Abstract
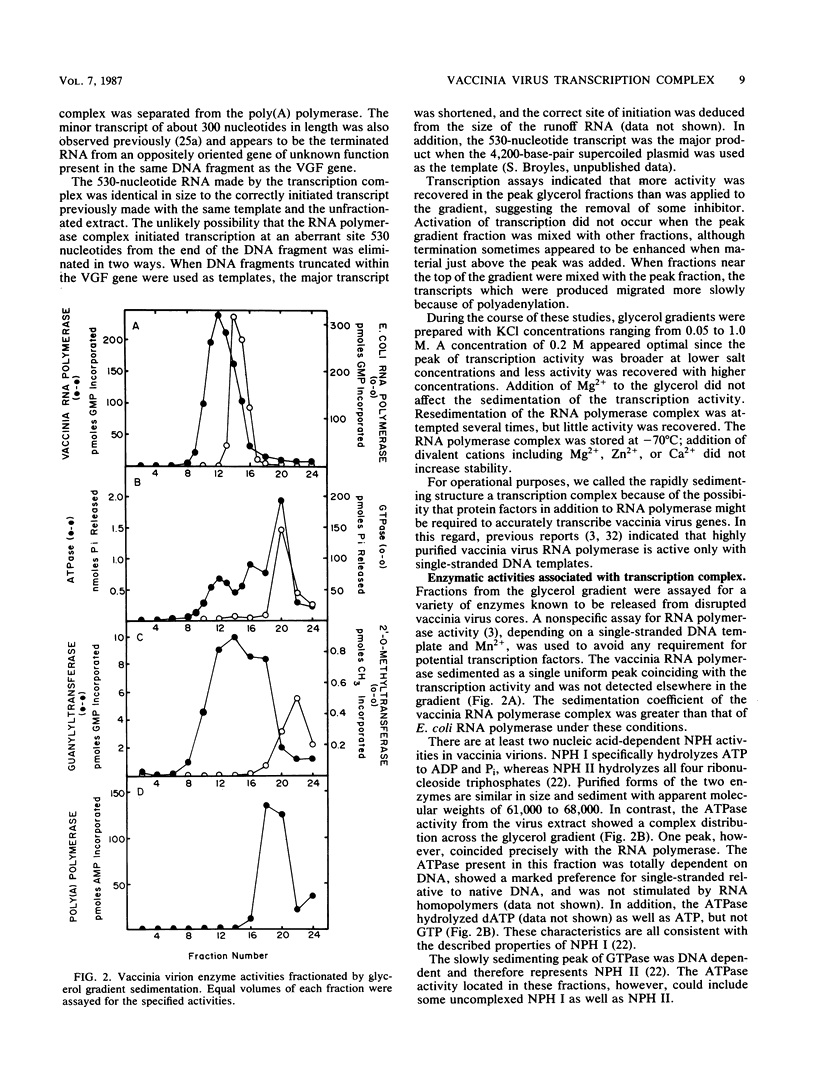
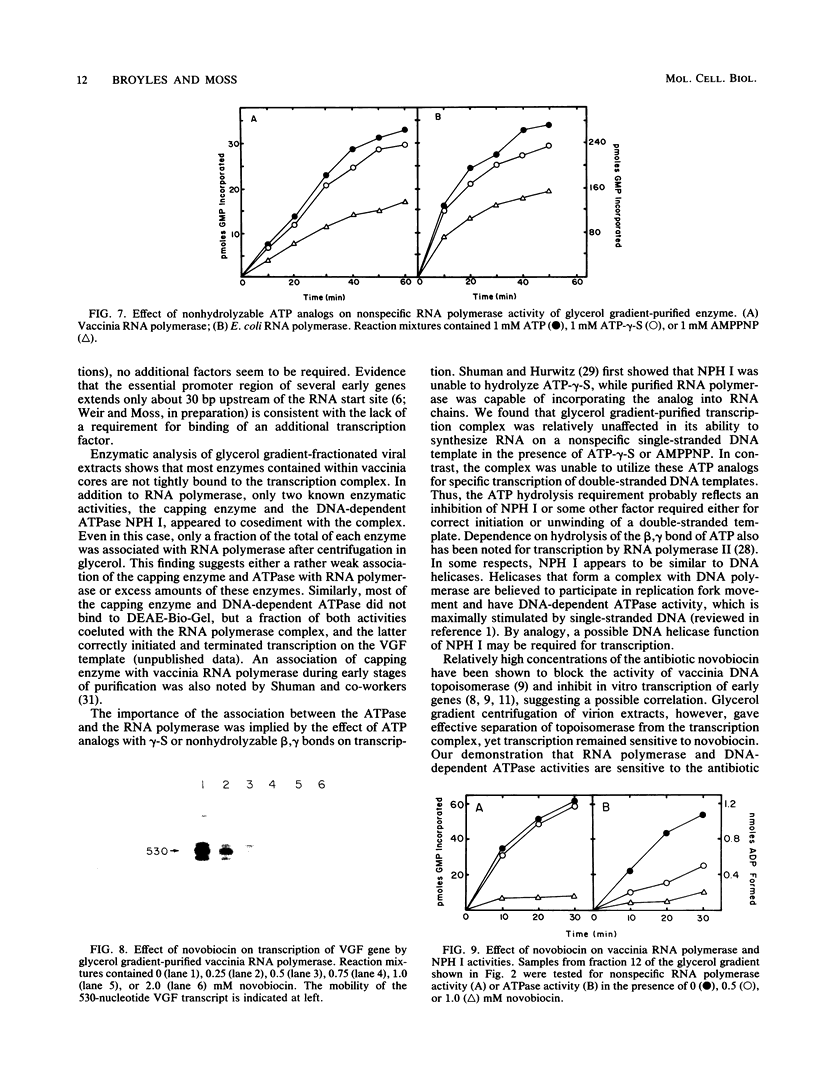
A high-molecular-weight protein complex that is capable of accurate transcription initiation and termination of vaccinia virus early genes without additional factors was demonstrated. The complex was solubilized by disruption of purified virions, freed of DNA by passage through a DEAE-cellulose column, and isolated by glycerol gradient sedimentation. All detectable RNA polymerase activity was associated with the transcription complex, whereas the majority of enzymes released from virus cores including mRNA (nucleoside-2'-O)methyltransferase, poly(A) polymerase, topoisomerase, nucleoside triphosphate phosphohydrolase II, protein kinase, and single-strand DNase sedimented more slowly. Activities corresponding to two enzymes, mRNA guanylyltransferase (capping enzyme) and nucleoside triphosphate phosphohydrolase I (DNA-dependent ATPase), partially sedimented with the complex. Silver-stained polyacrylamide gels, immunoblots, and autoradiographs confirmed the presence of subunits of vaccinia virus RNA polymerase, mRNA guanylyltransferase, and nucleoside triphosphate phosphohydrolase I, as well as additional unidentified polypeptides, in fractions with transcriptase activity. A possible role for the DNA-dependent ATPase was suggested by studies with ATP analogs with gamma-S or nonhydrolyzable beta-gamma-phosphodiester bonds. These analogs were used by vaccinia virus RNA polymerase to nonspecifically transcribe single-stranded DNA templates but did not support accurate transcription of early genes by the complex. Transcription also was sensitive to high concentrations of novobiocin; however, this effect could be attributed to inhibition of RNA polymerase or ATPase activities rather than topoisomerase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Arthur H. M., Benz I., Hoffmann-Berling H., Reygers U., Seiter A., Taucher-Scholz G. Functions of DNA helicases in the DNA metabolism of Escherichia coli. Adv Exp Med Biol. 1984;179:385–393. doi: 10.1007/978-1-4684-8730-5_40. [DOI] [PubMed] [Google Scholar]
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Purification and physical properties. J Biol Chem. 1978 Nov 10;253(21):7692–7697. [PubMed] [Google Scholar]
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Bauer W. R., Ressner E. C., Kates J., Patzke J. V. A DNA nicking-closing enzyme encapsidated in vaccinia virus: partial purification and properties. Proc Natl Acad Sci U S A. 1977 May;74(5):1841–1845. doi: 10.1073/pnas.74.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Homology between RNA polymerases of poxviruses, prokaryotes, and eukaryotes: nucleotide sequence and transcriptional analysis of vaccinia virus genes encoding 147-kDa and 22-kDa subunits. Proc Natl Acad Sci U S A. 1986 May;83(10):3141–3145. doi: 10.1073/pnas.83.10.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Foglesong P. D., Bauer W. R. Effects of ATP and inhibitory factors on the activity of vaccinia virus type I topoisomerase. J Virol. 1984 Jan;49(1):1–8. doi: 10.1128/jvi.49.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglesong P. D. In vitro transcription of a cloned vaccinia virus gene by a soluble extract prepared from vaccinia virus-infected HeLa cells. J Virol. 1985 Mar;53(3):822–826. doi: 10.1128/jvi.53.3.822-826.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershowitz A., Boone R. F., Moss B. Multiple roles for ATP in the synthesis and processing of mRNA by vaccinia virus: specific inhibitory effects of adenosine (beta,gamma-imido) triphosphate. J Virol. 1978 Aug;27(2):399–408. doi: 10.1128/jvi.27.2.399-408.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golini F., Kates J. R. A soluble transcription system derived from purified vaccinia virions. J Virol. 1985 Jan;53(1):205–213. doi: 10.1128/jvi.53.1.205-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M. Novobiocin inhibits RNA polymerase III transcription in vitro by a mechanism distinct from DNA topoisomerase II. Nucleic Acids Res. 1986 Mar 11;14(5):2075–2088. doi: 10.1093/nar/14.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Purification of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2420–2429. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Moyer R. W. Detection of a subunit of cellular Pol II within highly purified preparations of RNA polymerase isolated from rabbit poxvirus virions. Cell. 1986 Feb 28;44(4):587–596. doi: 10.1016/0092-8674(86)90268-0. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and properties of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1977 Oct 10;252(19):6930–6938. [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Paoletti E., Cooper N., Moss B. Regulation of synthesis of two immunologically distinct nucleic acid-dependent nucleoside triphosphate phosphohydrolases in vaccinia virus-infected HeLa cells. J Virol. 1974 Sep;14(3):578–586. doi: 10.1128/jvi.14.3.578-586.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Two nucleic acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Nucleotide substrate and polynucleotide cofactor specificities. J Biol Chem. 1974 May 25;249(10):3281–3286. [PubMed] [Google Scholar]
- Puckett C., Moss B. Selective transcription of vaccinia virus genes in template dependent soluble extracts of infected cells. Cell. 1983 Dec;35(2 Pt 1):441–448. doi: 10.1016/0092-8674(83)90177-0. [DOI] [PubMed] [Google Scholar]
- Rohrmann G., Moss B. Transcription of vaccinia virus early genes by a template-dependent soluble extract of purified virions. J Virol. 1985 Nov;56(2):349–355. doi: 10.1128/jvi.56.2.349-355.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G., Yuen L., Moss B. Transcription of vaccinia virus early genes by enzymes isolated from vaccinia virions terminates downstream of a regulatory sequence. Cell. 1986 Sep 26;46(7):1029–1035. doi: 10.1016/0092-8674(86)90702-6. [DOI] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Paoletti E., Moss B. Single-stranded deoxyribonucleic acid-specific nuclease from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3287–3291. [PubMed] [Google Scholar]
- Sarih L., Garret M., Aoyama H., Graves P. V., Tarrago-Litvak L., Litvak S. The in vitro inhibition of DNA polymerase alpha and avian reverse transcriptase by novobiocin. Biochem Int. 1983 Jul;7(1):79–88. [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Energy requirement for specific transcription initiation by the human RNA polymerase II system. J Biol Chem. 1984 Apr 25;259(8):5321–5326. [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Spencer E., Furneaux H., Hurwitz J. The role of ATP in in vitro vaccinia virus RNA synthesis effects of AMP-PNP and ATP gamma S. J Biol Chem. 1980 Jun 10;255(11):5396–5403. [PubMed] [Google Scholar]
- Shuman S., Surks M., Furneaux H., Hurwitz J. Purification and characterization of a GTP-pyrophosphate exchange activity from vaccinia virions. Association of the GTP-pyrophosphate exchange activity with vaccinia mRNA guanylyltransferase . RNA (guanine-7-)methyltransferase complex (capping enzyme). J Biol Chem. 1980 Dec 10;255(23):11588–11598. [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Sumiyoshi Y., Nishikawa T., Watanabe T., Kano K. Inhibition of retrovirus RNA-dependent DNA polymerase by novobiocin and nalidixic acid. J Gen Virol. 1983 Oct;64(Pt 10):2329–2333. doi: 10.1099/0022-1317-64-10-2329. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982 Nov;44(2):637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Modification of the 5' end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980 Feb 10;255(3):903–908. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wingender E., Jahn D., Seifart K. H. Association of RNA polymerase III with transcription factors in the absence of DNA. J Biol Chem. 1986 Jan 25;261(3):1409–1413. [PubMed] [Google Scholar]
- Yuen L., Moss B. Multiple 3' ends of mRNA encoding vaccinia virus growth factor occur within a series of repeated sequences downstream of T clusters. J Virol. 1986 Oct;60(1):320–323. doi: 10.1128/jvi.60.1.320-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]