Abstract
Purpose
To investigate infant tear film secretion and protein profile, and to compare major tear proteins, such as immunoglobulin A, lactoferrin, and lysozyme, with those of adult controls.
Methods
Tears were collected, with a cellulose rod, from 40 healthy infants (19 female infants and 21 male infants, gestational duration: 39.71 ± 1.27 weeks) within 48 hours of birth and 22 adults (10 female infants and 12 male infants, mean age: 24.95 ± 3.63 years). A second collection was obtained from 14 of the infants (8 female infants and 6 male infants, postnatal age: 7.76 ± 6.14 weeks). The tear volume was measured, and protein in the samples was analyzed by Bradford assay and gel electrophoresis.
Results
Median tear volume (interquartile range) was 0.5μL (0.6– 2 μL) for newborn infants, 2.5μL (1.4–7.75 μL) for these infants at an older age, and 6μL (2.73–12.75 μL) in adults (P < 0.001, Kruskall–Wallis test). Immunoglobulin A concentration was significantly lower in newborns (P < 0.001, analysis of variance). Lipocalin was present in 36% of the newborn tear samples, whereas serum albumin was found in 86%. Mean protein concentration (μg/μL 6 SD) was 10.95 ± 5.51 in the newborns, 12.93 ± 3.99 in the older infants, and 13.04 ± 3.46 in the adults (P > 0.5, analysis of variance).
Conclusions
This is the first study reporting an investigation of unstimulated infant tears, using a noninvasive collection method. Tear protein content demonstrated that the infant tear film is different to that in adults.
Keywords: infant, lacrimal gland, tear protein, blinking
The infant tear film has been of interest in its quantitative aspects for many years, and several studies have shown that newborns have aqueous production1,2 sufficient to maintain clear vision and moisten the ocular surface. More recent research has shown a remarkable infant tear film quality, which is reflected in a very low blink rate, typically 2–3 blinks per minute3 and an outstanding tear breakup time of 32.5 ± 5.2 (mean ± SD) seconds.4 This performance may be because of a thicker lipid layer4 combined with superior biophysical properties5 compared with that in adults. Parallel to the superior lipid layer, an increase in mucus expression in infant tears, with the example of MUC5AC, has been described recently.6 However, infant tear protein content has not been studied as much as the tear film in adults because of the difficulty of collecting from the tear film without provoking reflex tearing and a dilution of the protein content. Proteins regulate important tear functions such as defense7 and stability in the lipid–aqueous interface.8
The aim of this study was to investigate the newborn tear film by analyzing the protein profile and the concentration of the major proteins, such as immunoglobulin (Ig) A, lactoferrin, and lysozyme in unstimulated infant tears.
MATERIALS AND METHODS
Subjects
Tear samples were collected from 40 full-term newborn infants (21 male infants and 19 female infants) within the first 48 hours of birth (weight and age in Table 1). Fourteen of these newborn infants (6 male infants and 8 female infants) were presented within the next 4 months for a second collection. During sample collection, the infants were resting in a cot or were held by a parent. If an infant was alert, their eyelids were not kept open manually.
TABLE 1.
Subject Groups: Sex and Mean ± SD for Gestational Duration (GD), Postnatal Age (PNA), and Weight on the Day of Tear Collection (Equal to Birth Weight for Newborns)
| Sex | GD (Wk) | PNA (Wk) | Weight (kg) | |
|---|---|---|---|---|
| At birth (n = 40) | 21 males, 19 females | 39.71 ± 1.27 | — | 3.42 ± 0.53 |
| At follow-up (n = 14) | 6 males, 8 females | 40.04 ± 1.5 | 7.76 ± 6.14 | 5.04 ± 1.34 |
Twenty-two adult subjects (12 men and 10 women, mean age: 24.95 ± 3.63 years) were recruited as a control group from members of staff and students of Cardiff University. Subjects were excluded if they had a systemic or ocular surface disease, took medication known to affect the ocular surface, wore contact lenses, suffered from an allergy, were pregnant, or had dry eye. Dry eye was assessed using a modified McMonnies questionnaire9 and defined when one of its described symptoms (soreness, scratchiness, dryness, gritti-ness, and burning) was found to be present and when a fluorescein breakup time of less than 5 seconds was observed. This stringent breakup time limit was chosen to decrease the probability of including subjects with deficient tear volumes. Ethical approval was obtained from the Central and Local Office for Research Ethics Committees (reference number: 04/WSE02/125; date: 8/11/2004), and informed consent was obtained from all adult subjects and infant subjects’ parents before participation.
Tear Collection
Tears were collected with flexible rods of 2-mm diameter and 10-mm length, made of cellulose acetate (Filtrona, Hamburg, Germany). The safety and collection efficiency of the cellulose rods had been approved in previous experiments.10 Before use, rods were disinfected by 5 minutes of exposure to UV light. The cellulose rod was held with forceps, close to the lower eyelid and the tip was gently touching the tear meniscus at the lower eyelid using our previously published protocol.10 Tear collection was restricted to a maximum of 2 minutes. During the collection, the eyelids were allowed to close for blinking every 5 seconds to minimize the stress on the infant and to minimize reflex tearing. A sample was collected from the right eye only.
Tear Analysis
After tear collection, the rods were placed in a sealed 0.2-mL Eppendorf tube and kept on ice. All samples were processed on the same day. To extract the sample, a small hole was cut in the tip of the 0.2-mL tube, which was then placed in a larger 0.5-mL tube. This combination was then centrifuged at 15000g, 4°C for 3 minutes.10 Each sample was first assessed for volume, and if sufficient tear volume was obtained, the required volume was subjected to sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with blue staining (Pierce Biotechnology, Inc, Rockford IL).
Duplicate undiluted aliquots of 0.5 μL of samples were subjected nondenatured to SDS-PAGE to obtain IgA as a single band as described previously10 In summary, resolving gels were produced in the laboratory from 8%-12%, starting with 8% on the top (Fig. 1). A modified coomassie blue stain (Pierce Biotechnology, Inc) was used for visualization of protein bands. Major proteins, tear-specific IgA, lactoferrin, and lysozyme, were identified by their molecular weight compared with a marker (250-10 kDa) and their reference standards run on the gels: human secretory IgA (1.8 μg/|μL), human milk lactoferrin 2.5 μg/μL, hen egg lysozyme 2.5 μg/μL (all from Sigma-Aldrich, Co, Gillingham, United Kingdom) and IgM (1 μg/μL from Autogen Bioclear, Wiltshire, United Kingdom). All gels were measured by scanning densitometry (Epson Expression 1680 Pro; Hermel Hempstead United Kingdom and Image Acquisition and Analysis software; Ultra-Violet Products, Cambridge, United Kingdom). Western blotting was used to verify IgA and to differentiate it from IgM (Fig. 2) and serum albumin (primary antibodies for all 3 from Sigma-Aldrich, Co). Representative samples from adults, molecular weight marker, and standards were subjected to Western blotting. IgM was prevalent in the uppermost band on the stacking gel. Western blotting was also used to verify the location of lipocalin (primary antibody against recombinant lipocalin 2 from R&D systems, Abingdon, Oxfordshire, United Kingdom) on representative samples. Duplicate aliquots of 1 μL were analyzed for total protein content by the Bradford method (Bradford, 1976) following the kit directions for microanalysis (Pierce Biotechnology, Inc).
FIGURE 1.
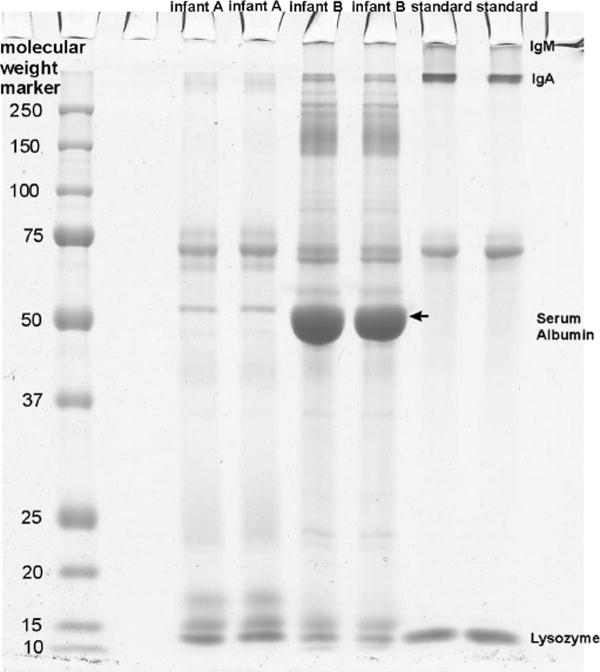
Representative tear protein profile on gel electro-phoresis from 2 different newborn infants. Although the bands from IgA, lactoferrin, and serum albumin look less strong for infant A compared with infant B, lipocalin and lysozyme (and unknown band with molecular weight of 15 kDa) are stronger.
FIGURE 2.
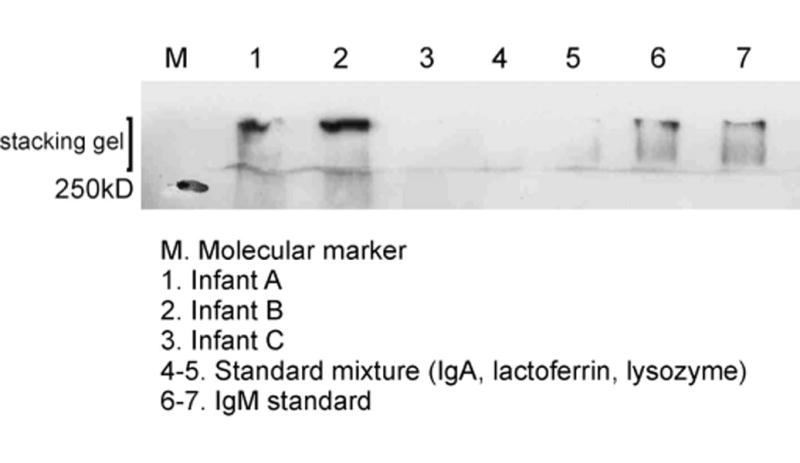
Western blotting of infant tear samples to confirm identity of IgM on stacking gel.
Statistical Analysis
For statistical analysis, SPSS 14 was used. Before analysis, data were checked for normality and appropriate parametric or nonparametric tests used.
RESULTS
Table 2 describes the distribution of tear volume collected within each group. Tear collection was performed on 40 newborn infants. Out of 40 collection rods, 31 rods contained a tear sample with a median interquartile range (IQR) tear volume of 0.5 mL (IQR: 0.6-2 mL). All collections from the infants at an older age contained samples with a median tear volume of 2.5 μL (IQR: 1.4-7.75 μL). When comparing the tear volumes collected from the infants at a newborn age with a median of 1.1 μL (IQR: 0.4-2.38 μL) with the tear volume collected from the same infants at the older age, a significant difference was found (P = 0.01, Wilcoxon signed rank test). In comparison to infant tears, the median tear volume in adults was 6 μL (IQR: 2.73-12.75 μL). A significant difference was found (P < 0.001, Kruskall-Wallis test) between these groups, and specifically, between the volumes obtained in the first and second collections of the newborns (P < 0.001, Mann-Whitney test).
TABLE 2.
Distribution of Tear Volume Size (in Percentage and Numbers)
| Tear Volumes | Newborns | Infants at Older Age | Adults |
|---|---|---|---|
| No tear volume | 22.5% (9) | 0 | 0 |
| Very low (0.1 and < 1 μL) | 22.5% (9) | 7% (1) | 14% (3) |
| Moderate (1 and < 5 μL) | 47.5% (19) | 64% (9) | 36% (8) |
| High (5-15 μL) | 7.5% (3) | 14% (2) | 27% (6) |
| Very high (> 15 μL) | 0 | 14% (2) | 23% (5) |
Volumes between 0.1 and < l μL were below the amount needed for protein analysis.
Samples from 22 newborns, 13 of the infants at an older age, and 19 adults were subjected to SDS-PAGE. Figure 1 shows the variety of protein bands found in 2 representative infant samples. Mean IgA concentrations were observed and found to differ significantly between the 3 subject groups [P = 0.001, analysis of variance (ANOVA); P < 0.05, Bonferroni] (Fig. 3). No difference in lactoferrin concentration (P = 0.05, ANOVA 1-way) or lysozyme concentration (P = 0.131, ANOVA) between the subject groups was identified (Fig. 3). However, the lysozyme concentration in infants showed a larger variation than that in adults. The mean protein concentration in the newborns (n = 8) was 10.95 ± 5.51 (μg/μL ± SD) and was 12.93 ± 3.99 in the older infants (n = 11). The tear protein concentrations tested of the 8 newborns and their second collection at an older age were statistically not correlated and no difference was found (P > 0.05, paired samples correlations and P > 0.05, paired t test). The 2 groups were treated as unrelated. In adults (n = 9), the total protein concentration was 13.04 ± 3.46 (μg/μL ± SD). No significant difference (P > 0.5, ANOVA) was found in the protein concentrations between any of the groups.
FIGURE 3.
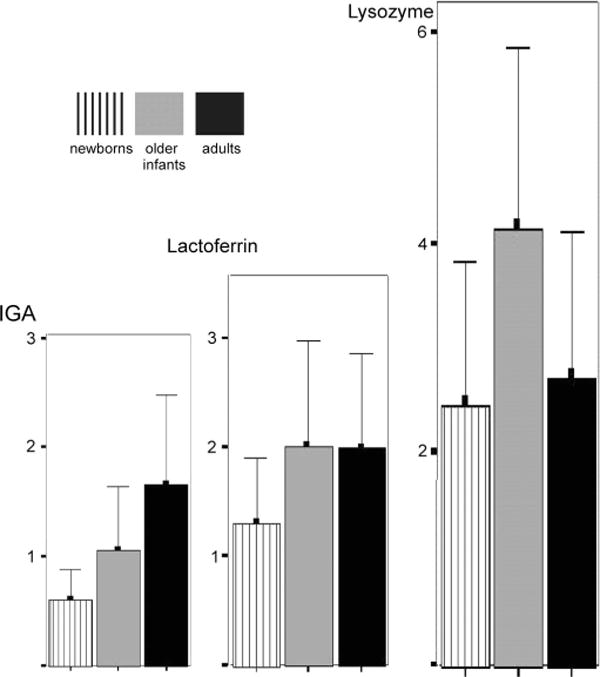
Major protein distribution in 3 experimental groups (y axis represents concentrations in μg/μL).
Observing protein profiles on SDS-PAGE, lipocalin bands were identified in 36% of the samples from newborns, 54% of older infants& samples, and 100% of adults. Serum albumin was absent in all adult samples (perhaps because of poor sensitivity of the gel stain for low protein concentrations) but was displayed in 86% of newborn and 54% of older full-term samples. In adults, no IgM band was observed whereas 55% of newborn and 31% of older infant gel images displayed an IgM band.
DISCUSSION
This study has found that, at birth, newborn infants have a lower tear volume than adults but that there seems to be a rapid increase to adult levels after birth. No difference was found between newborn and adult total protein concentrations, but there were differences in protein profile and major protein concentrations.
Tears were collected in all subjects, except for 9 (22.5%) of the newborns where this was not possible. If this was caused by the collection technique being unable to collect tears in some of the subjects, then it would be expected to have a similar high number of failed collections in the other groups, which did not occur. Instead a late onset of tear secretion is more likely to have caused the lack of large collection volume. This is in agreement with Sjogren1 who found alacrimy in 13% of full-term newborns, which decreased to only 3% of the subjects in 1–7 weeks. Many studies have looked at tear secretion rate, but a comparison of collection volume is difficult because most used Schirmer paper, an invasive method used over 5 minutes.2 Isenberg et al11 found a secretion from full-term infants of 9.2 (± 4.3) mm measured with topical anesthetics, and 13.2 (± 6.5) mm without. These values approach the normal secretion for adults.
The IgA concentration was lowest in full-term newborn infants. Only 1 investigator previously has measured the total protein concentration of infants, and they also found IgA at very low concentrations.12 This has led some investigators to believe that infants are essentially IgA free.13 However, these reports are not recent, and they may have used less sensitive assays for large tear volumes. IgA concentration decreases with stimulation,14 which may have occurred with the larger tear volume samples. In this study, small sample sizes and minimizing reflex tearing, indicated that there was no dilution effect on the IgA findings.
Lactoferrin is a tear protein, also present in milk.15 In adults, its concentration is 0.8–1.48 μg/μL and decreases with age.16 In patients with dry eye, lactoferrin is usually altered compared with healthy subjects.17 In this study, infant lactoferrin concentration showed no difference to that of adults. This is the first time that lactoferrin concentrations in infants have been measured. Lysozyme in infant tears was first identified in 1963.18 Its concentration cannot diminish by stimulation,14 which may be the reason why it attracted those researchers’ attention who used the invasive collection technique of Schirmer paper strips. A wide range of lysozyme concentrations has been reported in the literature (1.1, 1.6,12 and 1.118 μg/μL19). The current study also showed a large concentration variability (as suggested by the large SD) between infant tears. This may be because of uncontrolled factors such as feeding and sleeping patterns. Similarly interesting was the absence of lipocalin in some infant samples. Lipocalin originates in the lacrimal gland, and its production is under the same regulation as lysozyme.20 Lipocalin contributes to the spread of the superficial lipid layer.21 There is a need for further investigation because previous research with healthy infants has identified high-quality lipid layers with improved blink rates.3
Serum albumin has been demonstrated in infants,18 but this is the first report of identification in unstimulated tears. Serum albumin is not locally produced but derived from conjunctival blood vessels. In adults, its concentration is very low but rises in closed eye tears22 and with dry eye.23 There is also a marked decrease in concentration with stimulation from 0.042 ± 0.012 to 0.012 ± 0.004 μg/μL.24 Considering that the infant tear film is superior in its lipid and mucus content, it is unlikely that the high concentration of serum albumin in infants is caused by underdeveloped or leaking lacrimal blood vessels during tear secretion. High-intensity serum albumin bands on gel electrophoresis may be related to typical infant sleeping phases.
IgM has been found in very small concentrations in adult tears using sensitive high-performance liquid chromatography methodology,24 but because of its low abundance, it is not detectable in adult tears by blue staining in gel electrophoresis. Thus, it was not expected to be found in infant tears. However, a band on the top of the gel proved the existence of this protein in the quantity that is comparable to the other major proteins. In infants, IgM may reach the tear fluid via ocular surface vessels,25 possibly alongside serum albumin, but this would be unusual because IgM is a large molecule that cannot diffuse well. Further exploration of the source of inflammatory antibodies such as IgM and determination of the diagnostic value of tear protein testing are required.
The results from this study are helpful in understanding more about the development of the tear film during infancy. However, there are some methodological issues that limit the conclusions. The protein concentration determined from gel electrophoresis is only a semiquantitative method, and Western blotting was used only to verify the presence of the specific protein. Using a noninvasive tear collection technique with a flexible soft rod, which is comfortable to control, helped to win trust of the parent and hence enable recruitment. However, the time point for collection of the repeat measurement of these newborn infants depends on the infant’s health and the parents availability to participate, resulting in the repeat measurement being spread over the first 4 months of life.
In conclusion, newborn tear secretion is low but increases significantly within early infancy. Interesting differences in tear protein profiles were identified between infant and adult tears, including variations in lysozyme and lipocalin concentrations. This insight into infant tear protein offers many new ideas for future research. The investigation of protein profile in premature infants with respect to maturity and a comparison to full-term infants may help to provide a better understanding of tear secretion development.
Acknowledgments
The authors wish to thank Dr. Julie Albon, Dr. Bablin Molik, Dr. Christine Purslow, Prof Jonathan Jackson, and Dr. Patrick Cartlidge for their support with the study recruitment, analysis, and article writing.
References
- 1.Sjogren H. The lacrimal secretion in newborn premature and fully developed children. Acta Ophthalmol (Copenh) 1955;33:557–560. doi: 10.1111/j.1755-3768.1955.tb03329.x. [DOI] [PubMed] [Google Scholar]
- 2.Apt L, Cullen BF. Newborns do secrete tears. JAMA. 1964;189:951–953. doi: 10.1001/jama.1964.03070120073024. [DOI] [PubMed] [Google Scholar]
- 3.Lawrenson JG, Birhah R, Murphy PJ. Tear-film lipid layer morphology and corneal sensation in the development of blinking in neonates and infants. J Anat. 2005;206:265–270. doi: 10.1111/j.1469-7580.2005.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isenberg SJ, Del Signore M, Chen A, et al. The lipid layer and stability of the preocular tear film in newborns and infants. Ophthalmology. 2003;110:1408–1411. doi: 10.1016/S0161-6420(03)00451-2. [DOI] [PubMed] [Google Scholar]
- 5.Kaercher T, Mobius D, Welt R. Biophysical behaviour of the infant meibomian lipid layer. Int Ophthalmol. 1994;18:15–19. doi: 10.1007/BF00919408. [DOI] [PubMed] [Google Scholar]
- 6.Mantelli F, Tiberi E, Micera A, et al. MUC5AC overexpression in tear film of neonates. Graefes Arch Clin Exp Ophthalmol. 2007;245:1377–1381. doi: 10.1007/s00417-007-0602-9. [DOI] [PubMed] [Google Scholar]
- 7.Sen DK, Sarin GS. Immunoglobulin concentrations in human tears in ocular diseases. Br J Ophthalmol. 1979;63:297–300. doi: 10.1136/bjo.63.5.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miano F, Mazzone M, Giannetto A, et al. Interface properties of simplified tear-like fluids in relation to lipid and aqueous layers composition. Adv Exp Med Biol. 2002;506(pt A):405–417. doi: 10.1007/978-1-4615-0717-8_58. [DOI] [PubMed] [Google Scholar]
- 9.McMonnies CW. Key questions in a dry eye history. J Am Optom Assoc. 1986;57:512–517. [PubMed] [Google Scholar]
- 10.Esmaeelpour M, Cai J, Watts P, et al. Tear sample collection using cellulose acetate absorbent filters. Ophthalmic Physiol Opt. 2008;28:577–583. doi: 10.1111/j.1475-1313.2008.00603.x. [DOI] [PubMed] [Google Scholar]
- 11.Isenberg SJ, Apt L, McCarty J, et al. Development of tearing in preterm and term neonates. Arch Ophthalmol. 1998;116:773–776. doi: 10.1001/archopht.116.6.773. [DOI] [PubMed] [Google Scholar]
- 12.Patrick RK. Lacrimal secretion in full-term and premature babies. Neo-nates do secrete tears. Trans Ophthalmol Soc U K. 1974;94:283–289. [Google Scholar]
- 13.Watson RR, McMurray DN, Martin P, et al. Effect of age, malnutrition and renutrition on free secretory component and IgA in secretions. Am J Clin Nutr. 1985;42:281–288. doi: 10.1093/ajcn/42.2.281. [DOI] [PubMed] [Google Scholar]
- 14.Fullard RJ, Tucker DL. Changes in human tear protein levels with progressively increasing stimulus. Invest Ophthalmol Vis Sci. 1991;32:2290–2301. [PubMed] [Google Scholar]
- 15.Masson PL, Heremans JF. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971;39:119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- 16.Jensen OL, Gluud BS, Birgens HS. The concentration of lactoferrin in tears of normals and of diabetics. Acta Ophthalmol (Copenh) 1986;64:83–87. doi: 10.1111/j.1755-3768.1986.tb06877.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi Y, Ishida R, Kojima T, et al. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003;136:291–299. doi: 10.1016/s0002-9394(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 18.Allerhand MD, Karelitz S, Penbharkkul S, et al. Electrophoresis and immunoelectrophoresis of neonatal tears. J Pediatr. 1963;62:85–92. doi: 10.1016/s0022-3476(63)80076-1. [DOI] [PubMed] [Google Scholar]
- 19.Etches P, Leahy F, Harris D, et al. Lysozyme in the tears of newborn babies. Arch Dis Child. 1979;54:218–221. doi: 10.1136/adc.54.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dartt DA. Signal transduction and control of lacrimal gland protein secretion: a review. Curr Eye Res. 1989;8:619–636. doi: 10.3109/02713688908995762. [DOI] [PubMed] [Google Scholar]
- 21.Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function, and control. Adv Exp Med Biol. 1998;438:281–295. doi: 10.1007/978-1-4615-5359-5_40. [DOI] [PubMed] [Google Scholar]
- 22.Sack RA, Tan KO, Tan A. Diurnal tear cycle: evidence for a nocturnal inflammatory constitutive tear fluid. Invest Ophthalmol Vis Sci. 1992;33:626–640. [PubMed] [Google Scholar]
- 23.Mandel ID, Stuchell RN. The Lacrimal-Salivary Axis in Health and Disease. Lubbock, TX: Dry Eye Institute, Inc; 1986. [Google Scholar]
- 24.Fullard RJ, Snyder C. Protein levels in nonstimulated and stimulated tears of normal human subjects. Invest Ophthalmol Vis Sci. 1990;31:1119–1126. [PubMed] [Google Scholar]
- 25.Franklin RM, Prendergast RA, Silverstein AM. Secretory immune system of rabbit ocular adnexa. Invest Ophthalmol Vis Sci. 1979;18:1093–1096. [PubMed] [Google Scholar]


