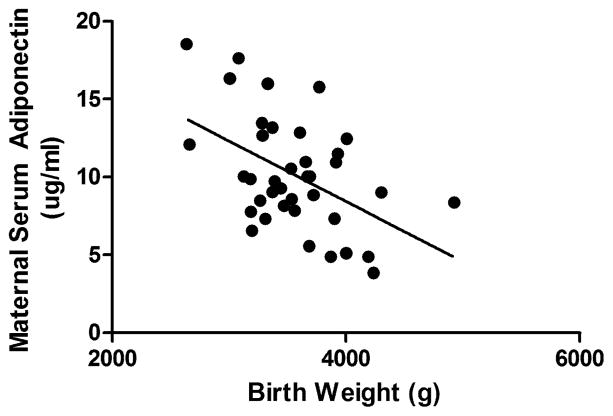
Several studies, including data from our laboratory (Figure 1), have demonstrated that serum levels of maternal adiponectin are negatively correlated with birth weight in healthy pregnant women with varying early pregnancy BMI [15, 16], as well as in women with gestational diabetes mellitus (GDM) [17]. Based on these clinical observations, we hypothesized that maternal adiponectin plays a causative role in regulating fetal growth by modulating placental nutrient transport. Here, we review the latest information on adiponectin receptors and signaling, summarize what is known with respect to maternal adiponectin in pregnancy, discuss recent findings demonstrating that adiponectin regulates placental function and briefly review emerging data implicating a role of fetal adiponectin in fetal adiposity and growth.
Figure 1. Relationship between maternal serum adiponectin and birth weight.
Maternal serum adiponectin was determined at 36 weeks of gestation in women with normal term pregnancies and varying early pregnancy BMI (16.9 – 44.44; Mean = 25.42). r = −0.4879, p = 0.0019, n = 39. Data from Jansson et al. 2008 [23].
2. Adiponectin and adiponectin signaling
Adiponectin was discovered by four independent groups using different approaches [8, 9, 18, 19]. Three of these labs identified the gene encoding adiponectin (known as AdipoQ) exclusively in adipose tissue [8, 9, 18], while another group reported circulating adiponectin [19]. Adiponectin was also reported to be the most abundantly expressed transcript in adipose tissue [18]. The AdipoQ gene encodes a protein (full-length adiponectin, fADN) with 248 amino acids and four domains based on the primary amino acid sequence [20]: an N-terminal signal peptide, a variable region, a collagenous domain and a globular domain at the C-terminal end [18]. The shorter globular adiponectin (gADN) possesses potent biological activities, which in many tissues display similar properties to fADN [21, 22]. In contrast, gADN and fADN have distinct, and sometimes opposing, biological effects in other tissues including the placenta [23, 24]. The physiological significance of gADN is currently unclear given that almost all circulating adiponectin exists as fADN [25], whereas gADN is only present in very low concentrations in human plasma [25, 26]. However, it has been proposed that gADN may be released by locally active proteinases and exert local paracrine effects [27].
Adiponectin exerts a multitude of tissue-specific effects, in part depending on its unique, tightly regulated multimerization behavior. fADN assembles into three oligomeric isoforms: low molecular weight (LMW) trimers, medium molecular weight (MMW) hexamers and high molecular weight (HMW) oligomers [28]. Low serum levels of HMW adiponectin, rather than the total or other oligomeric forms, are associated with several metabolic disorders including type 2 diabetes mellitus [29], childhood obesity [30] and the metabolic syndrome across different populations [31].
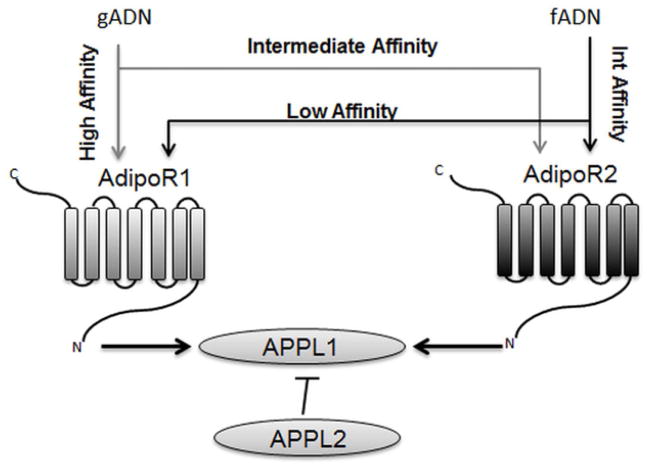
The biological properties of adiponectin are mediated via two receptors: AdipoR1 and AdipoR2. While adiponectin is secreted exclusively by the adipose tissue, adiponectin receptors display widespread tissue expression. Both AdipoRs are predicted to contain 7 helices, resembling an inverse transmembrane architecture of G-protein coupled receptors [32]. Although the amino acid sequence of AdipoR1 and AdipoR2 show extensive homology, the two receptors exhibit diverse tissue expression patterns and activity. AdipoR1 is ubiquitously expressed with high levels in the skeletal muscle, whereas AdipoR2 expression is more restricted, with high expression in the liver [33]. AdipoR1 binds gADN with high affinity and fADN with low affinity, whereas AdipoR2 binds both gADN and fADN with intermediate affinity (Figure 2).
Figure 2. Adiponectin receptors and adaptor proteins.
Adiponectin-signaling is mediated via AdipoR1 and AdipoR2. AdipoR1 binds gADN with high affinity and fADN with low affinity, whereas AdipoR2 binds both gADN and fADN with intermediate affinity. Adaptor protein APPL1 interacts with adiponectin receptors and mediates the activation of downstream targets, while APPL2 is bound to APPL1 in the receptor inactive state and inhibits signaling activity. AdipoR1/2, adiponectin receptor 1/2; APPL1/2, adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1/2; fADN, full-length adiponectin; gADN, globular adiponectin; Int, intermediate.
AdipoR signaling can be further modulated by the interaction with two adaptor proteins: Adaptor Protein Containing Pleckstrin Homology Domain, Phosphotyrosine Binding Domain and Leucine Zipper Motif 1 (APPL1) and APPL2 (Figure 2). Following adiponectin–AdipoR1 binding, APPL1 mediates a number of downstream signaling events associated with adiponectin function [34]. When the receptor is inactive APPL2 binds and inhibits APPL1 function, but APPL2 binding is displaced upon activation of AdipoR1 [35].
Adiponectin was also shown to bind to T-cadherin in myoblasts [36], implicating this protein as a novel adiponectin receptor. However, T-cadherin is absent in liver (a major site of adiponectin action) and because it lacks an intracellular domain it is not believed to mediate signal transduction. Hence T-cadherin may act as an adiponectin antagonist by competing for adiponectin binding.
Adiponectin activates three key signaling pathways in muscle and liver: AMP-activated protein kinase (AMPK), p38 mitogen-activated protein kinase (p38 MAPK) and peroxisome proliferator-activated receptor α (PPARα). Activation of these pathways results in fatty acid oxidation and glucose uptake in skeletal muscle, and inhibition of gluconeogenesis in liver [37]. These effects are believed to mediate the insulin-sensitizing actions of adiponectin. In skeletal muscle both gADN and fADN can induce the cellular energy sensor AMPK resulting in increased glucose utilization [37] and import of free fatty acids into mitochondria for β-oxidation [38]. The promoter activity of key genes regulating fatty acid transport and oxidation is controlled by the transcription factor PPARα [33]. p38 MAPK, which responds to cytokines and metabolic stress, is also activated by adiponectin. Recently, Yoon and coworkers demonstrated that adiponectin sequentially activates AMPK/p38 MAPK and PPARα in skeletal muscle [39]. In myotubes, inhibition of AMPK or p38 MAPK prevented PPARα activation by adiponectin [39].
In the liver fADN, but not gADN, increased AMPK phosphorylation [37]. Furthermore, disruption of adiponectin signaling using selective AdipoR knockout mice has implicated AdipoR2 in modulating PPARα activity in the liver, while AMPK was without effect [40]. Hence PPARα activity in the liver is likely to be dependent on adiponectin-mediated p38 MAPK signaling whereas in the muscle AMPK plays a dominant role.
3. Adiponectin in the pregnant mother
Accumulating evidence suggests that adiponectin plays disparate roles in maternal, placental and fetal physiology. This may be due to differential expression of AdipoRs and APPLs in the three compartments. Although mechanistic studies are largely lacking, clinical observations suggest that adiponectin plays a physiological role in the pregnant mother. In humans, early pregnancy is associated with metabolic changes resulting in the accumulation of fat. This is followed by the development of an insulin-resistant state to support increased hepatic gluconeogenesis and reduced glucose uptake in maternal skeletal muscle and adipose tissue, and increased lipolysis in adipose tissue, thereby making glucose and lipids available to the fetus. Interestingly, changes in serum adiponectin track changes in maternal insulin sensitivity. Compared to the pre-gravid state, serum adiponectin is increased in early gestation [41]. Subsequently, adiponectin levels in serum and adipose tissue decline over the second half of gestation [42]. These observations are consistent with the possibility that high levels of adiponectin in early pregnancy enhance maternal accretion of nutrients, whereas declining adiponectin levels later in gestation promotes allocation of nutrients to the fetus.
In line with a role in regulating insulin sensitivity in the mother, lower adiponectin levels were reported in mothers with gestational diabetes mellitus (GDM) [17], a condition characterized by insulin-resistance and glucose intolerance. Furthermore low adiponectin levels in these women are typically associated with increased risk of delivering large for gestational age or macrosomic infants [17]. In otherwise healthy pregnant women, maternal serum adiponectin was also inversely correlated with birth weight [16].
4. Adiponectin signaling in the placenta: effects on placental function and fetal growth
Unlike skeletal muscle and liver, adiponectin signaling in the placenta is relatively unknown. Although adiponectin has been reported to be expressed in the placenta [10, 11] a number of studies have not been able to confirm these findings [12–14]. The human placenta was also proposed to secrete adiponectin in abundance [10, 11]. However, recent reports using more sensitive techniques suggest that it is unlikely that adiponectin is expressed and produced in the placenta, and indicate that contamination by adiponectin from the maternal circulation or fetal bovine serum used in cell cultures may explain previous positive findings [14, 43].
AdipoR1 and AdipoR2 are expressed in placental trophoblasts at the mRNA level [10], but only AdipoR2 protein is reported in human [44] and mouse trophoblast plasma membranes [45]. In agreement with the differential expression of AdipoRs in human placenta, gADN and fADN often display distinct biological effects in primary human trophoblasts (PHTs) (Table 1 and [24]). However, almost all studies to date addressing the role of adiponectin in placental function have utilized gADN. McDonald and Wolfe demonstrated that gADN attenuates mRNA expression and/or production of placental lactogen, chorion gonadotropin and progesterone in trophoblast cells [14]. Adiponectin has been reported to promote syncytialization in BeWo cells and in PHTs isolated from early first trimester placentas [46], but inhibit syncytialization in PHT isolated later in gestation [14, 46]. Furthermore, fADN was shown to inhibit cell proliferation in placental cell lines [47]. These observations may provide an additional mechanism by which adiponectin limits fetal growth. Our laboratory recently explored the distinct effects of gADN and fADN in cultured PHTs from term placentas [24]. Treatment with gADN increased secretion of IL-6 and TNF-α from PHTs, while fADN increased TNF-α but decreased IL-6 production. The effects of gADN on cytokine secretion were corroborated by findings of others demonstrating a pro-inflammatory action of gADN in PHTs [48].
Table 1. Effects of globular and full-length adiponectin in primary human trophoblasts.
The table summarizes findings in Jones et al. [33].
| Biological Effect | Globular Adiponectin | Full-Length Adiponectin |
|---|---|---|
| Cell Signaling | ||
| Insulin-stimulated IRS-1 phosphorylation (Tyr612) | ? | ↓ |
| Insulin-stimulated Akt phosphorylation (Thr308 and Ser473) | ↔ | ↓ |
| AMPKα phosphorylation (Thr172) | ↑ | ↔ |
| PPARα phosphorylation (Ser21) | ? | ↑ |
| p38 MAPK phosphorylation (Thr180/Tyr182) | ? | ↑* |
| Inflammation | ||
| IL-6 secretion | ↑ | ↓ |
| TNF-α secretion | ↑ | ↑ |
| Nutrient Transport | ||
| Basal System A activity | ↑ | ↔ |
| Insulin-stimulated System A activity | ↔ | ↓ |
| Basal System L activity | ↔ | ↔ |
| Basal SNAT2 protein expression | ↑ | ↔ |
| Insulin stimulated SNAT2 protein expression | ↔ | ↓ |
AMPK, AMP-activated protein kinase; fADN, full-length adiponectin; gADN, globular adiponectin; IL-6, interleukin-6; IRS-1, insulin-receptor substrate-1; p38 MAPK, p38 mitogen-activated protein kinase; PPARα, peroxisome proliferator-activated receptor alpha; Thr, Threonine; TNF-α, tumor necrosis factor alpha; Tyr, tyrosine; Ser, serine; ? unknown; * unpublished observation; ↔ no change; ↑ increases; ↓ decreases.
Whereas the role of adiponectin in modulating insulin responsiveness in skeletal muscle and liver is well established, the factors regulating insulin sensitivity in the placenta are largely unknown. Recent findings indicate unique interactions between adiponectin and placental insulin signaling. In the absence of physiological concentrations of insulin, gADN stimulated trophoblast System A mediated amino acid transport, whereas fADN was without effect [24].
However, fADN prevented insulin stimulated System A activity, indicating a role for fADN in placental insulin-resistance. Moreover, fADN attenuated insulin-dependent activation of insulin receptor substrate-1 (IRS-1), as well as preventing the downstream activation of protein kinase B/Akt. In contrast, gADN did not modulate insulin signaling in PHTs. Full length adiponectin did not affect AMPK phosphorylation but increased phosphorylation of PPARα in PHTs. Collectively, these data suggest that the effects of fADN in trophoblasts are mediated by AdipoR2 signaling [33]. This conclusion is also supported by preliminary findings demonstrating that incubation of PHTs in fADN leads to p38 MAPK phosphorylation (unpublished observations).
In agreement with the findings in cultured PHTs, chronic infusion of fADN in pregnant mice decreased placental amino acid transport resulting in a 19% reduction in fetal weight [45]. Insulin signaling, as evidenced by IRS-1 and Akt phosphorylation, was also attenuated in the placentas of dams chronically infused with fADN. The mechanisms underlying the effect of fADN on insulin-mediated amino acid transport may involve mTORC1 signaling. Functional readouts of mTORC1 activity, including phosphorylation of ribosomal protein S6K1, ribosomal protein S6 and eukaryotic translation initiating factor 4E-binding protein 1, were attenuated in the placentas of fADN-infused dams. Because mTORC1 is a downstream target of insulin signaling and constitutes a positive regulator of System A transporter activity in the placenta [49] mTORC1 signaling may provide a link between fADN signaling and nutrient transport. Similar to the findings in PHTs, maternal infusion of fADN in mice led to increased PPARα phosphorylation but not to activation of AMPK. Collectively, the data obtained in mice in vivo support the findings in cultured PHTs.
PPARα regulates the transcription of genes involved in fatty acid oxidation and thus adiponectin provides beneficial effects in the liver and skeletal muscle partly through reduction in triglyceride levels [33]. However, several lines of evidence indicate that PPARα also influences sphingolipid metabolism. For example, rats treated with PPARα agonists show increased expression of enzymes associated with ceramide biosynthesis including sphingosine palmitoyl transferase in the liver [50] and sphingomyelinase in the heart [51], concomitant with elevated ceramide concentrations in these tissues. Therefore, we hypothesize that fADN-mediated activation of PPARα in the placenta leads to elevation in ceramide levels, which impairs insulin-signaling and down-regulates amino acid transport. In support of this hypothesis, PHTs treated with ceramide display decreased Akt phosphorylation, which is downstream of IRS-1 [52]. Moreover, ceramide exposure decreased System A activity and protein synthesis in L6 myotubes [53]. Recently, Holland et al. demonstrated a role for sphingolipids in mediating the beneficial effects of adiponectin in mice liver and heart [54], implicating sphingolipids as critical intermediaries in adiponectin and insulin-signaling in a variety of tissues.
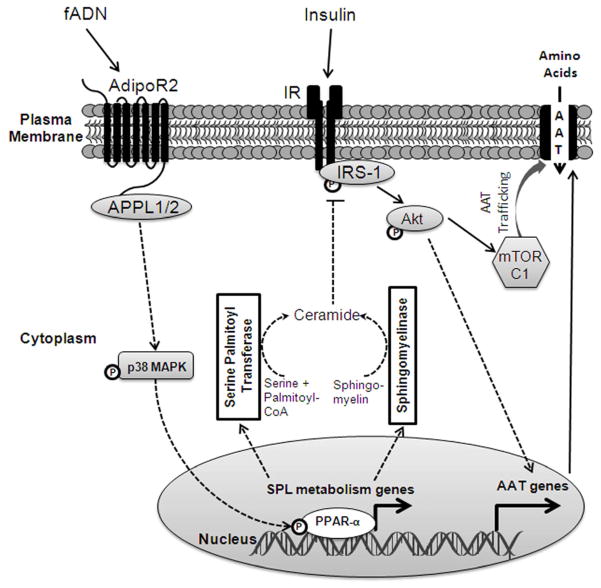
Based on the evidence presented above we propose a working model of fADN-mediated attenuation of insulin-stimulated amino acid transport in placental trophoblasts (Figure 3). Insulin signals via its receptor and activates IRS-1 and Akt, which results in increased System A activity mediated by mTORC1 signaling and other mechanisms. fADN signals via AdipoR2 and APPL1/2 to activate p38 MAPK and PPARα. We postulate that the activation of PPARα leads to transcription of genes favoring ceramide biosynthesis. Changes in sphingolipid metabolism may then promote ceramide-dependent inhibition of IRS-1 signaling and its downstream effects on amino acid transporter activity (Figure 3).
Figure 3. Proposed model of adiponectin signaling in syncytiotrophoblast cells.
fADN interacts with AdipoR2 on the syncytial plasma membrane. Mediated by interaction with APPLs, this results in the activation of p38 MAPK and PPARα. Activation of PPARα dependent gene transcription leads to changes in sphingolipid metabolizing enzymes promoting ceramide biosynthesis. Consequently, elevated intracellular ceramide impairs IRS-1 activity and its downstream signaling of Akt and mTORC1, thereby inhibiting amino acid transport. Solid lines represent previously established processes, dashed lines indicate hypothetical mechanisms. AAT, amino acid transporters; AdipoR1/2, adiponectin receptor 1/2; APPL1/2, adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1/2; fADN, full-length adiponectin; IR, insulin receptor; IRS-1, insulin receptor substrate-1; mTORC1, mammalian target of rapamycin complex 1; p38 MAPK, p38 mitogen-activated protein kinase; PPARα, peroxisome proliferator-activated receptor alpha; SPL, sphingolipid.
5. Emerging role of fetal adiponectin in fetal adiposity and growth
In contrast to the adult, neonatal concentrations of adiponectin positively correlate with several anthropometric indices of adiposity [12, 55]. Higher levels of cord blood adiponectin are associated with increased birth weight [55]. These findings suggest that maternal and fetal adiponectin have opposite roles in regulating fetal growth. At birth, cord blood concentrations of adiponectin are approximately 4–7 fold higher than maternal serum [56]. However, this is followed by a progressive decline in adiponectin levels in the first year of life [57]. Since maternal adiponectin does not cross the placenta [41], the associations between cord blood adiponectin and measures of fetal adiposity reflect an independent role of fetal adiponectin.
While the above studies were based on clinical associations, Qiao et al. recently used genetic approaches to manipulate fetal adiponectin gene expression in mice and reported data supporting a direct link between elevated adiponectin and increased size of fat depots in early life [58]. Similar to the findings in humans, neonatal adiposity in mice was positively correlated with circulating neonatal adiponectin concentrations, whereas adiponectin knockout fetuses displayed lower body weight and fat content. However, the effect of adiponectin gene-knockout on body weight and body fat was no longer observable after the 15th postnatal day. While the mechanisms underlying the delayed expansion of adipose tissue in adiponectin knockout fetuses remain unclear, it may be related to decreased transcription of lipogenic genes in the fetal liver.
6. Summary and future perspectives
Despite numerous clinical studies in the last decade reporting an inverse association between maternal adiponectin and birth weight, the underlying mechanisms remain largely unknown. Based on studies in cell-lines and non-pregnant animals, adiponectin signaling in skeletal muscle and liver of pregnant women would be expected to enhance insulin sensitivity (Figure 4). However, recent findings suggest that adiponectin has the opposite function in the placenta, i.e. promotes insulin resistance. Treatment of trophoblast cells in vitro as well as chronic adiponectin-infusion in pregnant mice impairs insulin signaling and attenuates insulin-stimulated amino acid transport, resulting in fetal growth inhibition in vivo. Regulation of placental function by adiponectin constitutes a novel physiological mechanism by which the endocrine functions of maternal adipose tissue influence fetal growth. The functional significance of fetal adiponectin is largely unknown; however, recent data suggest that fetal and neonatal adiponectin may promote expansion of adipose tissue and stimulate growth in early life. Mechanistic studies in both animal models and in vitro models such as PHTs will be instrumental in elucidating the precise role of adiponectin on maternal, placental and fetal physiology.
Figure 4. Specific roles of adiponectin in maternal, placental and fetal physiology.
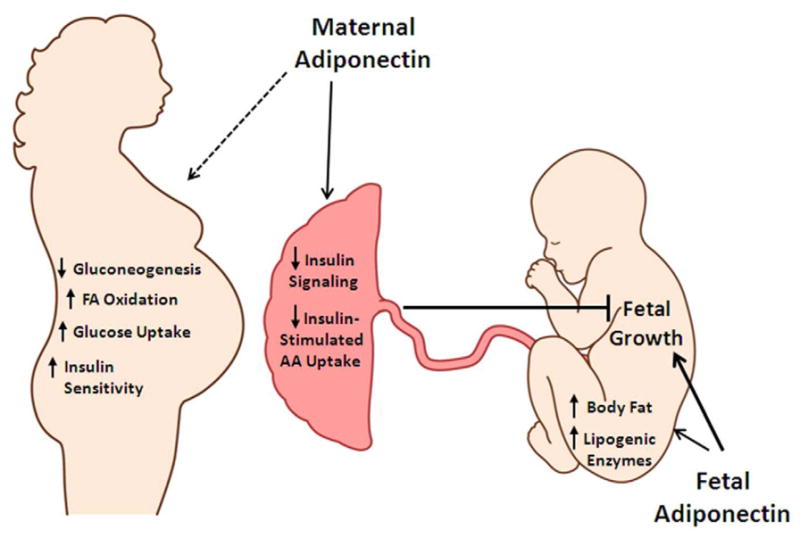
Maternal adiponectin is predicted to decrease gluconeogenesis in maternal liver, increase fatty acid oxidation and glucose utilization, and improve insulin sensitivity in liver and skeletal muscle. In the placenta, maternal adiponectin decreases placental insulin-signaling and reduces insulin-stimulated amino acid transport and subsequently decreases fetal growth. Fetal adiponectin is proposed to increase fetal adiposity and growth, possibly via increased lipogenic enzyme expression in the fetal liver. AA, amino acid; FA, fatty acid.
Acknowledgments
This work was supported by NIH grant HD065007.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198(4):416, e1–6. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- 2.Oken E, Gillman MW. Fetal origins of obesity. Obesity research. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 3.Jansson T, Powell T. In: Placental function in maternofetal exchange. Rodeck CH, Whittle MJ, editors. Churchill Livingstone; 2009. pp. 97–108. [Google Scholar]
- 4.Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signalling in placental nutrient-sensing. Placenta. 2012 doi: 10.1016/j.placenta.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? -- a review. Placenta. 2006;27 (Suppl A):S91–7. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Gaccioli F, Lager S, Powell TL, Jansson T. Placental transport in response to altered maternal nutrition. Journal of Developmental Origins of Health and Disease. 2012 doi: 10.1017/S2040174412000529. [DOI] [PMC free article] [PubMed]
- 7.Briana DD, Malamitsi-Puchner A. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16(10):921–37. doi: 10.1177/1933719109336614. [DOI] [PubMed] [Google Scholar]
- 8.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 9.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 10.Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, Casanueva FF, Dieguez C. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab. 2005;90(7):4276–86. doi: 10.1210/jc.2004-0930. [DOI] [PubMed] [Google Scholar]
- 11.Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186(3):457–65. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 12.Corbetta S, Bulfamante G, Cortelazzi D, Barresi V, Cetin I, Mantovani G, Bondioni S, Beck-Peccoz P, Spada A. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab. 2005;90(4):2397–402. doi: 10.1210/jc.2004-1553. [DOI] [PubMed] [Google Scholar]
- 13.Ichida K, Moriyama T, Morita H, Kondo T, Yoshida S, Ohara N, Maruo T. Plasma adiponectin concentrations and placental adiponectin expression in pre-eclamptic women. Gynecol Endocrinol. 2007;23(4):238–43. doi: 10.1080/09513590701297740. [DOI] [PubMed] [Google Scholar]
- 14.McDonald EA, Wolfe MW. Adiponectin attenuation of endocrine function within human term trophoblast cells. Endocrinology. 2009;150(9):4358–65. doi: 10.1210/en.2009-0058. [DOI] [PubMed] [Google Scholar]
- 15.Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthen L, Powell TL, Jansson T. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87(6):1743–9. doi: 10.1093/ajcn/87.6.1743. [DOI] [PubMed] [Google Scholar]
- 16.Lowe LP, Metzger BE, Lowe WL, Jr, Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95(12):5427–34. doi: 10.1210/jc.2010-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, Miled A, Grissa A, Jerbi M, Tabka Z, Khan NA. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91(10):4137–43. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 18.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221(2):286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 19.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. Journal of biochemistry. 1996;120(4):803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 20.Heiker JT, Kosel D, Beck-Sickinger AG. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biological chemistry. 2010;391(9):1005–18. doi: 10.1515/BC.2010.104. [DOI] [PubMed] [Google Scholar]
- 21.Palanivel R, Fang X, Park M, Eguchi M, Pallan S, De Girolamo S, Liu Y, Wang Y, Xu A, Sweeney G. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res. 2007;75(1):148–57. doi: 10.1016/j.cardiores.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Schmid PM, Resch M, Steege A, Fredersdorf-Hahn S, Stoelcker B, Birner C, Schach C, Buechler C, Riegger GA, Luchner A, Endemann DH. Globular and full-length adiponectin induce NO-dependent vasodilation in resistance arteries of Zucker lean but not Zucker diabetic fatty rats. American journal of hypertension. 2011;24(3):270–7. doi: 10.1038/ajh.2010.239. [DOI] [PubMed] [Google Scholar]
- 23.Bobbert P, Antoniak S, Schultheiss HP, Rauch U. Globular adiponectin but not full-length adiponectin induces increased procoagulability in human endothelial cells. Journal of molecular and cellular cardiology. 2008;44(2):388–94. doi: 10.1016/j.yjmcc.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells. Diabetes. 2010;59(5):1161–70. doi: 10.2337/db09-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278(41):40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 27.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146(2):790–6. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409(3):623–33. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 29.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56(8):2174–7. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 30.Araki S, Dobashi K, Kubo K, Asayama K, Shirahata A. High molecular weight, rather than total, adiponectin levels better reflect metabolic abnormalities associated with childhood obesity. J Clin Endocrinol Metab. 2006;91(12):5113–6. doi: 10.1210/jc.2006-1051. [DOI] [PubMed] [Google Scholar]
- 31.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55(1):249–59. [PubMed] [Google Scholar]
- 32.Deckert CM, Heiker JT, Beck-Sickinger AG. Localization of novel adiponectin receptor constructs. Journal of receptor and signal transduction research. 2006;26(5–6):647–57. doi: 10.1080/10799890600920670. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 34.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nature cell biology. 2006;8(5):516–23. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, Chen H, Mao X, Kikani CK, Liu F, Dong LQ. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009;284(46):31608–15. doi: 10.1074/jbc.M109.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101(28):10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 38.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99(25):16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55(9):2562–70. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 41.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E, Sivan E. Maternal serum adiponectin levels during human pregnancy. J Perinatol. 2007;27(2):77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 42.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-De Mouzon S. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49(7):1677–85. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Lu G, Wong WP, Vliegenthart JF, Gerwig GJ, Lam KS, Cooper GJ, Xu A. Proteomic and functional characterization of endogenous adiponectin purified from fetal bovine serum. Proteomics. 2004;4(12):3933–42. doi: 10.1002/pmic.200400826. [DOI] [PubMed] [Google Scholar]
- 44.Tie W, Yu H, Chen J, Wang X, Chen W, Zhou R. Expressions of adiponectin receptors in placenta and their correlation with preeclampsia. Reprod Sci. 2009;16(7):676–84. doi: 10.1177/1933719109334258. [DOI] [PubMed] [Google Scholar]
- 45.Rosario FJ, Schumacher MA, Jiang J, Kanai Y, Powell TL, Jansson T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol. 2012;590(Pt 6):1495–509. doi: 10.1113/jphysiol.2011.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benaitreau D, Dos Santos E, Leneveu MC, De Mazancourt P, Pecquery R, Dieudonne MN. Adiponectin promotes syncytialisation of BeWo cell line and primary trophoblast cells. Reprod Biol Endocrinol. 2010;8:128. doi: 10.1186/1477-7827-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benaitreau D, Dieudonne MN, Dos Santos E, Leneveu MC, Mazancourt P, Pecquery R. Antiproliferative effects of adiponectin on human trophoblastic cell lines JEG-3 and BeWo. Biol Reprod. 2009;80(6):1107–14. doi: 10.1095/biolreprod.108.070573. [DOI] [PubMed] [Google Scholar]
- 48.McDonald EA, Wolfe MW. The pro-inflammatory role of adiponectin at the maternal-fetal interface. Am J Reprod Immunol. 2011;66(2):128–36. doi: 10.1111/j.1600-0897.2010.00971.x. [DOI] [PubMed] [Google Scholar]
- 49.Roos S, Powell TL, Jansson T. Human placental taurine transporter in uncomplicated and IUGR pregnancies: cellular localization, protein expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R886–93. doi: 10.1152/ajpregu.00232.2004. [DOI] [PubMed] [Google Scholar]
- 50.Zabielski P, Blachnio-Zabielska A, Baranowski M, Zendzian-Piotrowska M, Gorski J. Activation of PPARalpha by bezafibrate negatively affects de novo synthesis of sphingolipids in regenerating rat liver. Prostaglandins Other Lipid Mediat. 2010;93(3–4):120–5. doi: 10.1016/j.prostaglandins.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Baranowski M, Blachnio A, Zabielski P, Gorski J. PPARalpha agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2007;58(1):57–72. [PubMed] [Google Scholar]
- 52.Singh AT, Dharmarajan A, Aye IL, Keelan JA. Ceramide biosynthesis and metabolism in trophoblast syncytialization. Mol Cell Endocrinol. 2012;362(1–2):48–59. doi: 10.1016/j.mce.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. Faseb J. 2005;19(3):461–3. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 54.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, Kanety H. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88(12):5656–60. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 56.Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, Kuroda Y. Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clinical endocrinology. 2004;61(4):418–23. doi: 10.1111/j.1365-2265.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- 57.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123(2):682–9. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiao L, Yoo HS, Madon A, Kinney B, Hay WW, Jr, Shao J. Adiponectin Enhances Mouse Fetal Fat Deposition. Diabetes. 2012 doi: 10.2337/db12-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]