Abstract
Energy metabolism is a highly coordinated process, and preferred fuel(s) differ among tissues. The hierarchy of substrate use can be affected by physiological status and environmental factors including high ambient temperature. Unabated heat eventually overwhelms homeothermic mechanisms resulting in heat stress, which compromises animal health, farm animal production, and human performance. Various aspects of heat stress physiology have been extensively studied, yet a clear understanding of the metabolic changes occurring at the cellular, tissue, and whole-body levels in response to an environmental heat load remains ill-defined. For reasons not yet clarified, circulating nonesterified fatty acid levels are reduced during heat stress, even in the presence of elevated stress hormones (epinephrine, glucagon, and cortisol), and heat-stressed animals often have a blunted lipolytic response to catabolic signals. Either directly because of or in coordination with this, animals experiencing environmental hyperthermia exhibit a shift toward carbohydrate use. These metabolic alterations occur coincident with increased circulating basal and stimulated plasma insulin concentrations. Limited data indicate that proper insulin action is necessary to effectively mount a response to heat stress and minimize heat-induced damage. Consistent with this idea, nutritional interventions targeting increased insulin action may improve tolerance and productivity during heat stress. Further research is warranted to uncover the effects of heat on parameters associated with energy metabolism so that more appropriate and effective treatment methodologies can be designed.
Introduction
Summer temperatures have been increasing worldwide, and some predict this trend will continue (1, 2). Average mean temperatures in North America are projected to increase 1.8°C to 4°C during this century (1). Regions of the United States that typically experience heat waves are expected to have a dramatic increase in the magnitude of these summer events. For example, in California, the number of heat wave days is expected to double and the “heat wave season” to lengthen an estimated 20% (3). As heat waves become more frequent and longer in duration, heat-related morbidity and/or mortality are also likely to increase (4, 5).
Heat claims the lives of more Americans annually than hurricanes, lightning, tornadoes, and floods combined (6). An estimated 1500 heat-related deaths are reported each summer (7), and the CDC (8) suggests that this is underestimated by at least 50%. Heat waves in Chicago during 1995 (9, 10) and 1999 (11) claimed >600 and nearly 100 lives, respectively. Worldwide, the numbers are even more staggering. In a 2003 heat wave that lasted ∼2 wk, an estimated 50,000 Europeans died (12, 13), 15,000 of those in France alone (14, 15). Increased body temperature disturbs biological systems, and this ranges from heat edema to heat stroke [heat stroke being the least common but most severe (16)]. The onset of heat-related illnesses can arise due to exposure to increased ambient temperatures (classic heat stress) or as a result of exercise [exertional heat stress (17)]. Diagnosing heat-related illness can be difficult because symptoms vary depending on the extent and magnitude of exposure and great individual variability to similar heat loads (18). Although many aspects of heat stress have been extensively researched, relatively little is known about the metabolic and biochemical changes that occur during heat exposure. In particular, it is unknown why certain populations are less tolerant to heat than others. Diabetic individuals, for example, have a higher risk of experiencing a heat-related illness than those who are non-diabetic (19), and death rates among those with diabetes increases significantly during the summer months (20). A review by Schuman (21) noted that during heat waves, diabetic individuals had the greatest increase (of all major chronic diseases) in death rates, although the reason for this increased susceptibility remains ill-defined.
In agriculturally relevant species, heat stress negatively affects many production parameters including milk yield and composition, growth, reproduction, and carcass traits. In addition, a heat load increases health care costs, and, depending on the severity, animals can die of severe thermal stress (especially lactating cows and animals without shade). A 2006 California heat wave purportedly resulted in the death of >30,000 dairy cows (22), and a recent heat wave in Iowa killed at least 4000 head of beef cattle (23). Furthermore, almost 50% of Canadian summer days are considered environmentally stressful to dairy cows (24). This illustrates that most geographic locales, including temperate and northern climates, can be susceptible to extreme and lethal heat. Therefore, environmental heat stress is an animal welfare issue and a financial burden to agri-industries [~$900 million/y for dairy and >$300 million/y in beef and swine in the US alone (25, 26)]. Improvements in farming infrastructure [i.e., cooling systems, barn construction (27, 28)] have alleviated some negative effects of thermal stress on animal agriculture, but production still decreases during the summer. Consequently, heat stress is one of the costliest issues facing progressive animal producers and certainly one of the primary constraints to efficient and profitable animal agriculture in developing countries (29).
Current status of knowledge
Cellular heat stress response
Exposure to increased ambient temperatures can result in significant alterations and damage at the cellular level. Many intracellular molecular structures rely on a variety of relatively weak interactions for stabilization, and these interactions are easily disrupted by changes in the microenvironment [i.e., increases or decreases in temperature and pH (30)]. Heat can negatively affect cell components directly, such as unfolding and subsequent aggregation of proteins (31–33). Protein synthesis appears to be particularly impaired by heat. For example, Mondovi et al. (34) and Henle and Leeper (35) noted that DNA, RNA, and protein synthesis was rapidly inhibited by heat treatment, with the reduction being greatest for protein synthesis followed by DNA and finally RNA synthesis (34, 35). Furthermore, reductions in protein, DNA, and RNA synthesis after heat exposure can be rapid and occur within 10 min at 42°C in HeLa and CHO (Chinese hamster ovary) cells (36). Protein synthesis is affected to the greatest extent but recovers the most quickly, whereas resumption of DNA synthesis requires an extended period of time (35).
The cellular response to a heat load includes activation of transcription factors such as heat shock factors (HSFs)6 (37–39), expression of proteins associated with acute homeostatic response such as heat shock proteins (HSPs), and altered gene expression [reviewed by Collier et al. (40)]. This coordinated response leads to changes in the expression of proteins necessary for restoring cellular function and directing cellular remodeling, such as regulatory proteins, cell-cycle control proteins, and structural proteins, as well as those determining cell fate, including proteins involved in pro- and antiapoptotic pathways (37–39, 41). Although multiple HSF isoforms exist, HSF1 is the central transcription factor involved in the heat shock response (42, 43) because mice lacking HSF1 are unable to mount a heat shock defense (44). HSF1 is activated in response to various stressors, including heat, oxidative stress, and non-native protein synthesis (45–47). HSF2 is primarily transcribed in response to inhibition of proteasome activity and thus complements the response of HSF1 to an increase in misfolded proteins (48).
Heat shock proteins.
Of particular importance to cell survival during hyperthermia are HSPs. Members of this protein family are ubiquitously expressed across species and are present at low levels in cells under normal conditions, but their levels increase greatly, but transiently, on a cellular insult (49–52). Classically known as molecular chaperones, HSPs bind to unfolded or misfolded proteins and help restore their native conformation (53–55). HSPs are grouped based on their molecular weight and amino acid sequence (56, 57) as well as by structure and function (58). Major HSPs in mammalian cells include HSP110, 90, 70, 60, and 27 (59), each having separate functions and cellular locations (56). Of these, HSP70 plays a heightened role in cryoprotection (60) and is frequently used as a biomarker of cellular stress. Expression levels of HSP70 are most closely indicative of the magnitude and duration of thermal stress (61). Ultimately, if damage from heat exposure is not/cannot be repaired (such as that caused by mitotic catastrophe), cell death via heat-induced apoptosis will occur (33). Intriguing work indicates that circulating HSP70 concentrations also increase during heat stress (62, 63), demonstrating a need to further investigate the origins and functions of extracellular HSP70.
In addition to their role in protecting cells from heat-induced protein misfolding, HSPs may enhance insulin function. Obesity increases activation of stress kinases, which then increase serine phosphorylation of the insulin receptor substrate 1 (IRS-1), and this ultimately reduces the functionality of the insulin signaling pathway (64–66). Expression of HSPs is reduced in obese patients, and heat therapy (e.g., saunas, spas) improves insulin function due to the ability of HSPs to reduce serine phosphorylation of IRS-1 through reducing stress kinase activation (67, 68). Taking this further, pharmacologically inducing HSP70 improves insulin sensitivity (62). Together, these studies demonstrate that HSPs are important for proper insulin function and suggest that strategically manipulating the insulin-HSP axis may improve human and animal health and productivity during heat stress.
Reactive oxygen species.
Heat stress likely increases and/or induces oxidative stress, possibly leading to mitochondrial damage, which is the primary intracellular source of reactive oxygen species (ROS). Damaged or malfunctioning mitochondria, due to the effects of heat directly or by increased oxidative stress, may be a contributing factor to alterations in metabolism during heat stress. It is widely accepted that mitochondrial respiration is the primary source of ROS (69, 70), with 0.2% of oxygen consumed converted to superoxide in the normal state (71). Levels of ROS are determined by production and ROS degradation rates and/or inactivation, and an appropriate balance is important for maintaining cellular homeostasis (72). Generation of ROS, such as superoxide, can damage proteins, DNA, and lipids (73, 74) and decrease mitochondrial function (75). Such oxidative damage results when a cell accumulates excessive amounts of ROS, a condition that occurs when ROS production exceeds cellular defenses. Detoxifying ROS occurs by several nonenzymatic antioxidants, including ascorbic acid and glutathione, in addition to ROS-scavenging enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and peroxiredoxin (76–78). Antioxidants are maintained at relatively low levels, but increase quickly and dramatically in response to increased ROS production. Cooperation among enzymes is common in ROS detoxification. For example, superoxide dismutase converts superoxide radicals into hydrogen peroxide and oxygen; catalase and peroxidases then convert the hydrogen peroxide to water (79, 80).
Heat stress increases ROS production, and there are many similarities in the pattern of gene expression of heat-stressed and oxidative-stressed cells (81, 82). Levels of ROS-scavenging enzymes were increased (transcript and protein levels) by heat stress (83–85). Rat intestinal epithelial cells exposed to severe and acute heat stress had increased ROS flux (86), and increased superoxide formation was detected in heat-stressed mouse diaphragm muscle (87). Studies by Mujahid et al. (88, 89) revealed an increase in mitochondrial superoxide generation and oxidative damage to mitochondrial proteins and lipids in the skeletal muscle of heat-stressed chickens. Similarly, heat stress causes oxidative stress in transition dairy cows (90). Acute heat-stressed broiler chickens had a 2-fold increase in malondialdehyde, a marker of lipid peroxidation, in skeletal muscle (91). In mitochondria of rat cardiomyocytes, oxidative metabolism was reduced, thereby significantly depleting ATP content (92) and uncoupling of oxidative phosphorylation leading to an increase in ROS production and oxidative stress. Superoxide production can be reduced by uncoupling of the electric transport chain by uncoupling proteins or adenine nucleotide translocator, however, in heat stress conditions, uncoupling proteins are downregulated (89), which is probably a protective mechanism to prevent further heat production. ROS are produced during normal aerobic metabolism; therefore, the increased energy demands imposed by elevated ambient temperatures may also contribute to ROS generation and oxidative stress.
Systemic heat stress response
The physiological basis by which heat stress affects production and performance in farm animals includes contributions from reduced feed intake, an altered endocrine status, metabolic shifts at the systemic and cellular levels, and changes in body composition. For information about the impact of heat stress on nutrient intake and the endocrine profile, the reader is referred to reviews by Collier et al. (93), West (94), and Bernabucci et al. (95). Here, the focus centers on heat-induced postabsorptive metabolic alterations.
Production and observational data suggest that heat stress may alter metabolism differently than would be expected based on calculated whole-body energy balance. For example, heat-stressed sows (96) and heifers (97) do not lose as much body weight and body condition, respectively, as do their pair-fed thermoneutral counterparts. In addition, pigs reared in heat-stress conditions have reduced muscle mass and increased adipose tissue, and this has been documented frequently in the past 40 y (98, 99). This phenomenon is not unique to pigs because heat stress also alters carcass composition similarly in rodents (100, 101) and growing poultry (102–105). It is counterintuitive that heat stress causes a decrease in nutrient intake and depresses growth but increases carcass lipid accretion and decreases carcass nitrogen content. In thermoneutral conditions, animals consuming a restricted diet will deposit protein at the expense of lipid accretion (i.e., the carcass lipid-to-protein ratio decreases, meaning that the carcass becomes leaner) and the quantity of lipid deposited per unit of energy consumed decreases (106–108). Hence, the reduced feed intake–to–body composition relationship is exactly the opposite in animals reared in heat-stress conditions compared with thermoneutral animals and is independent of the plane of nutrition.
We recently demonstrated that despite reduced feed intake and loss of body weight, heat-stressed cows and pigs do not have an increase in plasma nonesterified fatty acids (NEFAs) (109–111), and this agrees with other heat-stress models (97, 112, 113). The lack of an elevated NEFA response to body weight loss is especially surprising because acute heat stress causes a marked increase in circulating cortisol, norepinephrine and epinephrine levels (93), catabolic signals that normally stimulate lipolysis, and adipose mobilization. This is also surprising because calculated energy balance is traditionally thought to be closely associated with circulating NEFA levels (114). Furthermore, we recently demonstrated that heat-stressed cows have a blunted (compared with pair-fed thermoneutral controls) NEFA response to an epinephrine challenge (115). These observations agree with rodent results indicating that heat stress reduces in vivo lipolytic rates and in vitro lipolytic enzyme activity (116). The fact that heat-stressed animals fail to enlist this “shift” in postabsorptive energetic metabolism (despite inadequate nutrient intake) may indicate that heat stress directly (not mediated by feed intake) affects fuel selection and overall energetics.
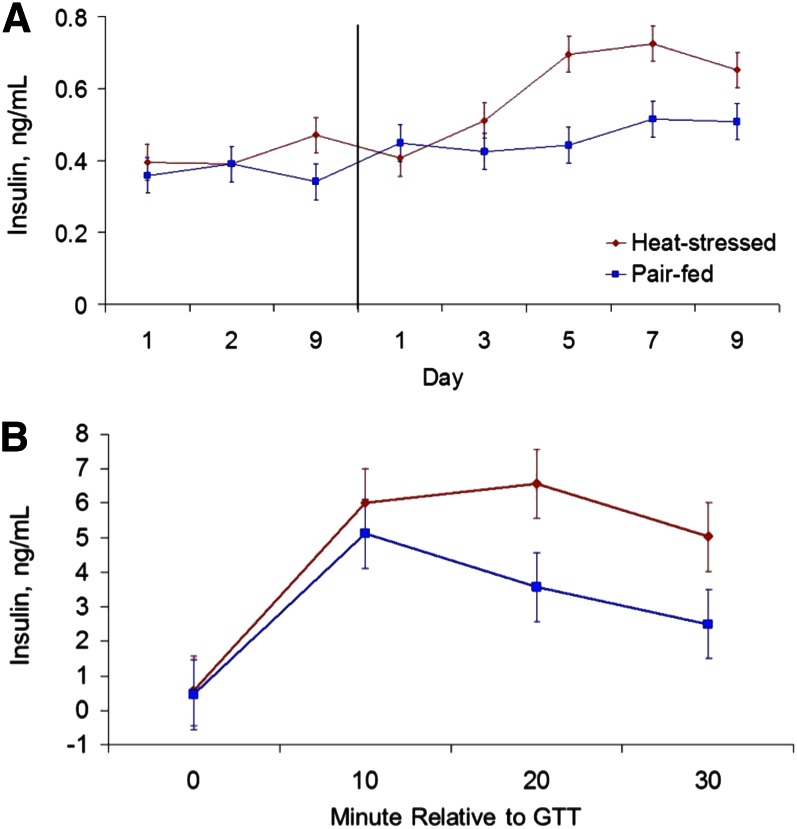
The aforementioned changes in lipid metabolism variables may be the result of increased insulin levels and/or enhanced insulin sensitivity because insulin is a potent lipogenic and antilipolytic hormone (117). In fact, despite the marked reductions in dry matter intake, heat stress increases insulin sensitivity in rodents [see review by DeSouza and Meier (118)], and basal insulin levels in rodents (116), heat-stressed pigs (111), a malignant hyperthermia porcine model (119), growing steers (120), and lactating cows (121, 122). In addition, heat-stressed sheep (123), growing cattle (120), and lactating cows (122) have an increased insulin response to a glucose tolerance test (Fig. 1). The increase in circulating insulin levels appears due to increased pancreas secretion rather than reduced circulating insulin removal because of the acute marked difference in insulin levels between heat-stressed and thermoneutral pair-fed animals after administration of an insulin secretagogue (29).
Figure 1.
(A) Effects of heat stress and pair-feeding on basal insulin concentrations in growing Holstein bull calves. The vertical line separates period 1 (thermoneutral conditions and ad libitum feed intake) from period 2 (either heat-stress conditions and ad libitum feed intake or thermoneutral conditions and pair-fed). (B) Effects of heat stress (ad libitum feed intake) and pair-feeding (thermoneutral conditions) on the insulin response to a glucose tolerance test (GTT) in growing Holstein bull calves. Conversion factor for insulin is 1 μg/L = 172.5 pmol/L. Adapted from Reference 120 with permission.
Due to the apparent lack of NEFA availability as a fuel substrate, it appears that heat-stressed animals increase both their production of, and reliance on, glucose as a fuel. For example, heat-stressed human athletes have increased endogenous glucose production and whole-body enhanced carbohydrate oxidation at the expense of lipids (124–126). In addition, endogenous glucose production typically decreases after ingesting carbohydrates, but exogenous sugars are unable to blunt the heat stress–induced liver glucose output (127). The increased hepatic glucose output during heat stress originates from both increased glycogenolysis (125) and increased gluconeogenesis (128). The increased liver glucose output during heat stress may in part be mediated by upregulated hepatic pyruvate carboxylase gene expression, a rate-limiting enzyme controlling lactate and alanine entry into the gluconeogenic pathway (129–131). This is supported by flux data indicating that lactate’s contribution to gluconeogenesis increases in hyperthermic rodents (128). Furthermore, heat-stressed chicks have increased intestinal sodium-dependent glucose transporter I activity and thus glucose absorption (132). Cell culture experiments demonstrate heat-induced activation of a high-affinity sodium-dependent glucose transporter and enhancement of sodium-glucose cotransport capacity during a thermal load (133, 134). We have also demonstrated that a larger percentage of hepatically derived glucose is used for non–milk-synthesizing purposes in heat-stressed cows, and this suggests an increase in glucose use as a fuel (115). Further, we have shown using stable isotopes that whole-body (presumed to be primarily from hepatic tissue) glucose production and the glucose response to an epinephrine challenge (used as a proxy for hepatic glycogenolytic sensitivity) does not differ between heat-stressed and pair-fed thermoneutral controls (115) despite reports suggesting that the liver becomes partially dysfunctional during heat stress (135, 136). Collectively, evidence from a variety of species suggests that hepatic carbohydrate metabolism is not compromised by heat stress, and presumably many of these changes occur to maximize glucose availability when the metabolic option of using adipose tissue–derived energy has been prevented.
Skeletal muscle is mobilized during periods of inadequate nutrient intake (in thermoneutral conditions) or because of muscular damage and/or disease. We have demonstrated that heat-stressed pigs (111), cows (110), and heifers (97) have increased plasma urea nitrogen levels compared with thermoneutral controls. Plasma urea nitrogen can sometimes be difficult to interpret because it originates from at least 2 sources (depending on species): inefficient rumen ammonia incorporation into microbial proteins or from hepatic deamination of amino acids mobilized from skeletal muscle. A better circulating indicator of muscle catabolism is either 3-methyl-histidine or creatine, both of which are increased in heat-stressed poultry (103), rabbits (137), pigs (111), and lactating cows (138). Additional evidence indicating that heat stress directly affects protein metabolism is decreased milk protein levels from heat-stressed cows (109, 110), and it appears that α and β casein synthesis is most susceptible (139). The reduction in muscle and mammary protein synthesis during heat stress is perplexing because an elevation in plasma insulin, typically observed during heat stress (Fig. 1), would reduce proteolysis and stimulate tissue protein synthesis (assuming adequate amino acid supply) during normal physiological states.
Although the reasons for increased insulin action and circulating concentrations during heat stress remain unclear, the increase in insulin is critical for its role in activating the cellular stress response (140). In addition to altered plasma insulin levels, heat stress markedly increases the insulin receptor expression in pig reproductive tissues (141). As stated previously, diabetic humans are more susceptible to heat-related illness and death (9, 21). Similarly, diabetic rats have an increased mortality rate when exposed to severe heat, and exogenous insulin increases their survival time (142). Furthermore, nonlethal heat stress ameliorates proxies of insulin insensitivity in diabetic rodents (143, 144) or rodents fed high-fat diets (145). This is similar to reports indicating that thermal therapy (saunas and hot baths) improves insulin sensitivity in humans (146). Insulin’s role in thermal stress adaptation appears to be an ancient mechanism preserved during evolution as even heat-stressed simple eukaryotes increase both insulin synthesis and insulin binding (147). Regardless of why, heat stress is one of the very few nondiabetic models of which we are aware in which nutrient intake is markedly reduced, but basal and stimulated insulin levels are increased. Hence, there is growing evidence to suggest that insulin action is a key component of the heat-stress response, and this may explain the observed phenotypic changes (increased carcass lipid) in farm animals during the summer.
Nutritional supplementation during heat stress
Because of the obvious health implications to humans and farm animals, there is a growing interest in strategically altering the diet in an attempt to improve the response to heat stress. A thorough description of agricultural dietary strategies is not within the scope of this article, and the reader is referred to other reviews (148–151). This section concentrates on dietary manipulation of systemic insulin sensitivity because it is clear that proper insulin action is one of the key components of successfully adapting to and surviving a heat load. Therefore, supplementing diet ingredients or pharmaceuticals that enhance insulin sensitivity may be an effective tactic to improve the likelihood of surviving an otherwise lethal heat load.
Lipoic acid.
Lipoic acid is synthesized from octanoic acid by the mitochondria (152–154), and it serves as a cofactor of mitochondrial enzymes that perform oxidative decarboxylation (155). Lipoic acid and its reduced form, dihydrolipoic acid, scavenge ROS and nitrogen species (156, 157) and enhance cellular glucose uptake due to their insulin mimetic action (158). Oral lipoic acid supplementation in poultry decreased plasma glucose and increased whole-body insulin sensitivity while increasing plasma triglycerides and adipose tissue lipolysis (159, 160). However, the effectiveness of lipoic acid supplementation to alter glucose availability may be dependent on the magnitude and duration of heat-stress events (161). Similar to chickens, oral lipoic acid supplementation reduced plasma glucose concentrations in young swine maintained in thermoneutral conditions (162). It appears that lipoic acid enhances insulin action in thermoneutral animals, and, thus, the ability of lipoic acid to promote thermal tolerance during chronic heat-stress conditions is of obvious interest. In horses, lipoic acid supplementation reduces blood lactate concentrations during exercise and increases citrate synthase activity before and after exercise (163), indicating that lipoic acid enhances oxidative metabolism. In addition, lipoic acid supplementation reduced exercise-induced increases in plasma amino aspartate transferase and creatine kinase, indicating that it may reduce muscle damage (164). Thus, dietary supplementation with either lipoic or dihydrolipoic acid may improve heat tolerance and animal performance during heat stress by enhancing insulin action.
Chromium.
Chromium is a micronutrient that facilitates insulin action on glucose, lipid, and protein metabolism (165). Little is known about actual dietary chromium requirements, and it is possible that in situations in which dietary chromium improves insulin function, the supplemental amount is actually replenishing a deficiency in the diet. Heifers supplemented with increasing amounts of chromium had increased insulin sensitivity (166), suggesting that chromium plays an essential role in glucose metabolism in ruminants. Because glucose use predominates during heat stress, chromium supplementation may improve thermal tolerance or production in heat-stressed animals. For instance, supplementing heat-stressed early-lactation dairy cows with chromium reduced the degree of weight loss, improved milk production, reduced plasma NEFA concentrations, and improved rebreeding rates (167, 168). In addition, broilers supplemented with dietary chromium and raised in heat-stress conditions had increased feed consumption, body weight gain, and carcass composition traits compared with nonsupplemented birds (169, 170). Limited research has been done on other species, but in heat-stressed swine, chromium supplementation did not improve the average daily gain or plasma glucose (171). Further research using varying concentrations and lengths of supplementation should be done to determine the ability of chromium to alleviate the deleterious effects of heat stress.
Thiazolidinediones.
Thiazolidinediones (TZDs) are a family of drugs that improve insulin sensitivity and are used to treat diabetes. TZDs are synthetic agonists of peroxisome PPAR-γ, an intracellular receptor and transcription factor that upregulates genes involved in cellular glucose uptake (172–175). Because of the improved insulin action, TZDs could be useful for improving and ensuring glucose use and upregulating HSP in heat-stress conditions. Further, TZDs improve heat tolerance in diabetic individuals (176, 177) and improves heat-induced HSP72 expression in cardiac muscle of obese diabetic rats (178). In thermoneutral conditions, TZD improves insulin responsiveness of dairy cows (179) and glucose and lipid transporters and insulin receptor gene expression in skeletal muscle of horses (180). If TZDs can enhance HSP production and improve glucose use, it could be a useful strategy during heat stress.
Conclusions and future directions
High ambient temperatures have a negative effect on animal and human health and performance. Heat waves can be lethal to humans and animals, and heat stress is responsible for billions of dollars in losses to global animal agriculture. Consequently, from a human medicine and agricultural perspective, research targeting the identification and implementation of strategies to improve welfare and performance is essential.
Nutrition is a potential avenue to aid human and animal adaptation in hot climates. For instance, heat-stressed animals exposed shift energy metabolism toward carbohydrate use and reduce lipid oxidation. Additionally, it is clear that those with diabetes are at a higher risk of experiencing a heat illness than those without diabetes, and this suggests that adequate insulin action may be essential for adapting to and surviving heat stress. Therefore, diets or nutritional supplements promoting glucose use may be beneficial. There are some indicators that insulin sensitivity is increased by heat stress because HSP expression is increased during heat stress, and these proteins improve insulin function by reducing IRS-1 serine phosphorylation. In fact, therapeutic heat stress increases insulin sensitivity in many species. However, the degree to which insulin action needs augmenting to maintain productivity in heat-stressed conditions remains unknown.
Several nutritional supplements and pharmaceuticals improve insulin action. These include lipoic acid, chromium, and TZDs. Both lipoic acid and chromium improve glucose metabolism in several species during thermoneutral conditions; however, only chromium has been shown to improve production parameters of heat-stressed animals. Similarly, although TZDs improve glucose metabolism in animals exposed to thermoneutral conditions, little is known about its potential to improve production in heat-stressed animals.
Acknowledgment
All authors have read and approved the final manuscript.
Footnotes
Abbreviations used: HSF, heat shock factor; HSP, heat shock protein; IRS-1, insulin receptor substrate 1; NEFA, nonesterified fatty acid; ROS, reactive oxygen species; TZD, thiazolidinedione.
Literature Cited
- 1.IPCC. The intergovernmental panel on climate change 4th assessment report. Available from: http://www.ipcc.ch/. Accessed May 12, 2011. 2007.
- 2.Luber G, McGeehin M. Climate change and extreme heat events. Am J Prev Med. 2008;35:429–35 [DOI] [PubMed] [Google Scholar]
- 3.Hayhoe K, Cayan D, Field CB, Frumhoff PC, Maurer EP, Miller NL, Moser SC, Schneider SH, Cahill KN, Cleland EE, et al. Emissions pathways, climate change, and impacts on California. Proc Natl Acad Sci U S A. 2004;101:12422–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305:994–7 [DOI] [PubMed] [Google Scholar]
- 5.Tan J, Zheng Y, Song G, Kalkstein LS, Kalkstein AJ, Tang X. Heat wave impacts on mortality in Shanghai, 1998 and 2003. Int J Biometeorol. 2007;51:193–200 [DOI] [PubMed] [Google Scholar]
- 6.Klinenberg E. Review of heat wave: social autopsy of disaster in Chicago. N Engl J Med. 2003;348:666–7 [DOI] [PubMed] [Google Scholar]
- 7.Epstein RP, Mills E. Climate change futures: health, ecological and economic dimensions. Boston, MA: Center for Health and Global Environment, Harvard Medical School; 2005. [Google Scholar]
- 8.CDC Heat-related deaths – United States, 1999–2003. MMWR Morb Mortal Wkly Rep. 2006;55:796–8 [PubMed] [Google Scholar]
- 9.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med. 1996;335:84–90 [DOI] [PubMed] [Google Scholar]
- 10.Dematte JE, O'Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB, Ndukwu IM. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173–81 [DOI] [PubMed] [Google Scholar]
- 11.Naughton MP, Henderson A, Mirabelli MC, Kaiser R, Wilhelm JL, Kieszak SM, Rubin CH, McGeehin MA. Heat-related mortality during a 1999 heat wave in Chicago. Am J Prev Med. 2002;22:221–7 [DOI] [PubMed] [Google Scholar]
- 12.Kosatsky T. The 2003 European heat waves. Euro Surveill. 2005;10:148–9 [PubMed] [Google Scholar]
- 13.Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005;438:310–7 [DOI] [PubMed] [Google Scholar]
- 14.Vandentorren S, Suzan F, Medina S, Pascal M, Maulpoix A, Cohen JC, Ledrans M. Mortality in 13 French cities during the August 2003 heat wave. Am J Public Health. 2004;94:1518–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirard P, Vandentorren S, Pascal M, Laaidi K, Le Tertre A, Cassadou S, Ledrans M. Summary of the mortality impact assessment of the 2003 heat wave in France. Euro Surveill. 2005;10:153–6 [PubMed] [Google Scholar]
- 16.Damanhouri ZA, Tayeb OS. Animal models for heat stroke studies. J Pharmacol Toxicol Methods. 1992;28:119–27 [DOI] [PubMed] [Google Scholar]
- 17. Wilkins IA, Wheeler DW. Regulation of temperature. Basic Sci. 2004:168a-168e.
- 18.Zumbach B, Misztal I, Tsuruta S, Sanchez JP, Azain M, Herring W, Holl J, Long T, Culbertson M. Genetic components of heat stress in finishing pigs: parameter estimation. J Anim Sci. 2008;86:2076–81 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz J. Who is sensitive to extremes of temperature? A case-only analysis. Epidemiology. 2005;16:67–72 [DOI] [PubMed] [Google Scholar]
- 20.Ellis FP. Mortality from heat illness and heat-aggravated illness in the United States. Environ Res. 1972;5:1–58 [DOI] [PubMed] [Google Scholar]
- 21.Schuman SH. Patterns of urban heat-wave deaths and implications for prevention: data from New York and St. Louis during July, 1966. Environ Res. 1972;5:59–75 [DOI] [PubMed] [Google Scholar]
- 22.CDFA. Hot topics affecting California agriculture. An update from Sec. Kawamura. Available from: http://www.cdfa.ca.gov/exec/Public_Affairs/pdf/AGOnAg080306.pdf. Accessed May 27, 2008. 2006.
- 23. Drovers Cattle Network. Heat wave kills as many as 4000 cattle last week in Iowa. 2011 [cited 2011 Aug 4]; Available from: http://www.cattlenetwork.com/cattle-resources/hot-topics/
- 24.Ominski KH, Kennedy AD, Wittenberg KM, Moshtaghi Nia SA. Physiological and production responses to feeding schedule in lactating dairy cows exposed to short-term, moderate heat stress. J Dairy Sci. 2002;85:730–7 [DOI] [PubMed] [Google Scholar]
- 25.St-Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci. 2003;86: Suppl:E52–77 [Google Scholar]
- 26.Pollman D. Seasonal effects on sow herds: industry experience and management strategies. [abstract]. J Anim Sci. 2010;88:9 [Google Scholar]
- 27.Armstrong DV. Heat stress interaction with shade and cooling. J Dairy Sci. 1994;77:2044–50 [DOI] [PubMed] [Google Scholar]
- 28.Stowell RR, Mader TR, Gaughan B. Livestock energetics and thermal environmental management. St. Joseph, MO: ASABE; 2009.
- 29.Baumgard LH, Rhoads RP. Ruminant Nutrition Symposium: Ruminant Production and Metabolic Responses to Heat Stress. J Anim Sci. 2012;90:1855–65 [DOI] [PubMed] [Google Scholar]
- 30.Streffer C. Aspects of metabolic change after hyperthermia. Recent Results Cancer Res. 1988;107:7–16 [DOI] [PubMed] [Google Scholar]
- 31.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–91 [DOI] [PubMed] [Google Scholar]
- 32.Caspani ML, Savioli M, Crotti S, Bruzzone P, Gattinoni L. Heat stress: characteristics, pathophysiology and avoidable mistakes. Minerva Anestesiol. 2004;70:617–24 [PubMed] [Google Scholar]
- 33.Roti Roti JL. Cellular responses to hyperthermia (40–46°C): cell killing and molecular events. Int J Hyperthermia. 2008;24:3–15 [DOI] [PubMed] [Google Scholar]
- 34.Mondovì B, Finazzi Agro A, Rotilio G, Strom R, Moricca G, Rossi Fanelli A. The biochemical mechanism of selective heat sensitivity of cancer cells. II. Studies on nucleic acids and protein synthesis. Eur J Cancer. 1969;5:137–46 [DOI] [PubMed] [Google Scholar]
- 35.Henle KJ, Leeper DB. Effects of hyperthermia (45 degrees) on macromolecular synthesis in Chinese hamster ovary cells. Cancer Res. 1979;39:2665–74 [PubMed] [Google Scholar]
- 36.McCormick W, Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969;39:315–33 [DOI] [PubMed] [Google Scholar]
- 37.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96 [DOI] [PubMed] [Google Scholar]
- 38.Nakai A. New aspects in the vertebrate heat shock factor system: HSF3 and HSF4. Cell Stress Chaperones. 1999;4:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–31 [DOI] [PubMed] [Google Scholar]
- 40.Collier RJ, Collier JL, Rhoads RP, Baumgard LH. Invited review: genes involved in the bovine heat stress response. J Dairy Sci. 2008;91:445–54 [DOI] [PubMed] [Google Scholar]
- 41.Sonna LA, Gaffin SL, Pratt RE, Cullivan ML, Angel KC, Lilly CM. Effect of acute heat shock on gene expression by human peripheral blood mononuclear cells. J Appl Physiol. 2002;92:2208–20 [DOI] [PubMed] [Google Scholar]
- 42.Baler R, Dahl G, Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol. 1993;13:2486–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–8 [DOI] [PubMed] [Google Scholar]
- 45.Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–69 [DOI] [PubMed] [Google Scholar]
- 46.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15 [DOI] [PubMed] [Google Scholar]
- 47.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–100 [DOI] [PubMed] [Google Scholar]
- 48.Mathew A, Mathur SK, Morimoto RI. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998;18:5091–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li GC. Induction of thermotolerance and enhanced heat shock protein synthesis in Chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol. 1983;115:116–22 [DOI] [PubMed] [Google Scholar]
- 50.Cairo G, Bardella L, Schiaffonati L, Bernelli-Zazzera A. Synthesis of heat shock proteins in rat liver after ischemia and hyperthermia. Hepatology. 1985;5:357–61 [DOI] [PubMed] [Google Scholar]
- 51.Ryan AJ, Gisolfi CV, Moseley PL. Synthesis of 70K stress protein by human leukocytes: effect of exercise in the heat. J Appl Physiol. 1991;70:466–71 [DOI] [PubMed] [Google Scholar]
- 52.Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413–7 [DOI] [PubMed] [Google Scholar]
- 53.Buchner J. Supervising the fold: functional principles of molecular chaperones. FASEB J. 1996;10:10–9 [PubMed] [Google Scholar]
- 54.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88 [DOI] [PubMed] [Google Scholar]
- 55.The stress response: a radiation study section workshop. Radiat Res. 1996;145:107–17 [PubMed] [Google Scholar]
- 56.Feige U, Polla BS. Hsp70–a multi-gene, multi-structure, multi-function family with potential clinical applications. Experientia. 1994;50:979–86 [DOI] [PubMed] [Google Scholar]
- 57.Westman J, Sharma HS. Heat shock protein response in the central nervous system following hyperthermia. Prog Brain Res. 1998;115:207–39 [DOI] [PubMed] [Google Scholar]
- 58.Park SH, Lee SJ, Chung HY, Kim TH, Cho CK, Yoo SY, Lee YS. Inducible heat-shock protein 70 is involved in the radioadaptive response. Radiat Res. 2000;153:318–26 [DOI] [PubMed] [Google Scholar]
- 59.Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci. 2007;32:595–610 [DOI] [PubMed] [Google Scholar]
- 60.Volloch V, Rits S. A natural extracellular factor that induces Hsp72, inhibits apoptosis, and restores stress resistance in aged human cells. Exp Cell Res. 1999;253:483–92 [DOI] [PubMed] [Google Scholar]
- 61.Mizzen LA, Welch WJ. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988;106:1105–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaughan JB, Bonner SL, Loxton I, Mader TL. Effects of chronic heat stress on plasma concentration of secreted heat shock protein 70 (Hsp70) in growing feedlot cattle. J Anim Sci. 2013;91:120–9 [DOI] [PubMed] [Google Scholar]
- 64.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE. 2005;2005:pe4. [DOI] [PubMed]
- 67.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simar D, Jacques A, Caillaud C. Heat shock proteins induction reduces stress kinases activation, potentially improving insulin signalling in monocytes from obese subjects. Cell Stress Chaperones. 2012;17:615–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–30 [DOI] [PubMed] [Google Scholar]
- 70.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–90 [DOI] [PubMed] [Google Scholar]
- 72.Covarrubias L, Hernandez-Garcia D, Schnabel D, Salas-Vidal E, Castro-Obregon S. Function of reactive oxygen species during animal development: passive or active? Dev Biol. 2008;320:1–11 [DOI] [PubMed] [Google Scholar]
- 73.Halliwell G. Oxygen radicals in biological systems: part B. In: Packer LP, Glazer AN, editors. Oxygen radicals and antioxidants. San Diego (CA): Academic Press; 1990. p. 1–85.
- 74.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99 [DOI] [PubMed] [Google Scholar]
- 75.Addabbo F, Montagnani M, Goligorsky MS. Mitochondria and reactive oxygen species. Hypertension. 2009;53:885–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. Amsterdam: Elsevier; 1987.
- 77.Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53:225–45 [DOI] [PubMed] [Google Scholar]
- 78.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–8 [DOI] [PubMed] [Google Scholar]
- 79.Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci. 2007;32:44–50 [DOI] [PubMed] [Google Scholar]
- 80.O'Brien PJ. Peroxidases. Chem Biol Interact. 2000;129:113–39 [DOI] [PubMed] [Google Scholar]
- 81.Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med. 1991;11:239–46 [DOI] [PubMed] [Google Scholar]
- 82.Schiaffonati L, Rappocciolo E, Tacchini L, Cairo G, Bernelli-Zazzera A. Reprogramming of gene expression in postischemic rat liver: induction of proto-oncogenes and hsp 70 gene family. J Cell Physiol. 1990;143:79–87 [DOI] [PubMed] [Google Scholar]
- 83.Rainwater DT, Gossett DR, Millhollon EP, Hanna HY, Banks SW, Lucas MC. The relationship between yield and the antioxidant defense system in tomatoes grown under heat stress. Free Radic Res. 1996;25:421–35 [DOI] [PubMed] [Google Scholar]
- 84.Sato Y, Murakami T, Funatsuki H, Matsuba S, Saruyama H, Tanida M. Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J Exp Bot. 2001;52:145–51 [PubMed] [Google Scholar]
- 85.Rizhsky L, Liang H, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002;130:1143–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998;431:285–6 [DOI] [PubMed] [Google Scholar]
- 87.Zuo L, Christofi FL, Wright VP, Liu CY, Merola AJ, Berliner LJ, Clanton TL. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:C1058–66 [DOI] [PubMed] [Google Scholar]
- 88.Mujahid A, Yoshiki Y, Akiba Y, Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult Sci. 2005;84:307–14 [DOI] [PubMed] [Google Scholar]
- 89.Mujahid A, Sato K, Akiba Y, Toyomizu M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult Sci. 2006;85:1259–65 [DOI] [PubMed] [Google Scholar]
- 90.Bernabucci U, Ronchi B, Lacetera N, Nardone A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci. 2002;85:2173–9 [DOI] [PubMed] [Google Scholar]
- 91.Mujahid A, Akiba Y, Toyomizu M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297:R690–8 [DOI] [PubMed] [Google Scholar]
- 92.Qian L, Song X, Ren H, Gong J, Cheng S. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones. 2004;9:281–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Collier RJ, Beede DK, Thatcher WW, Israel LA, Wilcox CJ. Influences of environment and its modification on dairy animal health and production. J Dairy Sci. 1982;65:2213–27 [DOI] [PubMed] [Google Scholar]
- 94.West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci. 2003;86:2131–44 [DOI] [PubMed] [Google Scholar]
- 95.Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal. 2010;4:1167–83 [DOI] [PubMed] [Google Scholar]
- 96.Prunier A, Louveau I. Influence of ovariectomy on metabolic and endocrine parameters during sexual development in the female pig. J Endocrinol. 1997;154:423–9 [DOI] [PubMed] [Google Scholar]
- 97.Ronchi B, Bernabucci U, Lacetera N, Supplizi AV, Nardone A. Distinct and common effects of heat stress and restricted feeding on metabolic status of Holstein heifers. Zoot Nutr Anim. 1999;25:11–20 [Google Scholar]
- 98.Close WH, Mount LE. Energy retention in the pig at several environmental temperatures and levels of feeding. Proc Nutr Soc. 1971;30:33A–4A [PubMed] [Google Scholar]
- 99.Verstegen MW, Close WH, Start IB, Mount LE. The effects of environmental temperature and plane of nutrition on heat loss, energy retention and deposition of protein and fat in groups of growing pigs. Br J Nutr. 1973;30:21–35 [DOI] [PubMed] [Google Scholar]
- 100.Schmidt P, Widdowson EM. The effect of a low-protein diet and a cold environment on calorie intake and body composition in the rat. Br J Nutr. 1967;21:457–65 [DOI] [PubMed] [Google Scholar]
- 101.Katsumata M, Yano H, Ishida N, Miyazaki A. Influence of a high ambient temperature and administration of clenbuterol on body composition in rats. J Nutr Sci Vitaminol (Tokyo). 1990;36:569–78 [DOI] [PubMed] [Google Scholar]
- 102.Geraert PA, Padilha JC, Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr. 1996;75:195–204 [DOI] [PubMed] [Google Scholar]
- 103.Yunianto VD, Hayashit K, Kaiwda S, Ohtsuka A, Tomita Y. Effect of environmental temperature on muscle protein turnover and heat production in tube-fed broiler chickens. Br J Nutr. 1997;77:897–909 [DOI] [PubMed] [Google Scholar]
- 104.Lu Q, Wen J, Zhang H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci. 2007;86:1059–64 [DOI] [PubMed] [Google Scholar]
- 105.Ain Baziz H, Geraert P, Padilha J, Guillaumin S. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poult Sci. 1996;75:505–13 [DOI] [PubMed] [Google Scholar]
- 106.Le Dividich J, Vermorel M, Noblet J, Bouvier JC, Aumaitre A. Effects of environmental temperature on heat production, energy retention, protein and fat gain in early weaned piglets. Br J Nutr. 1980;44:313–23 [DOI] [PubMed] [Google Scholar]
- 107.Oresanya TF, Beaulieu AD, Patience JF. Investigations of energy metabolism in weanling barrows: the interaction of dietary energy concentration and daily feed (energy) intake. J Anim Sci. 2008;86:348–63 [DOI] [PubMed] [Google Scholar]
- 108.Van Milgen J, Noblet J. Partitioning of energy intake to heat, protein, and fat in growing pigs. J Anim Sci. 2003;81:E86–93 [Google Scholar]
- 109.Rhoads ML, Rhoads RP, VanBaale MJ, Collier RJ, Sanders SR, Weber WJ, Crooker BA, Baumgard LH. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J Dairy Sci. 2009;92:1986–97 [DOI] [PubMed] [Google Scholar]
- 110.Shwartz G, Rhoads ML, VanBaale MJ, Rhoads RP, Baumgard LH. Effects of a supplemental yeast culture on heat-stressed lactating Holstein cows. J Dairy Sci. 2009;92:935–42 [DOI] [PubMed] [Google Scholar]
- 111.Pearce SC, Gabler NK, Ross JW, Escobar J, Patience JF, Rhoads RP, Baumgard LH. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J Anim Sci. Epub 2013 Mar 5 [DOI] [PubMed] [Google Scholar]
- 112.Sano H, Takahashi K, Ambo K, Tsuda T. Turnover and oxidation rates of blood glucose and heat production in sheep exposed to heat. J Dairy Sci. 1983;66:856–61 [DOI] [PubMed] [Google Scholar]
- 113.Itoh F, Obara Y, Rose MT, Fuse H. Heat influences on plasma insulin and glucagon in response to secretogogues in non-lactating dairy cows. Domest Anim Endocrinol. 1998;15:499–510 [DOI] [PubMed] [Google Scholar]
- 114.Bauman DE, Peel CJ, Steinhour WD, Reynolds PJ, Tyrrell HF, Brown AC, Haaland GL. Effect of bovine somatotropin on metabolism of lactating dairy cows: influence on rates of irreversible loss and oxidation of glucose and nonesterified fatty acids. J Nutr. 1988;118:1031–40 [DOI] [PubMed] [Google Scholar]
- 115.Baumgard LH, Wheelock JB, Sanders SR, Moore CE, Green HB, Waldron MR, Rhoads RP. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows. J Dairy Sci. 2011;94:5620–33 [DOI] [PubMed] [Google Scholar]
- 116.Torlińska T, Banach R, Paluszak J, Gryczka-Dziadecka A. Hyperthermia effect on lipolytic processes in rat blood and adipose tissue. Acta Physiol Pol. 1987;38:361–6 [PubMed] [Google Scholar]
- 117.Vernon RG. Effects of diet on lipolysis and its regulation. Proc Nutr Soc. 1992;51:397–408 [DOI] [PubMed] [Google Scholar]
- 118.DeSouza CJ, Meier AH. Alterations in body fat stores and plasma insulin levels with daily intervals of heat exposure in Holtzman rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:1109–14 [DOI] [PubMed] [Google Scholar]
- 119.Hall GM, Lucke JN, Lovell R, Lister D. Porcine malignant hyperthermia. VII: Hepatic metabolism. Br J Anaesth. 1980;52:11–7 [DOI] [PubMed] [Google Scholar]
- 120.O’Brien MD, Rhoads RP, Sanders SR, Duff GC, Baumgard LH. Metabolic adaptations to heat stress in growing cattle. Domest Anim Endocrinol. 2010;38:86–94 [DOI] [PubMed] [Google Scholar]
- 121.Itoh F, Obara Y, Rose MT, Fuse H, Hashimoto H. Insulin and glucagon secretion in lactating cows during heat exposure. J Anim Sci. 1998;76:2182–9 [DOI] [PubMed] [Google Scholar]
- 122.Wheelock JB, Rhoads RP, VanBaale MJ, Sanders SR, Baumgard LH. Effects of heat stress on energetic metabolism in lactating Holstein cows. J Dairy Sci. 2010;93:644–55 [DOI] [PubMed] [Google Scholar]
- 123.Achmadi J, Yanagisawa T, Sano H, Terashima Y. Pancreatic insulin secretory response and insulin action in heat-exposed sheep given a concentrate or roughage diet. Domest Anim Endocrinol. 1993;10:279–87 [DOI] [PubMed] [Google Scholar]
- 124.Fink WJ, Costill DL, Van Handel PJ. Leg muscle metabolism during exercise in the heat and cold. Eur J Appl Physiol Occup Physiol. 1975;34:183–90 [DOI] [PubMed] [Google Scholar]
- 125.Febbraio MA. Alterations in energy metabolism during exercise and heat stress. Sports Med. 2001;31:47–59 [DOI] [PubMed] [Google Scholar]
- 126.Jentjens RLPG, Wagenmakers AJM, Jeukendrup AE. Heat stress increases muscle glycogen use but reduces the oxidation of ingested carbohydrates during exercise. J Appl Physiol. 2002;92:1562–72 [DOI] [PubMed] [Google Scholar]
- 127.Angus DJ, Febbraio MA, Lasini D, Hargreaves M. Effect of carbohydrate ingestion on glucose kinetics during exercise in the heat. J Appl Physiol. 2001;90:601–5 [DOI] [PubMed] [Google Scholar]
- 128.Collins FG, Mitros FA, Skibba JL. Effect of palmitate on hepatic biosynthetic functions at hyperthermic temperatures. Metabolism. 1980;29:524–31 [DOI] [PubMed] [Google Scholar]
- 129.Rhoads RP, La Noce AJ, Wheelock JB, Baumgard LH. Short communication: alterations in expression of gluconeogenic genes during heat stress and exogenous bovine somatotropin administration. J Dairy Sci. 2011;94:1917–21 [DOI] [PubMed] [Google Scholar]
- 130.White HM, Koser SL, Donkin SS. Regulation of bovine pyruvate carboxylase mRNA and promoter expression by thermal stress. J Anim Sci, in press [DOI] [PubMed] [Google Scholar]
- 131.O’Brien M, Cole L, Wheelock J, Sanders S, Duff G, Baumgard L, Rhoads R. Thermal and nutritional regulation of hepatic gluconeogenic genes in growing beef cattle. [abstract]. J Anim Sci. 2008;86:455 [Google Scholar]
- 132.Garriga C, Hunter RR, Amat C, Planas JM, Mitchell MA, Moretó M. Heat stress increases apical glucose transport in the chicken jejunum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R195–201 [DOI] [PubMed] [Google Scholar]
- 133.Sussman CR, Renfro JL. Heat shock-induced protection and enhancement of Na+-glucose cotransport by LLC-PK1 monolayers. Am J Physiol. 1997;273:F530–7 [DOI] [PubMed] [Google Scholar]
- 134.Ikari A, Nakano M, Suketa Y, Harada H, Takagi K. Reorganization of ZO-1 by sodium-dependent glucose transporter activation after heat stress in LLC-PK1 cells. J Cell Physiol. 2005;203:471–8 [DOI] [PubMed] [Google Scholar]
- 135.Willis WT, Jackman MR, Bizeau ME, Pagliassotti MJ, Hazel JR. Hyperthermia impairs liver mitochondrial function in vitro. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1240–6 [DOI] [PubMed] [Google Scholar]
- 136.Ramnath V, Rekha PS, Sujatha KS. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by brahma rasayana. Evid Based Complement Alternat Med. 2008;5:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Marder J, Eylath U, Moskovitz E, Sharir R. The effect of heat exposure on blood chemistry of the hyperthermic rabbit. Comp Biochem Physiol A Physiol. 1990;97:245–247. [DOI] [PubMed]
- 138.Schneider PL, Beede DK, Wilcox CJ. Nycterohemeral patterns of acid-base status, mineral concentrations and digestive function of lactating cows in natural or chamber heat stress environments. J Anim Sci. 1988;66:112–25 [DOI] [PubMed] [Google Scholar]
- 139.Bernabucci U, Lacetera N, Ronchi B, Nardone A. Effects of the hot season on milk protein fractions in Holstein cows. Anim Res. 2002;51:25–33 [Google Scholar]
- 140.Li G, Ali IS, Currie RW. Insulin induces myocardial protection and HSP70 localization to plasma membranes in rat hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1709–21 [DOI] [PubMed] [Google Scholar]
- 141.Nteeba J, Ullerich EE, Pearce SC, Boddicker R, Ross JW, Baumgard LH. Keating AF. Effect of heat stress on phosphatidylinositol-3 kinase signaling in gilt ovaries. [abstract]. J Anim Sci. 2012;90:573 [Google Scholar]
- 142.Niu CS, Lin MT, Liu IM, Cheng JT. Role of striatal glutamate in heatstroke-induced damage in streptozotocin-induced diabetic rats. Neurosci Lett. 2003;348:77–80 [DOI] [PubMed] [Google Scholar]
- 143.Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, Nakabe N, Handa O, Takagi T, Naito Y, et al. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperthermia. 2007;23:259–65 [DOI] [PubMed] [Google Scholar]
- 144.Kokura S, Adachi S, Mizushima K, Okayama T, Hattori T, Okuda T, Nakabe N, Manabe E, Ishikawa T, Handa O, et al. Gene expression profiles of diabetic mice treated with whole body hyperthermia: A high-density DNA microarray analysis. Int J Hyperthermia. 2010;26:101–7 [DOI] [PubMed] [Google Scholar]
- 145.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58:567–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCarty MF, Barroso-Aranda J, Contreras F. Regular thermal therapy may promote insulin sensitivity while boosting expression of endothelial nitric oxide synthase–effects comparable to those of exercise training. Med Hypotheses. 2009;73:103–5 [DOI] [PubMed] [Google Scholar]
- 147.Csaba G, Pallinger E. Effect of stress and stress hormones on the hormone (insulin) binding of Tetrahymena. Cell Biochem Funct. 2009;27:448–51 [DOI] [PubMed] [Google Scholar]
- 148.Burke LM. Nutritional needs for exercise in the heat. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:735–48 [DOI] [PubMed] [Google Scholar]
- 149.Sawka MN, Montain SJ. Fluid and electrolyte supplementation for exercise heat stress. Am J Clin Nutr. 2000;72:564S–72S. [DOI] [PubMed] [Google Scholar]
- 150.Lin H, Jiao H, Buyse J, Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult Sci J. 2006;62:71–86 [Google Scholar]
- 151.Marai I, El-Darawany A, Fadiel A, Abdel-Hafez M. Physiological traits as affected by heat stress in sheep—a review. Small Rumin Res. 2007;71:1–12 [Google Scholar]
- 152.Reed LJ. The chemistry and function of lipoic acid. Adv Enzymol Rel Areas Mol Biol. 2009:319. [DOI] [PubMed]
- 153.Jordan SW, Cronan JE. Biosynthesis of lipoic acid and posttranslational modification with lipoic acid in Escherichia coli. Methods Enzymol. 1997;279:176–83 [DOI] [PubMed] [Google Scholar]
- 154.Wada H, Shintani D, Ohlrogge J. Why do mitochondria synthesize fatty acids? Evidence for involvement in lipoic acid production. Proc Natl Acad Sci U S A. 1997;94:1591–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reed LJ. Multienzyme complexes. Acc Chem Res. 1974;7:40–6 [Google Scholar]
- 156.Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–50 [DOI] [PubMed] [Google Scholar]
- 157.Gregus Z, Stein AF, Varga F, Klaassen CD. Effect of lipoic acid on biliary excretion of glutathione and metals. Toxicol Appl Pharmacol. 1992;114:88–96 [DOI] [PubMed] [Google Scholar]
- 158.Diesel B, Kulhanek-Heinze S, Höltje M, Brandt B, Höltje HD, Vollmar AM, Kiemer AK. α-Lipoic acid as a directly binding activator of the insulin receptor: protection from hepatocyte apoptosis. Biochemistry. 2007;46:2146–55 [DOI] [PubMed] [Google Scholar]
- 159.Hamano Y. Effects of dietary lipoic acid on plasma lipid, in vivo insulin sensitivity, metabolic response to corticosterone and in vitro lipolysis in broiler chickens. Br J Nutr. 2006;95:1094–101 [DOI] [PubMed] [Google Scholar]
- 160.Hamano Y. Influence of lipoic acid on lipid metabolism and β-adrenergic response to intravenous or oral administration of clenbuterol in broiler chickens. Reprod Nutr Dev. 2002;42:307–16 [DOI] [PubMed] [Google Scholar]
- 161.Hamano Y. Alleviative effects of α-lipoic acid supplementation on acute heat stress-induced thermal panting and the level of plasma nonesterified fatty acids in hypothyroid broiler chickens. Br Poult Sci. 2012;53:125–33 [DOI] [PubMed] [Google Scholar]
- 162.Maddock K, Carroll J, Berg E. Evaluation of the potential role of alpha-lipoic acid with regard to health and performance of weanling pigs. J Anim Vet Adv. 2003;2.
- 163.Kinnunen S, Hyyppä S, Oksala N, Laaksonen DE, Hannila ML, Sen CK, Atalay M. α-Lipoic acid supplementation enhances heat shock protein production and decreases post exercise lactic acid concentrations in exercised standardbred trotters. Res Vet Sci. 2009;87:462–7 [DOI] [PubMed] [Google Scholar]
- 164.Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000;72:653S–69S [DOI] [PubMed] [Google Scholar]
- 165.Mertz W. Chromium in human nutrition: a review. J Nutr. 1993;123:626–33 [DOI] [PubMed] [Google Scholar]
- 166.Spears JW, Whisnant CS, Huntington GB, Lloyd KE, Fry RS, Krafka K, Lamptey A, Hyda J. Chromium propionate enhances insulin sensitivity in growing cattle. J Dairy Sci. 2012;95:2037–45 [DOI] [PubMed] [Google Scholar]
- 167.Soltan MA. Effect of dietary chromium supplementation on productive and reproductive performance of early lactating dairy cows under heat stress. J Anim Physiol Anim Nutr (Berl). 2010;94:264–72 [DOI] [PubMed] [Google Scholar]
- 168. Mirzaei M, Ghorbani GR, Khorvash M, Rahmani HR, Nikkhah A. Chromium improves production and alters metabolism of early lactation cows in summer. J Anim Physiol Anim Nutr (Berl). 2011;95:81–9. [DOI] [PubMed]
- 169.Toghyani M, Shivazad M, Gheisari A, Bahadoran R. Chromium supplementation can alleviate the negative effects of heat stress on growth performance, carcass traits, and meat lipid oxidation of broiler chicks without any adverse impacts on blood constituents. Biol Trace Elem Res. 2012;146:171–80 [DOI] [PubMed] [Google Scholar]
- 170.Lien TF, Horng YM, Yang KH. Performance, serum characteristics, carcase traits and lipid metabolism of broilers as affected by supplement of chromium picolinate. Br Poult Sci. 1999;40:357–63 [DOI] [PubMed] [Google Scholar]
- 171.Kim BG, Lindemann MD, Cromwell GL. The effects of dietary chromium (III) picolinate on growth performance, blood measurements, and respiratory rate in pigs kept in high and low ambient temperature. J Anim Sci. 2009;87:1695–704 [DOI] [PubMed] [Google Scholar]
- 172.Kletzien RF, Clarke SD, Ulrich RG. Enhancement of adipocyte differentiation by an insulin-sensitizing agent. Mol Pharmacol. 1992;41:393–8 [PubMed] [Google Scholar]
- 173.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPARγ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52 [DOI] [PubMed] [Google Scholar]
- 174.Ranganathan G, Unal R, Pokrovskaya I, Yao-Borengasser A, Phanavanh B, Lecka-Czernik B, Rasouli N, Kern PA. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res. 2006;47:2444–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sandouk T, Reda D, Hofmann C. The antidiabetic agent pioglitazone increases expression of glucose transporters in 3T3–F442A cells by increasing messenger ribonucleic acid transcript stability. Endocrinology. 1993;133:352–9 [DOI] [PubMed] [Google Scholar]
- 176.Petrofsky JS, McLellan K, Bains GS, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E, III, Schwab E. Skin heat dissipation: the influence of diabetes, skin thickness, and subcutaneous fat thickness. Diabetes Technol Ther. 2008;10:487–93 [DOI] [PubMed] [Google Scholar]
- 177.Petrofsky JS, Lee S, Cuneo-Libarona M. The impact of rosiglitazone on heat tolerance in patients with type 2 diabetes. Med Sci Monit. 2005;11:CR562–9 [PubMed] [Google Scholar]
- 178.Taniguchi Y, Ooie T, Takahashi N, Shinohara T, Nakagawa M, Yonemochi H, Hara M, Yoshimatsu H, Saikawa T. Pioglitazone but not glibenclamide improves cardiac expression of heat shock protein 72 and tolerance against ischemia/reperfusion injury in the heredity insulin-resistant rat. Diabetes. 2006;55:2371–8 [DOI] [PubMed] [Google Scholar]
- 179.Schoenberg KM, Overton TR. Effects of plane of nutrition and 2,4-thiazolidinedione on insulin responses and adipose tissue gene expression in dairy cattle during late gestation. J Dairy Sci. 2011;94:6021–35 [DOI] [PubMed] [Google Scholar]
- 180.Suagee JK, Corl BA, Wearn JG, Crisman MV, Hulver MW, Geor RJ, McCutcheon LJ. Effects of the insulin-sensitizing drug pioglitazone and lipopolysaccharide administration on insulin sensitivity in horses. J Vet Intern Med. 2011;25:356–64 [DOI] [PubMed] [Google Scholar]