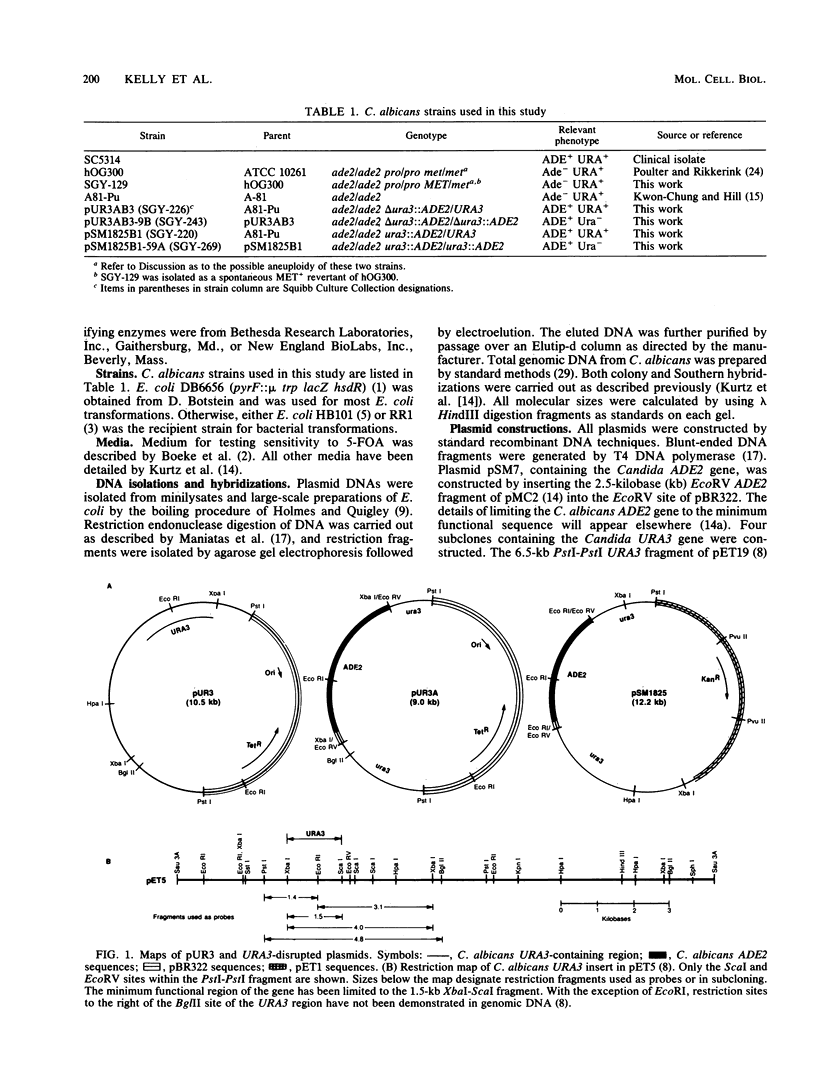
Abstract
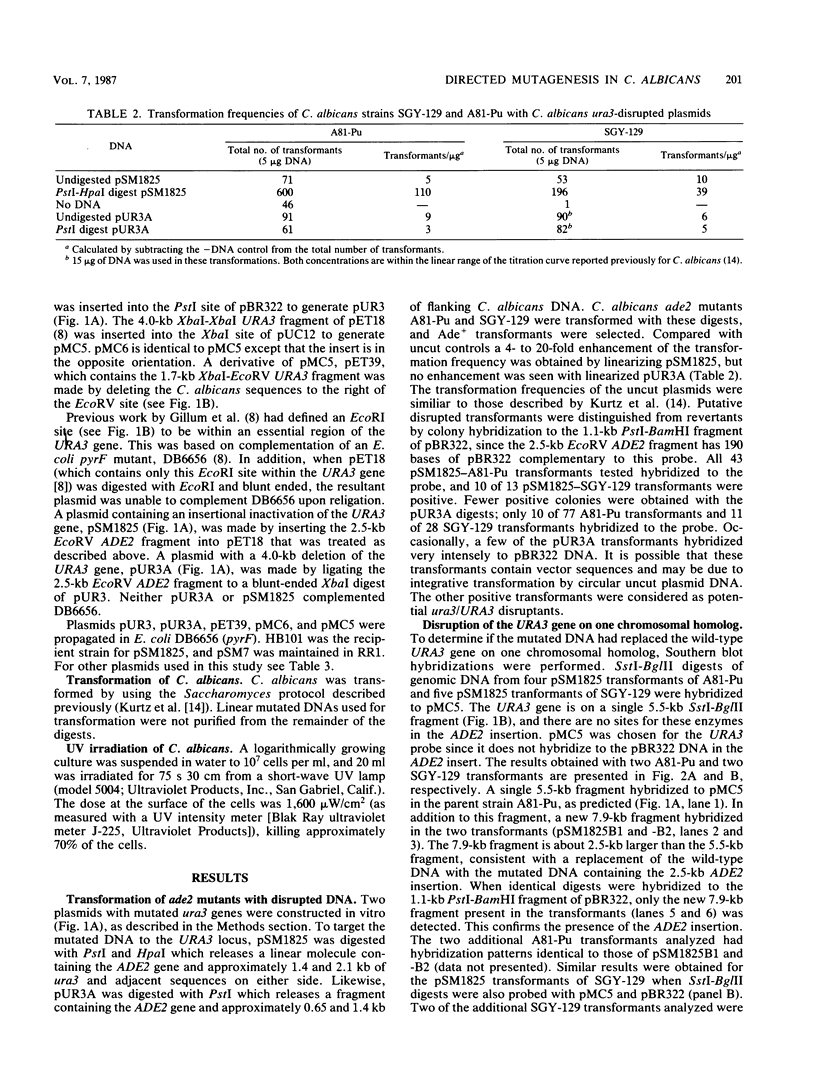
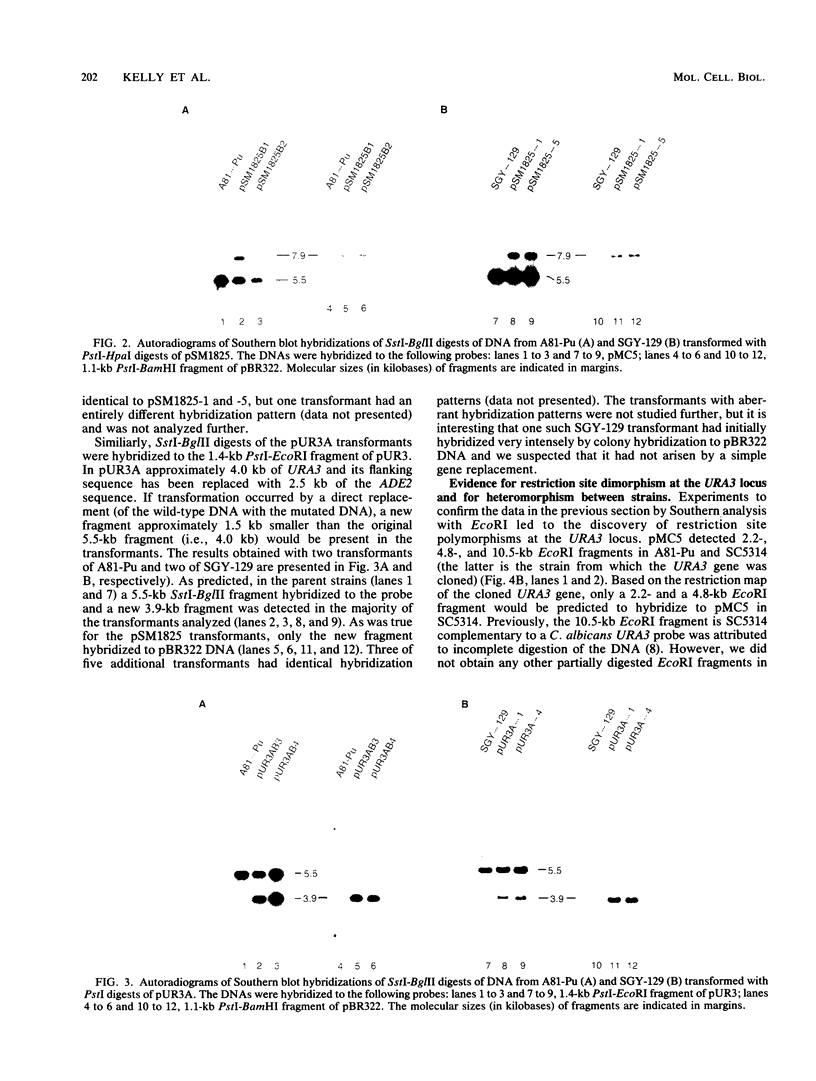
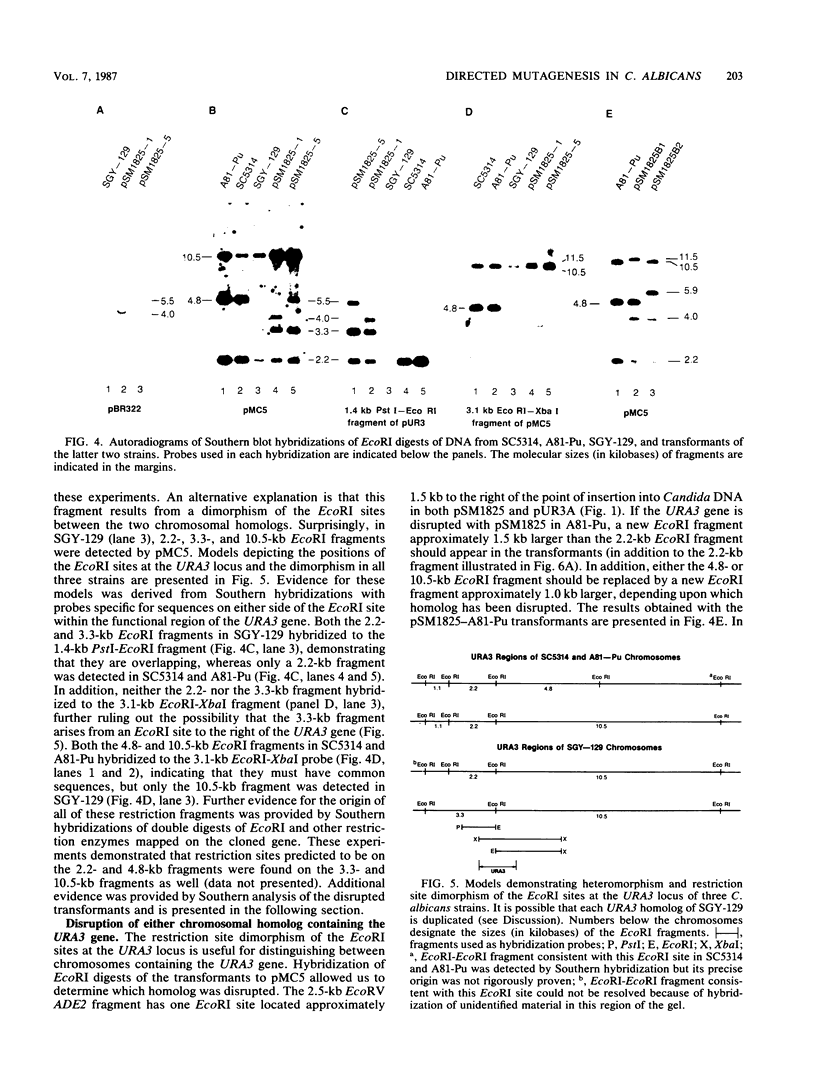
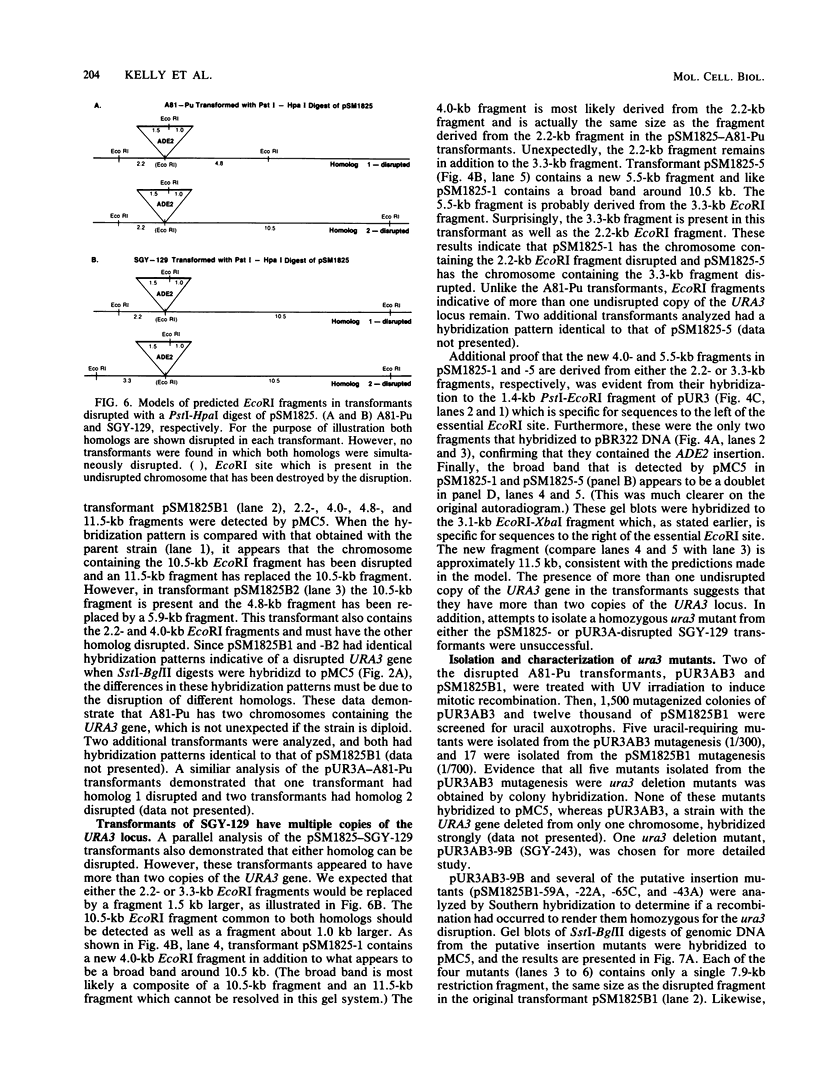
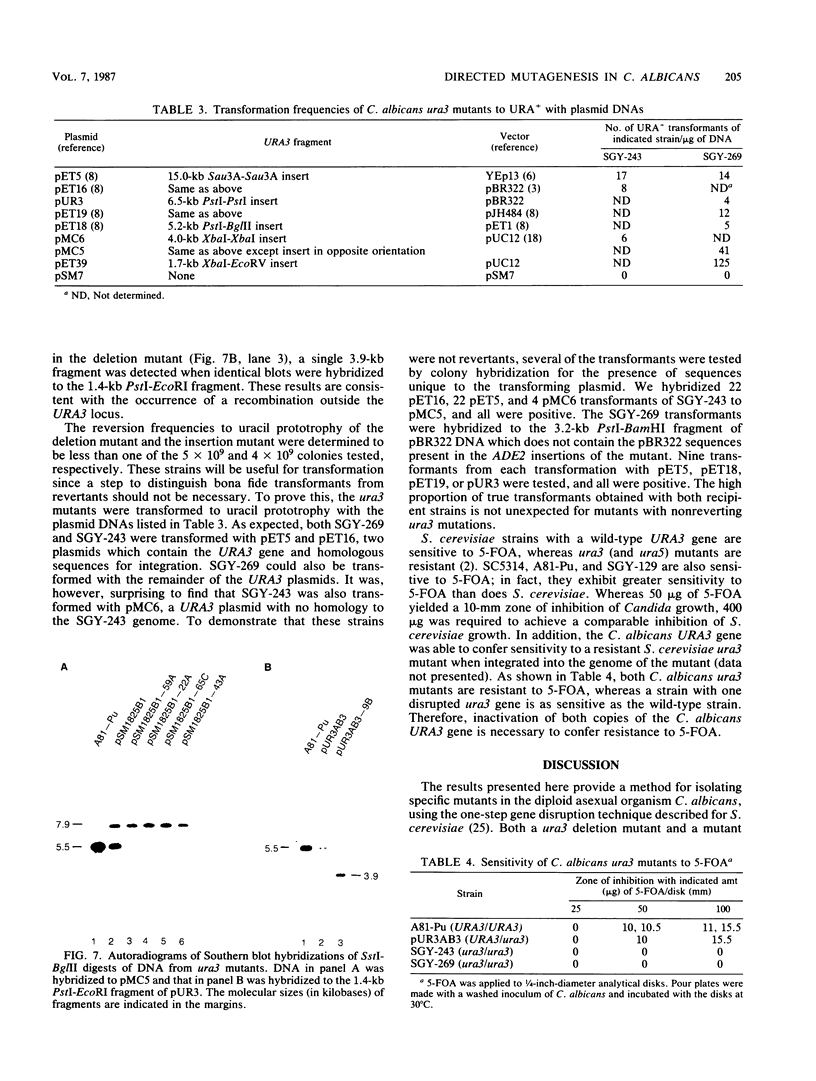
A method for introducing specific mutations into the diploid Candida albicans by one-step gene disruption and subsequent UV-induced recombination was developed. The cloned C. albicans URA3 gene was disrupted with the C. albicans ADE2 gene, and the linearized DNA was used for transformation of two ade2 mutants, SGY-129 and A81-Pu. Both an insertional inactivation of the URA3 gene and a disruption which results in a 4.0-kilobase deletion were made. Southern hybridization analyses demonstrated that the URA3 gene was disrupted on one of the chromosomal homologs in 15 of the 18 transformants analyzed. These analyses also revealed restriction site dimorphism of EcoRI at the URA3 locus which provides a unique marker to distinguish between chromosomal homologs. This enabled us to show that either homolog could be disrupted and that disrupted transformants of SGY-129 contained more than two copies of the URA3 locus. The A81-Pu transformants heterozygous for the ura3 mutations were rendered homozygous and Ura- by UV-induced recombination. The homozygosity of a deletion mutant and an insertion mutant was confirmed by Southern hybridization. Both mutants were transformed to Ura+ with plasmids containing the URA3 gene and in addition, were resistant to 5-fluoro-orotic acid, a characteristic of Saccharomyces cerevisiae ura3 mutants as well as of orotidine-5'-phosphate decarboxylase mutants of other organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Chung K. J., Hill W. B. Studies on the pink, adenine-deficient strains of Candida albicans. I. Cultural and morphological characteristics. Sabouraudia. 1970 May;8(1):48–59. [PubMed] [Google Scholar]
- Gillum A. M., Tsay E. Y., Kirsch D. R. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(2):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kakar S. N., Magee P. T. Genetic analysis of Candida albicans: identification of different isoleucine-valine, methionine, and arginine alleles by complementation. J Bacteriol. 1982 Sep;151(3):1247–1252. doi: 10.1128/jb.151.3.1247-1252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar S. N., Partridge R. M., Magee P. T. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics. 1983 Jun;104(2):241–255. doi: 10.1093/genetics/104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunes S., Botstein D., Fox M. S. Transformation of yeast with linearized plasmid DNA. Formation of inverted dimers and recombinant plasmid products. J Mol Biol. 1985 Aug 5;184(3):375–387. doi: 10.1016/0022-2836(85)90288-8. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Kirsch D. R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986 Jan;6(1):142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Miller S. M., Lai M., Kirsch D. R. Development of autonomously replicating plasmids for Candida albicans. Mol Cell Biol. 1987 Jan;7(1):209–217. doi: 10.1128/mcb.7.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller B. L., Miller K. Y., Timberlake W. E. Direct and indirect gene replacements in Aspergillus nidulans. Mol Cell Biol. 1985 Jul;5(7):1714–1721. doi: 10.1128/mcb.5.7.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paietta J. V., Marzluf G. A. Gene disruption by transformation in Neurospora crassa. Mol Cell Biol. 1985 Jul;5(7):1554–1559. doi: 10.1128/mcb.5.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R. T., Rikkerink E. H. Genetic analysis of red, adenine-requiring mutants of Candida albicans. J Bacteriol. 1983 Dec;156(3):1066–1077. doi: 10.1128/jb.156.3.1066-1077.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R., Hanrahan V., Jeffery K., Markie D., Shepherd M. G., Sullivan P. A. Recombination analysis of naturally diploid Candida albicans. J Bacteriol. 1982 Dec;152(3):969–975. doi: 10.1128/jb.152.3.969-975.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rudolph H., Koenig-Rauseo I., Hinnen A. One-step gene replacement in yeast by cotransformation. Gene. 1985;36(1-2):87–95. doi: 10.1016/0378-1119(85)90072-1. [DOI] [PubMed] [Google Scholar]
- Savage N., Balish E. Morphology, Physiology, and Virulence of Some Mutants of Candida albicans. Infect Immun. 1971 Jan;3(1):141–148. doi: 10.1128/iai.3.1.141-148.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imai Y., Yamashita I., Fukui S. In vivo ligation of linear DNA molecules to circular forms in the yeast Saccharomyces cerevisiae. J Bacteriol. 1983 Aug;155(2):747–754. doi: 10.1128/jb.155.2.747-754.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Magee P. T. Natural heterozygosity in Candida albicans. J Bacteriol. 1981 Feb;145(2):896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]
- Winston F., Chaleff D. T., Valent B., Fink G. R. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics. 1984 Jun;107(2):179–197. doi: 10.1093/genetics/107.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]