Background: Advanced glycation end products (AGEs) can act as neoantigens to trigger immune responses.
Results: Natural IgM antibodies against AGEs recognize multiple molecules, including DNA and chemically modified proteins.
Conclusion: There is a close relationship between the formation of AGEs and innate immune responses.
Significance: Our findings highlight AGEs and related modified proteins as a source of multispecific natural antibodies.
Keywords: Antibodies, Antigen, Glycation, Innate Immunity, Protein Chemical Modification, AGEs, Natural Antibodies
Abstract
Advanced glycation end products (AGEs) are a heterogeneous and complex group of compounds that are formed when reducing sugars, such as dehydroascorbic acid, react in a nonenzymatic way with amino acids in proteins and other macromolecules. AGEs are prevalent in the diabetic vasculature and contribute to the development of atherosclerosis. The presence and accumulation of AGEs in many different cell types affect the extracellular and intracellular structure and function. In the present study, we studied the immune response to the dehydroascorbic acid-derived AGEs and provide multiple lines of evidence suggesting that the AGEs could be an endogenous source of innate epitopes recognized by natural IgM antibodies. Prominent IgM titers to the AGEs were detected in the sera of normal mice and were significantly accelerated by the immunization with the AGEs. Patients with systemic lupus erythematosus (SLE), a potentially fatal systemic autoimmune disease characterized by the increased production of autoantibodies, showed significantly higher serum levels of the IgM titer against the AGEs than healthy individuals. A progressive increase in the IgM response against the AGEs was also observed in the SLE-prone mice. Strikingly, a subset of monoclonal antibodies, showing a specificity toward the AGEs, prepared from normal mice immunized with the AGEs and from the SLE mice cross-reacted with the double-stranded DNA. Moreover, they also cross-reacted with several other modified proteins, including the acetylated proteins, suggesting that the multiple specificity of the antibodies might be ascribed, at least in part, to the increased electronegative potential of the proteins. These findings suggest that the protein modification by the endogenous carbonyl compounds, generating electronegative proteins, could be a source of multispecific natural antibodies.
Introduction
Advanced glycation end products (AGEs)2 are a heterogeneous group of molecules formed from the nonenzymatic reaction of reducing sugars with the free amino groups of proteins, lipids, and nucleic acids (1). The initial product of this reaction is called a Schiff base, which spontaneously rearranges itself into an Amadori product. The formation and accumulation of AGEs has been implicated in a variety of age-related diseases, including diabetic complications and atherosclerosis (2, 3). AGEs can initiate a wide range of responses in cells and tissues, such as the inappropriate expression of growth factors, alterations in growth dynamics, accumulation of the extracellular matrix, promotion of vasoregulatory dysfunction, and initiation of death pathways. AGEs can also act as neoantigens to trigger proinflammatory cellular immune responses. A reservoir of soluble AGEs, derived from proteins during the circulation and degradation of tissue-bound AGE, has been shown to contribute to pathogenesis by interacting with vascular tissues via specific receptors, including the receptor for AGEs and scavenger receptors (4). Indeed, Nϵ-(carboxymethyl)lysine, one of the major AGEs, binds the receptor for AGEs and modulates cellular properties (5). Ligation of AGEs to the receptors mediates the uptake and triggers the cellular activation or proliferation, leading to inflammation and tissue destruction (4).
Dehydroascorbic acid (DHA), an oxidized form of vitamin C, is known to be an endogenous dicarbonyl compound, which can serve as an excellent intermediate of the glycation reaction. As a powerful antioxidant, vitamin C can neutralize harmful free radicals, and it aids in neutralizing pollutants and toxins. Although the direct antioxidant protection afforded by vitamin C is limited to water-soluble environments, it plays an antioxidant role in lipids through its regeneration of the fat-soluble antioxidant vitamin E (α-tocopherol). When used as an antioxidant, vitamin C is readily oxidized to DHA. In addition, DHA can be rearranged into additional degradation products, such as 2,3-diketogulonic acid, 3-deoxythreosone, xylosone, and threosone (6). These vitamin C-derived products can covalently modify proteins through nonenzymatic glycation to generate AGEs (7–9). It has been suggested that, because the vitamin C levels are high in a variety of tissues, DHA and its degradation products could be causally involved in the formation of AGEs in vivo.
Modified forms, such as AGEs, generated on self-antigens could be an important target of the immune response. The existence of antibodies (Abs) against AGEs was indeed reported by Witztum et al. (10), demonstrating that plasma from patients with diabetes could react with glycated proteins. Subsequently, Nϵ-(carboxymethyl)lysine was identified as a major target for these Abs (11). They also reported increased levels of Abs against Nϵ-(carboxymethyl)lysine in patients with diabetic nephropathy. More recently, Engelbertsen et al. (12) observed an association between high levels of IgM against the methylglyoxal-modified apolipoprotein B100 and reduced coronary artery calcification in patients with type 2 diabetes and suggested that the IgM against the methylglyoxal-modified protein may be protective in diabetic vasculopathy. However, the linkage between AGEs and innate immunity, especially focusing on the production of natural Abs, has never been studied. Moreover, the exact nature of the anti-AGEs Abs remains to be elucidated. In the present study, we studied the innate immune response to the DHA-derived AGEs and provided multiple lines of evidence suggesting that the AGEs could be an endogenous source of innate epitopes recognized by natural antibodies. In addition, based on the findings that the natural Abs cross-reacted with dsDNA and several other modified proteins, including the acetylated proteins, we suggest a mechanism, in which the electronegative potential of antigens might be involved, at least in part, in the recognition by the natural Abs.
EXPERIMENTAL PROCEDURES
Materials
DHA, methylglyoxal, and calf thymus dsDNA were obtained from Sigma-Aldrich. BSA was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All of other reagents used in the study were of analytical grade and obtained from commercial sources.
Animals
Balb/c mice were purchased from the Japan SLC (Hamamatsu, Japan). Female MRL-lpr and MRL-MpJ mice were purchased from Chubu Kagaku Shizai Co., Ltd. (Nagoya, Japan). All animal protocols were approved by the Animal Experiment Committee in the Graduate School of Bioagricultural Sciences of Nagoya University.
Plasma Samples
Plasma samples were obtained from 5 healthy individuals, 20 patients with IgA nephropathy, and 26 patients with SLE who underwent diagnostic evaluation at the Nagoya University Hospital (Nagoya, Japan). The antibody titers against dsDNA and AGEs in the plasma samples were measured by ELISA using calf thymus dsDNA and DHA-modified BSA, respectively, as the coating antigens. This study was approved by the Ethical Committee of the Nagoya University School of Medicine.
Preparation of Modified Proteins in Vitro
Modification of the protein by DHA was performed by incubating BSA (1.0 mg/ml) with DHA (25.0 mm) in PBS buffer (pH 7.4) at 37 °C under atmospheric oxygen. After 7 days, aliquots were collected and dialyzed against PBS. The oxidized LDL was prepared as previously described (13). The acetylated BSA was prepared according to a published procedure (14).
Statistical Analysis
Differences were analyzed by the unpaired two-tailed Student's t test or Welch's t test as appropriate, and p values < 0.05 were considered significant.
ELISA
We used direct antigen ELISAs to measure the antibody reactivity. The calf thymus DNA and native and modified proteins were used as the antigens. A 100-μl aliquot of the antigen solution (50 μg/ml) was added to each well of a 96-well microtiter plate and incubated for 20 h at 4 °C. The antigen solution was then removed, and the plate was washed with PBS containing 0.5% Tween 20 (PBS/Tween). Each well was incubated with 200 μl of 4% Blockace (Yukijirushi, Sapporo, Japan) in PBS/Tween for 60 min at 37 °C to block the unsaturated plastic surface. The plate was then washed three times with PBS/Tween. A 100-μl aliquot of a 500× dilution of serum was added to each well and incubated for 2 h at 37 °C. After discarding the supernatants and washing three times with PBS/Tween, 100 μl of a 5 × 103 dilution of goat anti-mouse IgG or IgM conjugated to horseradish peroxidase in PBS/Tween was added. After incubation for 1 h at 37 °C, the supernatant was discarded, and the plates were washed three times with PBS/Tween. The enzyme-linked Ab bound to the well was revealed by adding 100 μl/well of 1,2-phenylenediamine (0.5 mg/ml) in a 0.1 m citrate/phosphate buffer (pH 5.5) containing 0.003% hydrogen peroxide. The reaction was terminated by the addition of 2 m sulfuric acid (50 μl/well), and the absorbance at 492 nm was read using a micro-ELISA plate reader. The signals were within the dynamic range of the assays with respect to Ab levels.
Polyacrylamide Gel Electrophoresis
Mobility shifts of modified proteins were demonstrated in a nondenaturing polyacrylamide gel (8% acrylamide) in 2× TAE buffer (80 mm Tris acetate, 2 mm EDTA, pH 8.0). Tris-glycine (pH 8.4) was used as the running buffer. 10 μl of 1 mg/ml protein was mixed with 2 μl of 6× loading buffer (0.03% bromophenol blue, 10 mm Tris-HCl, pH 7.6, 60 mm EDTA, 60% glycerol) and electrophoresed in the gel at 100 V. After electrophoresis, the gel was stained with Coomassie Brilliant Blue.
Preparation of IgM mAbs against AGEs
Spleen cells from the SLE-prone MRL-lpr mice were fused with P3/U1 murine myeloma cells and cultured in hypoxanthine/aminopterin/thymidine selection medium. The culture supernatants of the hybridoma were screened using an ELISA, employing pairs of wells in microtiter plates on which were absorbed calf thymus dsDNA and DHA-treated BSA (MRL-lpr mice) as antigens (5 μg of protein or DNA/well). After incubation with 100 μl of the hybridoma supernatants, and with intervening washes with PBS/Tween, the wells were incubated with horseradish peroxidase goat anti-mouse IgG in PBS/Tween, followed by a substrate solution containing 100 μl/well of 1,2-phenylenediamine (0.5 mg/ml) in a 0.1 m citrate/phosphate buffer (pH 5.5) containing 0.003% hydrogen peroxide. Hybridoma cells, corresponding to the supernatants that were positive on either dsDNA or DHA-modified BSA and negative on native BSA, were then cloned by limited dilution. After repeated screenings, three clones showing the most distinctive recognition of DNA and two clones showing the most distinctive recognition of both DHA-modified BSAs were obtained.
The IgM mAbs were also prepared from Balb/c mice immunized with DHA-modified proteins. The immunogen was prepared by incubating keyhole limpet hemocyanin (KLH; 1.0 mg/ml) with 25 mm DHA in 5 ml of PBS (pH 7.4) at 37 °C for 7 days. We immunized the female Balb/c mice on day 1 with complete Freund adjuvant and 0.05 mg of immunogen (DHA-modified KLH) and boosted on days 7, 17, and 27 with incomplete Freund adjuvant, by emulsifying and intraperitoneal injection. The IgM mAbs were prepared from the immunized mice following the same protocol as for the SLE-prone mice.
Antibody Sequence Analysis
Immunoglobulin variable region genes were cloned and sequenced following amplification by PCR. The total RNA was prepared from 5 × 106 hybridoma cells by the phenol-guanidine isothiocyanate method (TRIzol reagent; Invitrogen) according to the manufacturer's protocol. The first strand cDNA synthesis was performed with RevertAid reverse transcriptase (Thermo Scientific) using the manufacturer's protocol. A 5-μg sample of the total RNA was primed with 10 pmol of random primers. Variable region genes were amplified using degenerate sense primers homologous to the mouse heavy and light chain leader sequences and antisense constant primers (Novagen), as previously described (15). The amplification products were ligated into the pGEM-T Easy Vector (Promega) using standard protocols, and both strands of inserts were sequenced using an automated dye chain termination DNA sequencer. The Basic Local Alignment Search Tool (BLAST) protocol was used to search the GenBankTM database to determine the homology with the V regions of other murine Abs that have been sequenced (16).
B Cell Responses after Immunization
Six-week-old Balb/c mice were immunized with 50 μg of KLH or DHA-modified KLH in complete Freund's adjuvant by intraperitoneal injection. The mice were then boosted every 2 weeks with 50 μg of the immunogens in incomplete Freund's adjuvant by emulsifying and intraperitoneal injection. The Abs used for the lymphocyte stainings were anti-mouse PE/Cy5 CD5 (clone 53-7.3; BioLegend) and anti-mouse CD45R/B220 (clone RA3-6B2; LifeSpan BioSciences). The anti-mouse CD45R/B220 was labeled by Alexa Fluor 488 carboxylic acid, succinimidyl ester, and mixed isomers (Molecular Probes). Mouse peritoneal cells were isolated by peritoneal lavage with 5 ml of blocking buffer (PBS with 3% FBS) (17). To block the Fc receptors, 1 × 106/50 μl cells were incubated in Blocking Buffer for 15 min at 4 °C. The cells were stained in staining buffer (1:100 anti-CD5 Ab, 1:20 anti-CD5 antibody in blocking buffer) for 30 min at 4 °C. Flow cytometry was performed on a FACS JSAN (Bay Bioscience). The flow cytometric profiles were analyzed using FlowJo software (Tree Star).
Analysis of Antigen-Antibody Interaction
Dynabeads M-270 carboxylic acid (Dynal; Invitrogen) were coupled with BSA and blocked according to instructions from the manufacturer. Subsequently, the BSA-coupled beads were incubated with 25 mm DHA in 0.2 m sodium phosphate buffer (pH 7.4) for 7 days to obtain the AGE-BSA-coupled beads. Independently, the acetylated BSA-coupled beads were prepared according to the published procedure (14). The beads (2 × 107) were then added to microcentrifuge tubes and incubated with 30 μg of normal mouse IgM (ab18400; Abcam, Cambridge, UK) or BDM1 in 1 ml of PBS/Tween for 1 h at room temperature. After washing three times with PBS/Tween, the beads were stained with 100 μl of a 5 × 102 dilution of the Alexa Fluor 488-conjugated antibody against mouse IgM (A21042; Invitrogen) for 30 min at room temperature. Flow cytometry was performed on a FACS JSAN (Bay Bioscience). Flow cytometric profiles were analyzed using FlowJo software (Tree Star). A total of 20,000 events/sample were counted and analyzed.
RESULTS
Innate Immune Response to AGEs
To characterize the immune response to the AGEs, specific Ab titers to the AGEs in the sera from the Balb/c mice that had not received experimental immunization or in vitro stimulation were assessed. Prominent IgM titers to the DHA-modified BSA, in addition to the native dsDNA and oxidized LDL, were detected in the sera, whereas the IgM titers to the native BSA and LDL were minimal or undetectable (Fig. 1A). The IgG responses to the dsDNA and AGEs were also detected, but their titers were severalfold lower than the IgM titers (Fig. 1, B and C). Similar IgM titers to the dsDNA and AGEs were observed in the sera from other normal mice, such as the C57BL/6 and MRL-MpJ mice (supplemental Fig. S1). Specific pathogen free-maintained mice contained IgM titers to the dsDNA and AGEs compared with titers against the native protein (supplemental Fig. S2), suggesting that the basal IgM titers against the dsDNA and AGEs are largely independent of noncommensal exposure to microbial pathogens. These data and the fact that the post-translational modification of proteins, such as glycation, is enhanced in aging and stressed cells and occurs under physiological conditions suggest the existence of an association between the formation of AGEs and innate immune responses.
FIGURE 1.
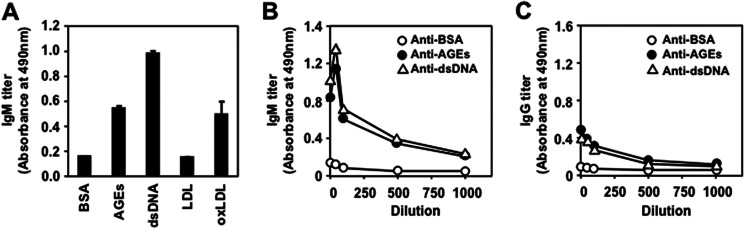
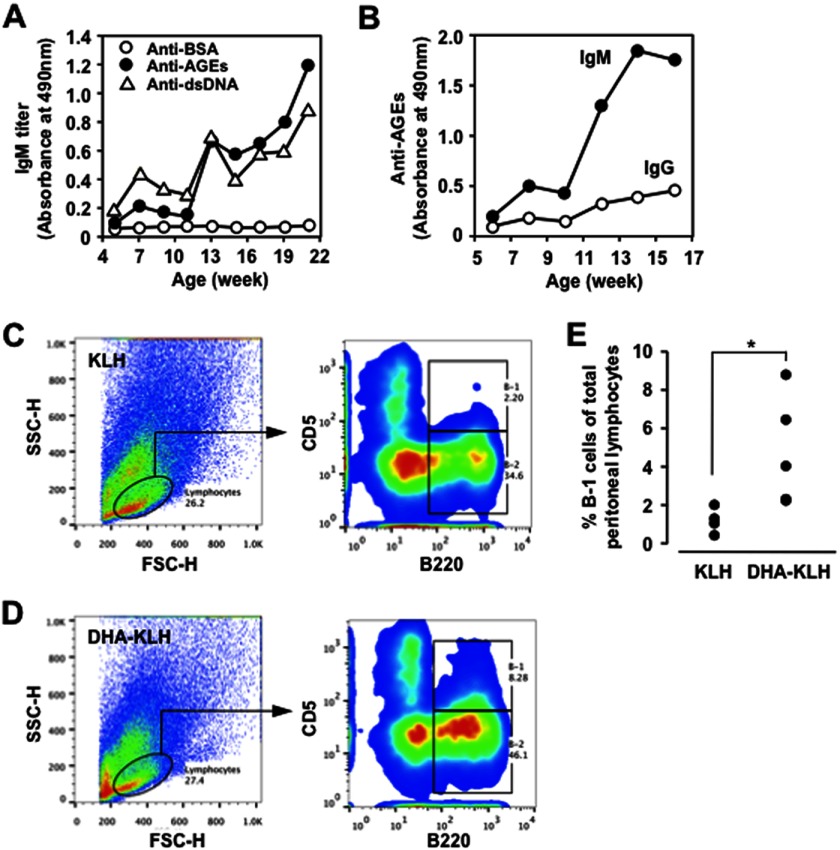
Presence of natural Abs against AGEs in normal mice. A, immunoreactivity of the Balb/c mice sera with the AGEs and other endogenous antigens. The IgM titer in the mouse sera was examined by an ELISA employing pairs of wells in microtiter plates on which were absorbed native BSA, DHA-modified BSA, dsDNA, LDL, and oxidized LDL (oxLDL) as the antigens. B, the titer of IgM Abs in the Balb/c mice sera to the endogenous antigens. C, the titer of IgG Abs in the Balb/c mice sera to the endogenous antigens. In B and C, native BSA (open circle), DHA-modified BSA (closed circle), and dsDNA (open triangle) were used as the antigens.
Stimulation of Innate Immune Response by AGEs
We then determined whether the production of natural Abs specific for the AGEs could be accelerated by the immunization with the same antigens. To this end, Balb/c mice were immunized every 2 weeks with the DHA-modified KLH emulsified with complete Freund's adjuvant, and the IgG and IgM responses to the dsDNA and AGEs were examined. The IgM titers to both the AGEs and dsDNA were dramatically enhanced by the immunization with the DHA-modified KLH (Fig. 2A). It was also revealed that the mice immunized with the AGEs mainly developed an IgM response, but not an IgG response, to the AGEs (Fig. 2B).
FIGURE 2.
Immunization of AGEs accelerates IgM response. Female Balb/c mice were immunized with complete Freund adjuvant and 50 μg of the immunogen (KLH or DHA-modified KLH) and then boosted every 2 weeks with incomplete Freund adjuvant by emulsifying and intraperitoneal injection. A, age-dependent elevation of the anti-AGEs and anti-dsDNA IgM Abs in the Balb/c mice immunized with the DHA-modified KLH. The IgG and IgM titers were determined by ELISA using the BSA (open circle), DHA-modified BSA (closed circle), and dsDNA (open triangle) as the absorbed antigens. B, age-dependent elevation of the anti-AGEs IgG and IgM Abs in the Balb/c mice immunized with the DHA-modified KLH. The IgG and IgM titers were determined by ELISA using the DHA-modified BSA (AGEs) as the absorbed antigens. C, flow cytometry analysis of peritoneal cells prepared from the KLH-immunized Balb/c mice. D, flow cytometric analysis of peritoneal cells prepared from the AGEs-immunized Balb/c mice. E, the proportion of B-1 cells in the peritoneum of Balb/c mice treated with KLH (n = 4) or DHA-modified KLH (n = 5). In C–E, cells were analyzed with forward (FSC) and side (SSC) light scatter. The gated B lymphocyte population (circled) was stained for B220 (anti-mouse CD45R/B220) versus CD5 (anti-mouse PE/Cy5 CD5). The results shown were obtained 4 weeks after the first immunization.
Because B-1 cells are known to be the major source of natural antibodies and most of the circulating IgM is contributed by B-1 cells in the absence of previous antigen stimulation (18), the percentage of B-1 cells in the peritoneal cavity among the B cell-enriched preparations from Balb/c mice treated with KLH or DHA-modified KLH was measured 4 weeks after the primary immunization. Fig. 2 (C and D) shows that the injection of the DHA-modified KLH into Balb/c mice increased the resident B-1 cell population as compared with the KLH-injected controls. The increase in the B-1 cell number upon AGE stimulation was statistically significant as compared with the controls (Fig. 2E). These results suggest that the IgM response, accelerated by the AGEs, might account for the outgrowth of the peritoneal B-1 cells.
Human SLE Patients Have Higher Ab Titers to AGEs
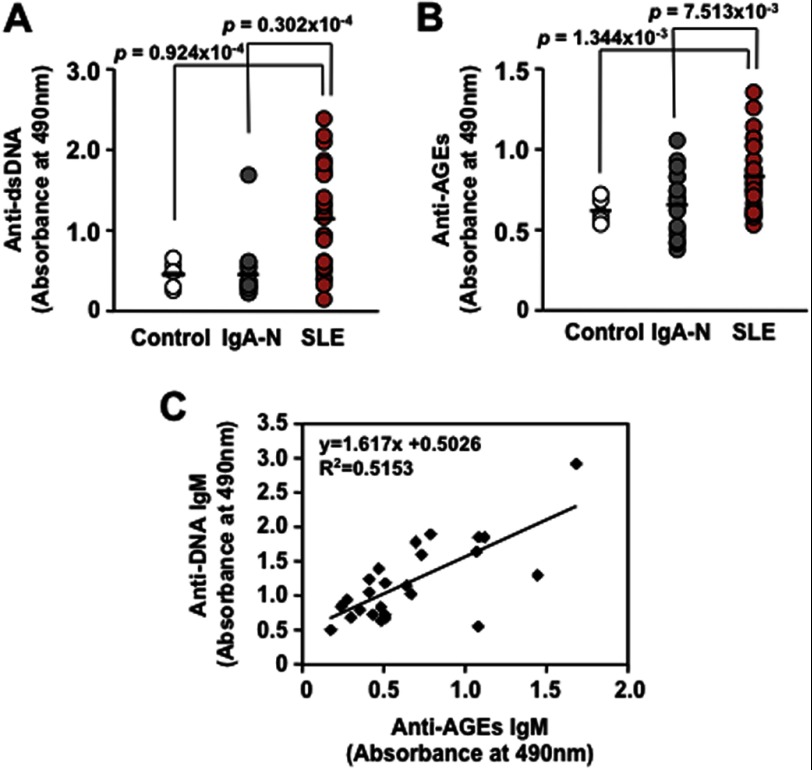
Innate immune signaling plays an important role in the protection from pathogens but has the potential for the development of autoimmune or autoinflammatory diseases, such as SLE, by inducing innate immune activation. Autoinflammatory patients often have high proinflammatory cytokine levels, which may come from activation of the innate immunity (19). SLE is a potentially fatal systemic autoimmune disease characterized by the increased production of auto-Abs, immune complex deposition in the microvasculature, leukocyte infiltration, and, ultimately, tissue damage in a number of organs. Of the multiple auto-Abs described in this disease, the Abs against the native DNA are among the most characteristic, yet the triggering antigen in the disease is still unknown. The appearance of these Abs in humans and in murine models of lupus correlates with the progression of the disease, and by comparison with all the other lupus auto-Abs, those against the dsDNA are thought to be the most pathogenic and involved in the development of renal pathology. To evaluate the presence of IgM Abs in the autoimmune diseases, we measured the plasma Ab titers directed against the dsDNA and AGEs. The patients with SLE indeed exhibited significant increases in the Ab titers to the DHA-modified proteins (Fig. 3B), as well as to the dsDNA (Fig. 3A) compared with the controls and the patients with IgA nephropathy. A high correlation was observed between the anti-dsDNA IgM and anti-AGEs IgM titers in the SLE patients (Fig. 3C).
FIGURE 3.
Elevation of innate immune response to the AGEs in autoimmune diseases. A, the immunoreactivity of human plasma with the dsDNA. B, the immunoreactivity of human plasma with DHA-modified proteins. C, linear regression analysis between serum anti-dsDNA IgM and anti-DHA-modified protein IgM titers in SLE patients. The plasma samples were prepared from 5 healthy individuals, 20 patients with IgA nephropathy (IgA-N), and 26 patients with SLE. The levels of the IgM Abs against the dsDNA and AGEs in the plasma samples were measured by ELISA using calf thymus dsDNA and DHA-modified protein, respectively, as the coating antigens. The calculated p values were obtained by Welch's t test analysis.
Elevated Immune Response to AGEs in SLE-prone MRL-lpr Mice
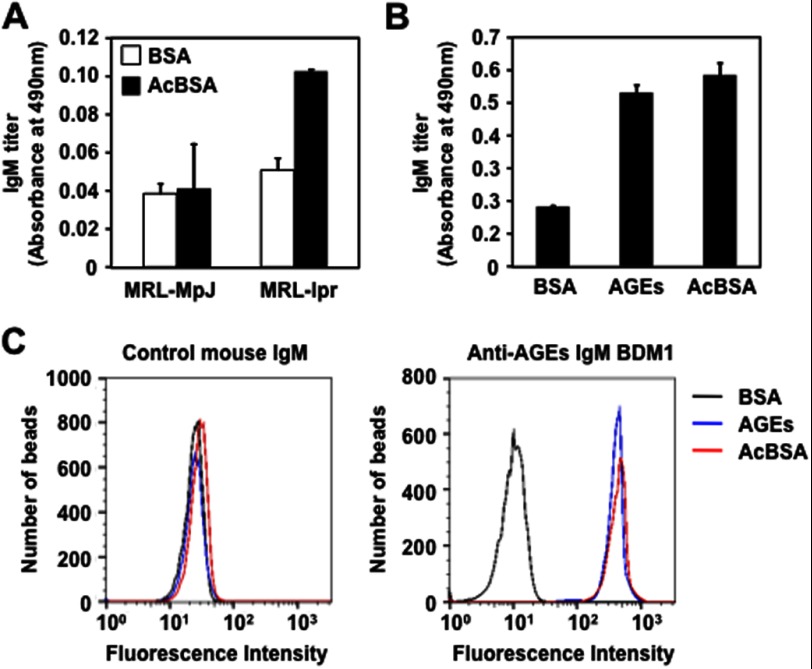
The presence of natural IgM Abs against the AGEs was also evaluated in the SLE-prone mice. We first determined whether the AGEs could be recognized by the sera from the MRL-lpr mice, which carry a defective Fas gene and develop a spontaneous lupus-like disease as they age, and from the control MRL-MpJ mice. The AGEs prepared upon incubation of BSA with DHA showed a significant cross-reactivity with the sera from the MRL-lpr mice, whereas no cross-reactivity with the sera from the control MRL-MpJ mice was observed (supplemental Fig. S3). When the age-dependent change in the Ab titers was measured in the sera from the MRL-lpr mice and control MRL-MpJ mice, only the MRL-lpr mice displayed a spontaneous age-dependent elevation of the IgG and IgM responses to both dsDNA and AGEs (Fig. 4). Specificity of Abs that deposited in the glomeruli of the MRL-lpr mice also showed that the titers of the IgG and IgM Abs against the AGEs were significantly higher in the MRL-lpr mice than in the MRL-MpJ mice (supplemental Fig. S4). These clinical and animal data suggest that the Ab response against the AGEs may be an immunological characteristic common to SLE.
FIGURE 4.
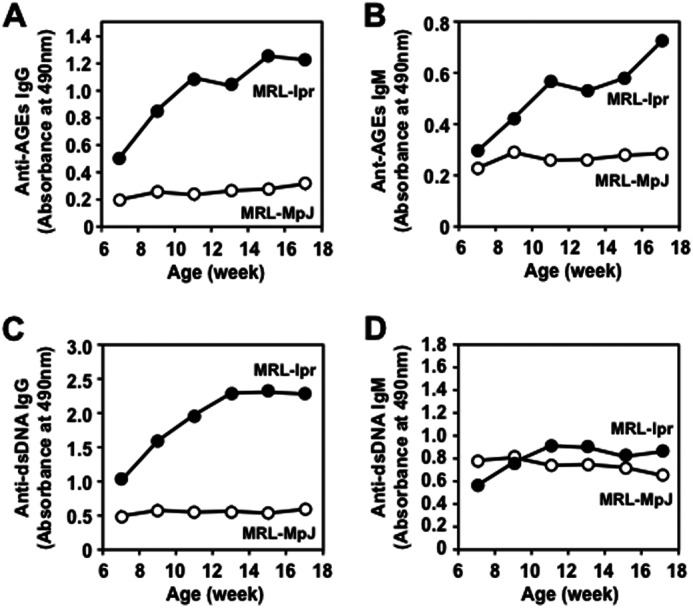
Age-dependent elevation of IgG and IgM responses to both dsDNA and AGEs in SLE-prone MRL-lpr mice compared with those in the wild-type MRL-MpJ mice. A, anti-AGEs IgG. B, anti-AGEs IgM. C, anti-dsDNA IgG. D, anti-dsDNA IgM. The antibody titers were determined by ELISA using the DHA-modified BSA and dsDNA as the absorbed antigens. The IgG and IgM titers were determined by ELISA using the dsDNA and DHA-modified protein as the absorbed antigens. Each point represents the average value of six animals.
Sequence Analysis of mAbs against AGEs
To establish the mAbs that recognize the AGEs, we sought to isolate the hybridoma clones, producing the Abs specific for the AGEs, from the Balb/c mice immunized with the AGEs and from the MRL-lpr mice. Three IgM mAbs, clones BDM1, BDM2, and BDM3, showing a recognition specificity toward the AGEs, was established from the immunized Balb/c mice after screening based on the specific binding to the corresponding antigen (AGEs). We also established three hybridoma clones, ADL7, ADL19, and DDL17, producing the IgM Abs, and three hybridoma clones, ADL13, DDL18, and DDL20, producing the IgG Abs, from the MRL-lpr mice.
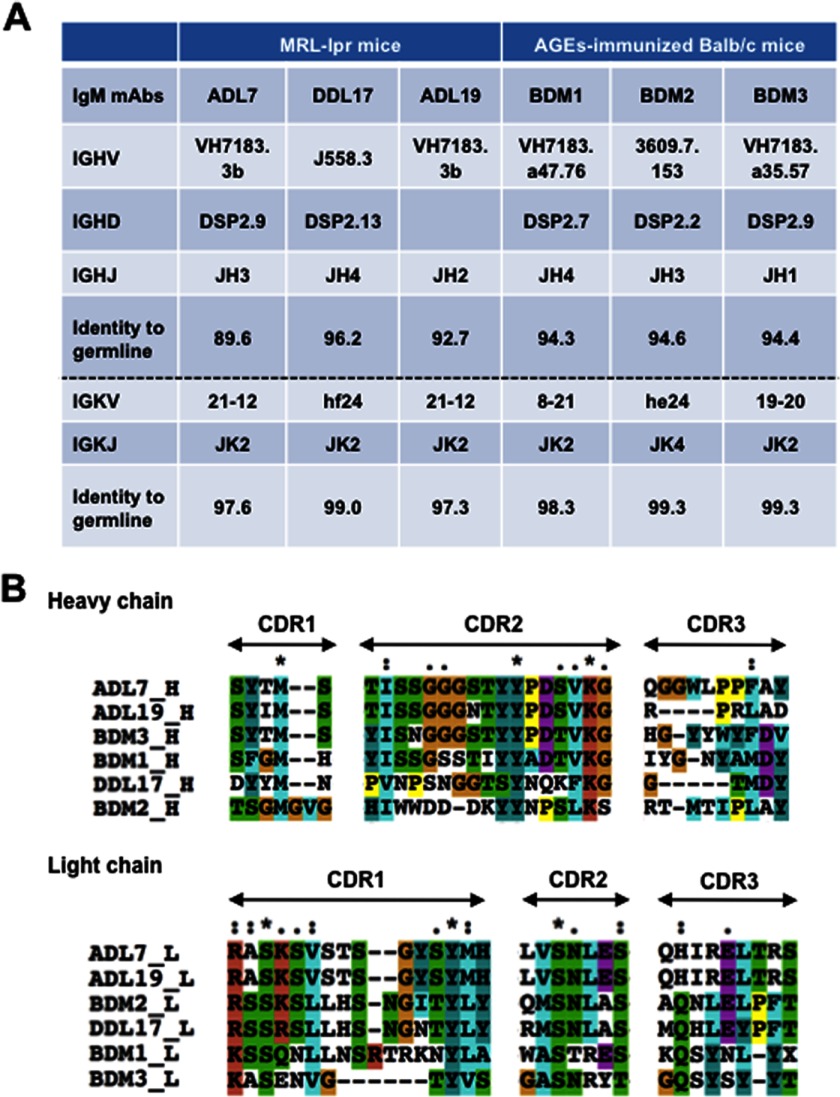
The VH and VL nucleotide sequences and the corresponding gene families were determined for the six anti-AGEs IgM mAbs (Fig. 5). V region gene usage and sequence alignment of the anti-AGEs mAbs are shown in A and B of Fig. 5, respectively. With the exception of the two IgM mAbs, clones DDL17 and BDM2, selected for the J558 and 3609 gene expressions, respectively, these alignments clearly showed preferential VH usage. Most notably, two IgM mAbs, ADL7 and ADL19, arising from the MRL-lpr mice and two IgM mAbs, BDM1 and BDM3, arising from the Balb/c mice immunized with the AGEs are all encoded by the VH7183 subfamilies. In addition, all of these IgM mAbs indeed showed a high nucleotide homology of 90–96% with the VH7183 subfamilies. The increased VH7183 family usage is known to be reminiscent of the signatures of natural Abs typically produced by B-1 cells (20–22). Thus, these IgM mAbs cloned from these mice might represent an expansion of the population of natural Abs ubiquitously expressed in healthy individuals.
FIGURE 5.
Sequence analysis of mAbs against AGEs. A, V region gene usage of the IgM mAbs directed against the AGEs. B, sequence alignment of the hypervariable regions of anti-AGEs mAbs. The completely conserved residues are indicated by an asterisk (*), and the essentially conserved ones are indicated by a period (.) or a colon (:). The sequences were aligned using program ClustalW (version 1.82) and manually modified. The residues are colored according to the standard ClustalX color scheme. The sequences have the following DDBJ accession numbers: ADL7_L, AB793279; DDL17_L, AB793506; ADL19_L, AB793508; ADL19_H, AB793509; BDM1_L, AB793510; BDM1_H, AB793511; BDM2_L, AB793512; BDM2_H, AB793513; BDM3_L, AB793514; and BDM3_H, AB793515.
Multispecificity of Anti-AGEs mAbs
Because IgM titers to the native dsDNA, in addition to the AGEs, were detected in the sera from the Balb/c mice (Fig. 1), the cross-reactivity of the anti-AGEs IgM mAbs toward the dsDNA were evaluated. As expected, the IgM mAbs, BDM1, BDM2, and BDM3, established from the Balb/c mice immunized with the AGEs, cross-reacted not only with the DHA-modified BSA but also with the dsDNA (Fig. 6A). In addition, the IgG mAbs, ADL13, DDL18, and DDL20, and the IgM mAbs, ADL7, ADL19, and DDL17, cloned from the SLE mice, also cross-reacted with both the AGEs and dsDNA (Fig. 6B). Thus, a subset of IgG and IgM mAbs, showing a specificity toward the AGEs, were found to be the multispecific mAbs that could recognize the dsDNA as an alternative antigen.
FIGURE 6.
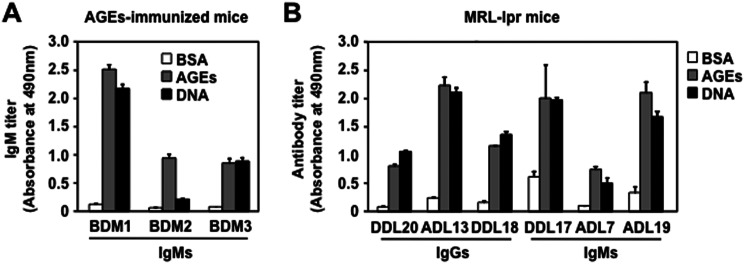
Multispecificity of the anti-AGEs mAbs. A, the immunoreactivity of anti-AGEs mAbs prepared from the Balb/c mice immunized with the DHA-modified KLH. Three hybridoma clones, BDM1, BDM2, and BDM3, producing IgM Abs specific for the AGEs were prepared from the mice. B, the immunoreactivity of the anti-AGEs mAbs prepared from MRL-lpr mice against the AGEs. Three hybridoma clones, ADL7, ADL19, and DDL17, producing the IgM Abs specific for the AGEs, and three hybridoma clones, ADL13, DDL18, and DDL20, producing IgG Abs specific for the AGEs, were prepared from the female MRL-lpr mice (20 weeks old). The IgM titers were determined by ELISA using dsDNA and native and DHA-modified proteins as the absorbed antigens.
Involvement of Electronegative Potential of Antigens in the Recognition by the Anti-AGEs mAbs
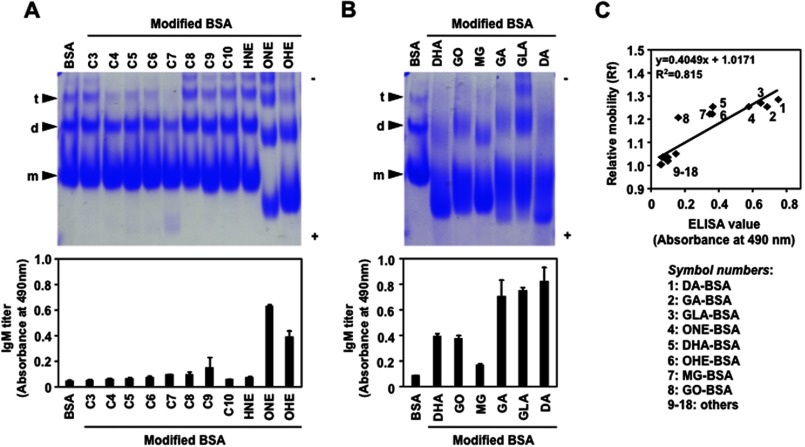
It is well known that the Abs to dsDNA often bind to other antigens in addition to dsDNA. The additional reactivities provide a view of antibody-antigen interactions that may partly depend on the structural similarity of the antigens (molecular mimicry). Thus, we speculated that the multispecificity of the Abs directed against the AGEs might arise through a molecular and/or immunologic mimicry among the antigens, including the dsDNA and AGEs. To gain an insight into this mechanism, we examined the effect of the DHA treatment on the mobility of the protein by native gel electrophoresis and observed an enhanced anodic mobility compared with the native protein (supplemental Fig. S5). The data implicate that DHA gave rise to the increased negative charge of the protein probably because of the modification of the ϵ-amino group of the lysine residues. The data also raised the possibility that the electronegative potential of antigens might be responsible for the recognition by the anti-AGEs mAbs. It has indeed been suggested that a large number of proteins, including the anti-DNA Abs, may recognize and be guided to their binding sites on the dsDNA helix through specific arginines reading the electronegative potential in the minor groove (23). Hence, using a variety of carbonyl compounds, we prepared several modified proteins and evaluated the correlation between the electronegative potential of the modified proteins and their cross-reactivity with the anti-AGEs mAbs. Among the carbonyl compounds tested, the lipid peroxidation products, such as 4-oxo-2-hexenal and 4-oxo-2-nonenal, and most of the glycolytic aldehydes, such as glyoxal, methylglyoxal, glyceraldehyde, glycolaldehyde, and dihydroxyacetone, gave rise to a significant anodic mobility shift in the protein on the native gel electrophoresis (Fig. 7, A and B), which was well correlated with the cross-reactivity of the Ab against the AGEs (Fig. 7C). Of interest, despite the fact that 2-alkenals (C3–C10) are strong electrophiles, which could readily react with lysine residues of proteins, a slight mobility shift of the protein bands was observed. The data suggest that the covalent binding of 2-alkenals hardly affect the charge on the protein.
FIGURE 7.
Electronegative potential of the aldehyde-modified proteins and their cross-reactivity with the anti-AGEs IgM mAb. The modified proteins were prepared by incubating BSA (1.0 mg/ml) with 1 mm lipid peroxidation-derived aldehydes in 1 ml of PBS (pH 7.4) at 37 °C for 24 h (A) or with 25 mm glycolytic aldehydes (B) in 5 ml of PBS (pH 7.4) at 37 °C for 7 days. The anti-AGEs IgM mAb BDM1 prepared from the Balb/c mice immunized with the DHA-modified KLH was used. Mobility shifts of modified proteins were examined in a nondenaturing polyacrylamide gel. The native BSA gave three bands, representing monomer (m), dimer (d), and trimer (t) of the protein. The IgM antibody titer was determined by ELISA using the DHA-modified BSA as the absorbed antigens. The aldehydes used were: C3, acrolein; C4, crotonaldehyde; C5, 2-pentenal; C6, 2-hexenal; C7, 2-heptenal; C8, 2-octenal; C9, 2-nonenal; C10, 2-decenal; HNE, 4-hydroxy-2-nonenal; ONE, 4-oxo-2-nonenal; OHE, 4-oxo-2-hexenal; DHA, dehydroascorbic acid; GO, glyoxal; MG, methylglyoxal; GA, glycolaldehyde; GLA, glyceraldehyde; DA, dihydroxyacetone. C, correlation between relative mobility of the modified proteins and their cross-reactivity with the anti-AGEs IgM. The relative mobility value was obtained by dividing the protein migration distance by the dye front distance in the gel electrophoresis test (A and B). The Ab titer was determined by ELISA using the DHA-modified BSA (AGEs) as the absorbed antigens.
To provide further insight on the involvement of the electronegative potential of the antigens in the recognition by the anti-AGEs Abs, we tested whether the sera from the SLE mice could recognize electronegative molecules, such as acetylated proteins. As expected, the ELISA analysis showed that the SLE sera cross-reacted with the acetylated BSA (Fig. 8A). In addition, the acetylated protein was recognized by the anti-AGEs mAbs IgM BDM1 (Fig. 8B). We also observed that the SLE sera and anti-AGEs IgM showed a remarkable specificity toward other electronegative molecules, including nucleic acids and phospholipids (supplemental Figs. S6 and S7).
FIGURE 8.
Involvement of electronegative potential of antigens in the recognition by the anti-AGEs mAbs. A, cross-reactivity of the sera from the MRL-MpJ and MRL-lpr mice with native BSA (open bar) and acetylated BSA (AcBSA, closed bar). B, cross-reactivity of the anti-AGEs IgM BDM1 established from the Balb/c mice immunized with the DHA-modified KLH with acetylated BSA. The antibody titers were determined by ELISA using the native BSA and DHA-modified and acetylated BSA as the absorbed antigens. C, flow cytometric analysis of antigen-antibody interaction. The solid phase paramagnetic beads coupled to the antigens, BSA (black line), AGEs (blue line), and acetylated BSA (red line), were used as the probes. The control mouse IgM (left panel) and representative anti-AGEs IgM BDM1 (right panel) were tested for binding to the protein-coupled beads. The number of binding events is plotted against fluorescence intensity.
To obtain more direct and convincing evidence for the recognition of the electronegative proteins by the anti-AGEs mAbs, we analyzed the antibody-antigen interaction by flow cytometry assays. The assay using the solid phase paramagnetic beads coupled to the antigens (BSA, AGEs, and acetylated BSA) as the probes showed that the binding of the normal IgM to these antigens was negligible, whereas the anti-AGEs mAb specifically recognized the AGEs and acetylated protein (Fig. 8C). These data support our hypothesis that the electronegative potential of the antigens might be involved, at least in part, in the recognition by the natural mAbs directed against the AGEs.
DISCUSSION
Natural Abs, predominantly IgMs, are produced at tightly regulated levels in the complete absence of external antigenic stimulation. They provide immediate, early, and broad protection against pathogens, making them a crucial nonredundant component of the humoral immune system (24, 25). They have also been shown to play an important function in the host response to the consequences of oxidative stress during oxidative events that occur when cells undergo apoptosis (reviewed in Ref. 26). These Abs are mainly produced by a subset of long-lived, self-replenishing B cells termed B-1 cells. It has been shown that B-1 cells can also respond to certain antigenic stimuli, e.g., 1,3-dextran, when presented on an appropriate carrier or in an appropriate immunization vehicle (reviewed in Ref. 27). In our current study, we detected prominent IgM titers not only to the dsDNA and oxidized LDL, but also to the AGEs in the sera of normal, conventionally housed mice (Fig. 1) and specific pathogen-free-maintained mice (supplemental Fig. S2). The apparent high prevalence of the AGEs as targets of natural Abs likely reflects the ubiquitous presence of these epitopes consequent to glycation events. The observation (Fig. 2, A and B) that the immunization of Balb/c mice with the AGEs in adjuvant strongly induced the IgM response, whereas virtually no IgG response was observed, suggests that the immunization of AGEs may not induce the typical B cell memory. Rather, the B-1 cells of the appropriate specificity may respond to the immunization of the AGEs by IgM production and limited isotype switching but do not produce high affinity Abs that might efficiently react with the self-antigens that selected these Abs into the B-1 repertoire. This hypothesis can be supported by the observations that the treatment of Balb/c mice with the AGEs intraperitoneally enriched B-1 cells in the peritoneal cavity after stimulation (Fig. 2, C–E). The mechanism(s) for elevation of the IgM titers in the Balb/c mice immunized with the AGEs is presently unknown. However, it may not be unlikely that the IgM Abs found associated with aging and oxidative stress represent an expansion of the population of these Abs ubiquitously expressed in normal healthy mice or, alternatively, represent the products of an antigen-selected B lymphocyte population. It has indeed been noted that the established B-1 cell clones can be expanded by antigen exposure, leading to the increased IgM levels in the plasma (28).
Based on the finding that the high IgM titers to the AGEs were observed in normal and SLE mice sera, we isolated several hybridoma clones, producing the Abs specific for the AGEs from the AGEs-immunized Balb/c and MRL-lpr mice and characterized their genetic and structural origin and specificity in detail. V gene analysis revealed several striking features of these anti-AGEs mAbs, most notably the frequent use of the VH7183 gene subfamilies by four IgM mAbs established from the AGEs-immunized Balb/c mice and disease-associated MRL-lpr mice (Fig. 5A). It is clear that immunization with the AGEs results in the expansion of the VH7183 encoded B cell pool, suggesting that the immune responses have a similar B cell origin. VH7183 is the smallest and most JH-proximal of the mouse VH families and is expressed in the early repertoire encoding for a wide range of polyreactive and autoreactive specificities (15). Thus, there is a very strong indication that the anti-AGEs specificity is preferentially encoded by VH7183, although it is also clear that the anti-AGEs IgM mAbs can on occasion be encoded by other VH gene families, such as J558 and 3609.
Of interest, most of the anti-AGEs IgG and IgM mAbs established from these SLE-prone mice cross-reacted with the dsDNA. In addition, similar multispecific IgM Abs were obtained from the Balb/c mice immunized with the AGEs. The six anti-AGEs IgM mAbs differ in both their heavy and light chain sequences (Fig. 5B). Therefore, the relative importance of the heavy and light chains in the actual binding to the antigens, such as AGEs and dsDNA, remains to be clarified. One possibility is that different VH and VL germ line genes are used to produce similar combining sites. A second possibility is that the different combinations result from the difference in the fine antigenic specificities of the Abs. It is also notable that the one IgM mAb BDM2 presented here differs in its specificity for dsDNA. The mAb possesses only low avidity to dsDNA, whereas other anti-AGEs IgM mAbs bound dsDNA with relatively high affinity. This may be associated with the observation that the amino acid sequence of the mAb BDM2 VH gene has limited identity (40–42%) with that of other mAb VH genes. It is therefore likely that a mutation in the mAb BDM2 may interfere with the recognition of dsDNA. Several different antigenic cross-reactivities have been identified for the anti-DNA Abs (29). These Abs share structural similarities with the Abs against bacterial polysaccharide, and some cross-react with the bacterial polysaccharide and protect mice against a lethal bacterial infection (30, 31). Other studies have also demonstrated a cross-reactivity of the anti-DNA Abs with microbial protein antigens, non-nucleic acid autoantigens, cell membranes and extracellular matrix components (32, 33). These cross-reactivities for an endogenous self- or neoself-antigen and an exogenous pathogen have also been described as a characteristic of natural Abs (34). A pattern of broad reactivity of a preformed pool of Abs has been suggested to be required for the rapid and immediate recognition and protection against invading pathogens. Recognition of multiple epitopes by the same Ab is of value to an organism, because this may result in an increase in the Ab repertoire and immune diversity without the need for additional lymphocytes expressing distinct Abs.
The present data suggest that the multispecificity of the natural Abs toward two or more distinct antigenic determinants could arise from antigens associated with the covalent modification of proteins by the reactive molecules that originated from the glycation and lipid peroxidation reactions. It is not unlikely that, because these reactive molecules are known to generate a variety of structures, some specific adducts generated in the modified proteins may be associated with the production of the natural Abs that simultaneously recognize other distinct molecules, such as dsDNA. However, such adducts commonly produced upon modification of protein by the reactive molecules that originated from the glycation and lipid peroxidation reactions are not known. In addition, we found that the anti-AGEs mAbs recognized a variety of electronegative molecules, including the acetylated proteins, nucleic acids, and phospholipids (Fig. 8 and supplemental Figs. S6 and S7). These data suggest an alternative mechanism in which the electronegative potential could be involved, at least in part, in the multiple cross-reactivity of the mAbs. This hypothesis may be supported by the mechanism for the cross-reactivity of the anti-DNA Abs, in which there are flexible phosphodiester polymers, such as RNA, teichoic acid, or other molecules, whose distribution of phosphates or similar negatively charged epitopes conform with the available contacts in the anti-dsDNA Ab combining site (35).
It has been shown that the natural IgM Abs could facilitate the removal of damaged cells in vivo. Chang et al. (36) demonstrated that the IgM Abs against the lipid peroxidation-modified proteins showed specific binding to the surface of apoptotic cells, but not to the surface of normal cells. They have also shown that the Abs inhibited the macrophage uptake of apoptotic cells, whereas the control IgM did not. Based on these data, they made a hypothesis that oxidized lipids and/or oxidized lipid-protein adducts on the surface of apoptotic cells, presumably derived from membrane peroxidation that occurs during apoptosis, could be ligands for the phagocytosis of apoptotic cells. We have also observed that the natural IgM Abs against the AGEs established from the AGEs-immunized Balb/c and MRL-lpr mice could bind the apoptosis-induced cells.3 Of interest, it has been suggested that, by altering the surface charge, the lipid changes that accompany apoptosis may cause redistribution of cationic proteins, potentially contributing to the execution of apoptotic cells. Indeed, the accumulation of anionic lipids in certain cellular membranes has been shown to serve to target and retain proteins with polycationic motifs (37). Thus, the binding of the Abs against the AGEs to the surface of apoptotic cells may also be associated with the increased electronegative potential of the cells.
The clearance of dying cells is one of the most essential responsibilities of the immune system, which is required to prevent uncontrolled inflammation and autoimmunity. In the murine immune system, natural IgM Abs that recognize apoptotic cells have been shown to enhance the phagocytic clearance of dead and dying cells and to suppress innate immune signaling pathways. In patients with SLE, the IgM auto-Abs, which bind to the neo-epitopes on apoptotic cells, have been demonstrated to be present at significantly higher levels in patients with a lower disease activity and with less severe organ damage (38). On the other hand, Witte (39) proposed a possible mechanism of protection by the IgM auto-Abs against the dsDNA, in which the IgM Abs may improve the clearance of pathogenic immune complexes containing IgG. Others have also suggested that the IgM Abs remove cellular debris and therefore may prevent the formation of IgG Abs (40). Indeed, in the absence of secreted IgM, normal mice spontaneously develop autoreactive IgG specific for the dsDNA (41). It has also been suggested that the IgM Abs have a protective role against the development of glomerulonephritis (42). In addition, in the SLE-prone MRL-lpr mice, the absence of secreted IgM accelerates the development of IgG auto-Abs and glomerulonephritis, and the mice succumb to the disease at an earlier age. These findings demonstrate that the secreted IgM can suppress the development of IgG auto-Abs and autoimmune disease under physiological conditions. Because autoantigenic epitopes would be generated even in healthy individuals, this IgM function is important for maintaining homeostasis. Thus, the high prevalence of the IgM Abs against the AGEs in the SLE-prone mice and SLE patients likely reflects the highest reactivity of DHA and its metabolites toward protein and the ubiquitous presence of such AGEs consequent to oxidative events.
In conclusion, the results of this study raised the possibility that the AGEs may be a ubiquitous target of natural Abs in both mice and humans. In addition, the apparent high prevalence of the AGEs as targets of the natural Abs likely reflects the ubiquitous presence of these epitopes consequent to glycation events. These findings offer an attractive hypothesis that the AGEs could be an important trigger of innate immunity and therefore contribute to the protection against certain exogenous invading pathogens and endogenous damage-associated molecules.
Acknowledgments
We thank Dr. Sohei Ito (University of Shizuoka) for the antibody sequence analysis and Dr. Takaaki Kojima (Nagoya University) for flow cytometric analysis. We also thank Yuki Hondo for excellent editorial support.
This work was supported by the project “Signaling Functions of Reactive Oxygen Species (ROS Signal),” Grants-in-Aid for Scientific Research on Innovative Areas (Research in a Proposed Research Area), Ministry of Education, Culture, Sports, Science, and Technology, Japan.

This article contains supplemental Figs. S1–S7.
M. Chikazawa and K. Uchida, unpublished data.
- AGE
- advanced glycation end product
- DHA
- dehydroascorbic acid
- SLE
- systemic lupus erythematosus
- Ab
- antibody
- KLH
- keyhole limpet hemocyanin.
REFERENCES
- 1. Monnier V. M., Sell D. R., Genuth S. (2005) Glycation products as markers and predictors of the progression of diabetic complications. Ann. N.Y. Acad. Sci. 1043, 567–581 [DOI] [PubMed] [Google Scholar]
- 2. Brownlee M., Cerami A., Vlassara H. (1988) Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 318, 1315–1321 [DOI] [PubMed] [Google Scholar]
- 3. Baynes J. W., Monnier V. M. (1989) Prog. Clin. Biol. Res. 304, 1–41 [PubMed] [Google Scholar]
- 4. Vlassara H. (2001) The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab. Res. Rev. 17, 436–443 [DOI] [PubMed] [Google Scholar]
- 5. Kislinger T., Fu C., Huber B., Qu W., Taguchi A., Du Yan S., Hofmann M., Yan S. F., Pischetsrieder M., Stern D., Schmidt A. M. (1999) Nϵ-(Carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 274, 31740–31749 [DOI] [PubMed] [Google Scholar]
- 6. Nemet I., Monnier V. M. (2011) Vitamin C degradation products and pathways in the human lens. J. Biol. Chem. 286, 37128–37136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunn J. A., Ahmed M. U., Murtiashaw M. H., Richardson J. M., Walla M. D., Thorpe S. R., Baynes J. W. (1990) Reaction of ascorbate with lysine and protein under autoxidizing conditions: formation of Nϵ-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry 29, 10964–10970 [DOI] [PubMed] [Google Scholar]
- 8. Nagaraj R. H., Sell D. R., Prabhakaram M., Ortwerth B. J., Monnier V. M. (1991) High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. U.S.A. 88, 10257–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tessier F., Obrenovich M., Monnier V. M. (1999) Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 274, 20796–20804 [DOI] [PubMed] [Google Scholar]
- 10. Witztum J. L., Steinbrecher U. P., Kesaniemi Y. A., Fisher M. (1984) Autoantibodies to glucosylated proteins in the plasma of patients with diabetes mellitus. Proc. Natl. Acad. Sci. U.S.A. 81, 3204–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shibayama R., Araki N., Nagai R., Horiuchi S. (1999) Autoantibody against Nϵ-(carboxymethyl)lysine. An advanced glycation end product of the Maillard reaction. Diabetes 48, 1842–1849 [DOI] [PubMed] [Google Scholar]
- 12. Engelbertsen D., Anand D. V., Fredrikson G. N., Hopkins D., Corder R., Shah P. K., Lahiri A., Nilsson J., Bengtsson E. (2012) High levels of IgM against methylglyoxal-modified apolipoprotein B100 are associated with less coronary artery calcification in patients with type 2 diabetes. J. Intern. Med. 271, 82–89 [DOI] [PubMed] [Google Scholar]
- 13. Shibata T., Shimozu Y., Wakita C., Shibata N., Kobayashi M., Machida S., Kato R., Itabe H., Zhu X., Sayre L. M., Uchida K. (2011) Lipid peroxidation modification of protein generates Nϵ-(4-oxononanoyl)lysine as a pro-inflammatory ligand. J. Biol. Chem. 286, 19943–19957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. (1976) Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 73, 3178–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antone S. M., Adderson E. E., Mertens N. M., Cunningham M. W. (1997) Molecular analysis of V gene sequences encoding cytotoxic anti-streptococcal/anti-myosin monoclonal antibody 36.2.2 that recognizes the heart cell surface protein laminin. J. Immunol. 159, 5422–5430 [PubMed] [Google Scholar]
- 16. Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Gapped BLAST and PSI-BLAST. A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wardemann H., Boehm T., Dear N., Carsetti R. (2002) B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayakawa K., Hardy R. R. (2000) Development and function of B-1 cells. Curr. Opin. Immunol. 12, 346–353 [DOI] [PubMed] [Google Scholar]
- 19. Kawasaki T., Kawai T., Akira S. (2011) Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol. Rev. 243, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diaw L., Magnac C., Pritsch O., Buckle M., Alzari P. M., Dighiero G. (1997) Structural and affinity studies of IgM polyreactive natural autoantibodies. J. Immunol. 158, 968–976 [PubMed] [Google Scholar]
- 21. Holmberg D. (1987) High connectivity, natural antibodies preferentially use 7183 and QUPC 52 VH families. Eur. J. Immunol. 17, 399–403 [DOI] [PubMed] [Google Scholar]
- 22. Pennell C. A., Arnold L. W., Haughton G., Clarke S. H. (1988) Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J. Immunol. 141, 2788–2796 [PubMed] [Google Scholar]
- 23. Lindemose S., Nielsen P. E., Hansen M., Møllegaard N. E. (2011) A DNA minor groove electronegative potential genome map based on photo-chemical probing. Nucleic Acids Res. 39, 6269–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller Y. I., Choi S. H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C. J., Que X., Montano E., Shaw P. X., Tsimikas S., Binder C. J., Witztum J. L. (2011) Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108, 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weismann D., Binder C. J. (2012) The innate immune response to products of phospholipid peroxidation. Biochim. Biophys. Acta 1818, 2465–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grönwall C., Vas J., Silverman G. J. (2012) Protective roles of natural IgM antibodies. Front. Immunol. 3, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baumgarth N. (2011) The double life of a B-1 cell. Self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46 [DOI] [PubMed] [Google Scholar]
- 28. Hartvigsen K., Chou M. Y., Hansen L. F., Shaw P. X., Tsimikas S., Binder C. J., Witztum J. L. (2009) The role of innate immunity in atherogenesis. J. Lipid Res. 50, S388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spatz L., Iliev A., Saenko V., Jones L., Irigoyen M., Manheimer-Lory A., Gaynor B., Putterman C., Bynoe M., Kowal C., Kuo P., Newman J., Diamond B. (1997) Studies on the structure, regulation, and pathogenic potential of anti-dsDNA antibodies. Methods 11, 70–78 [DOI] [PubMed] [Google Scholar]
- 30. Kowal C., Weinstein A., Diamond B. (1999) Molecular mimicry between bacterial and self antigen in a patient with systemic lupus erythematosus. Eur. J. Immunol. 29, 1901–1911 [DOI] [PubMed] [Google Scholar]
- 31. Limpanasithikul W., Ray S., Diamond B. (1995) Cross-reactive antibodies have both protective and pathogenic potential. J. Immunol. 155, 967–973 [PubMed] [Google Scholar]
- 32. Chan T. M., Leung J. K., Ho S. K., Yung S. (2002) Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus. J. Am. Soc. Nephrol. 13, 1219–1229 [DOI] [PubMed] [Google Scholar]
- 33. Jacob L., Lety M. A., Choquette D., Viard J. P., Jacob F., Louvard D., Bach J. F. (1987) Presence of antibodies against a cell-surface protein, cross-reactive with DNA, in systemic lupus erythematosus. A marker of the disease. Proc. Natl. Acad. Sci. U.S.A. 84, 2956–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson K. M., Sutherland J., Barden G., Melamed M. D., Wright M. G., Bailey S., Thorpe S. J. (1992) Human monoclonal antibodies specific for blood group antigens demonstrate multispecific properties characteristic of natural autoantibodies. Immunology 76, 146–157 [PMC free article] [PubMed] [Google Scholar]
- 35. Radic M. Z., Weigert M. (1994) Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu. Rev. Immunol. 12, 487–520 [DOI] [PubMed] [Google Scholar]
- 36. Chang M. K., Bergmark C., Laurila A., Hörkkö S., Han K. H., Friedman P., Dennis E. A., Witztum J. L. (1999) Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages. Evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. U.S.A. 96, 6353–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeung T, Terebiznik M, Yu L, Silvius J, Abidi W. M., Philips M, Levine T, Kapus A, Grinstein S. (2006) Receptor activation alters inner surface potential during phagocytosis. Science 313, 347–351 [DOI] [PubMed] [Google Scholar]
- 38. Grönwall C., Akhter E., Oh C., Burlingame R. W., Petri M., Silverman G. J. (2012) IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin. Immunol. 142, 390–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Witte T. (2008) IgM antibodies against dsDNA in SLE. Clin. Rev. Allergy Immunol. 34, 345–347 [DOI] [PubMed] [Google Scholar]
- 40. Hahn B. H. (1998) Antibodies to DNA. N. Engl. J. Med. 338, 1359–1368 [DOI] [PubMed] [Google Scholar]
- 41. Boes M., Schmidt T., Linkemann K., Beaudette B. C., Marshak-Rothstein A., Chen J. (2000) Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc. Natl. Acad. Sci. U.S.A. 97, 1184–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shoenfeld Y., Toubi E. (2005) Protective autoantibodies. Role in homeostasis, clinical importance, and therapeutic potential. Arthritis Rheum. 52, 2599–2606 [DOI] [PubMed] [Google Scholar]