VOLUME 288 (2013) PAGES 1047–1054
PAGE 1050:
The proton translocation rate of Fig. 3 was monitored at A457 (y axis) and not A556 as shown in the original figure.
PAGE 1053:
Fig. 7 should be modified as shown below.
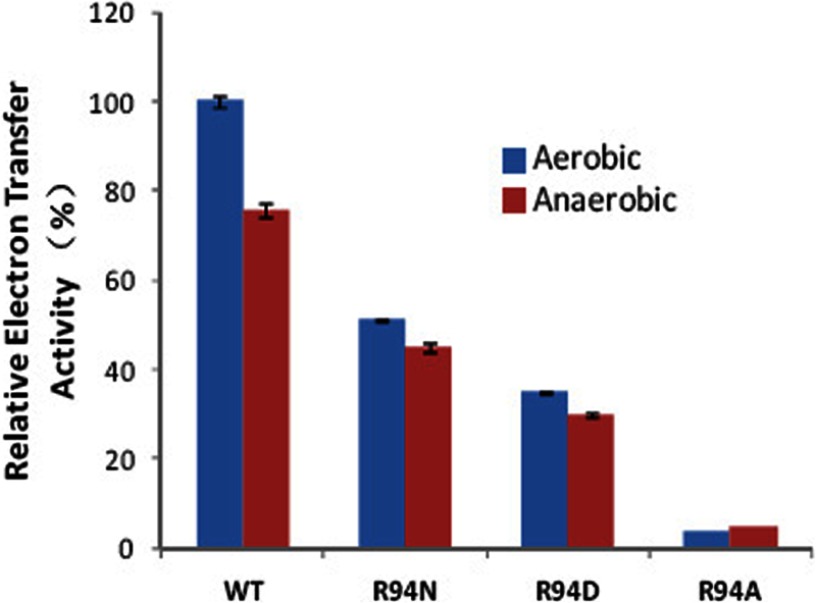
FIGURE 7.
Electron transfer activity changes in the wild-type and mutant bc1 complexes under aerobic or anaerobic conditions. The activity of wild-type bc1 under the aerobic conditions is referenced as 100%. Data represent an average of three experiments. Error bars indicate S.D.
Fig. 8 should be modified as shown below.
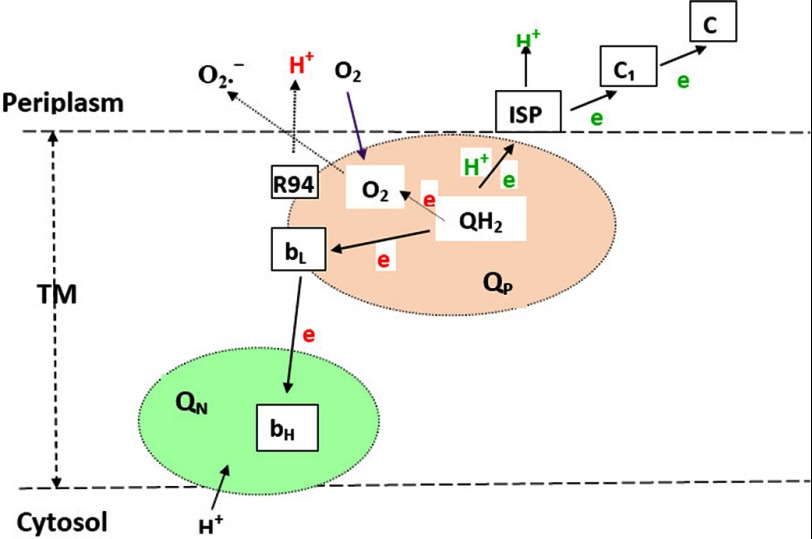
FIGURE 8.
Schematic depiction of the transfer of the two electrons and two protons of a ubiquinol at the QP pocket when oxidized in the bc1 complex. The first electron and proton are in green, and the second electron and proton are in red. The purple arrow indicates oxygen molecules that are diffused to the QP pocket. Dashed arrows indicate plausible electron, proton, and superoxide transfer routes. QP and QN denote quinol oxidation and the quinone reduction pocket. QH2, ubiquinol; TM, transmembrane region; ISP, Rieske iron-sulfur protein.