Summary
The type III secretion system (TTSS) of Pseudomonas aeruginosa is induced by contact with eukaryotic cells and by growth in low-calcium media. We have identified a protein, RtsM, that is necessary for expression of the TTSS genes in P. aeruginosa. RtsM possesses both histidine kinase and response regulator domains common to two-component signalling proteins, as well as a large predicted periplasmic domain and seven transmembrane domains. Deletion of rtsM resulted in a defect in production and secretion of the type III effectors. Northern blot analysis revealed that mRNAs encoding the effectors ExoT and ExoU are absent in the ΔrtsM strain under TTSS-inducing conditions. Using transcriptional fusions, we demonstrated that RtsM is required for transcription of the operons encoding the TTSS effectors and apparatus in response to calcium limitation or to host cell contact. The operon encoding the TTSS regulator ExsA does not respond to calcium limitation, but the basal transcription rate of this operon was lower in ΔrtsM than in the wild-type parent, PA103. The defect in TTSS effector production and secretion of ΔrtsM could be complemented by overexpressing ExsA or Vfr, two transcriptional activators involved in TTSS regulation. ΔrtsM was markedly less virulent than PA103 in a murine model of acute pneumonia, demonstrating that RtsM is required in vivo. We propose that RtsM is a sensor protein at the start of a signalling cascade that induces expression of the TTSS in response to environmental signals.
Introduction
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen that causes acute and chronic infections in hospitalized individuals, burn victims and cystic fibrosis patients. One of the virulence factors most strongly correlated with severe infection both in animal models and in human patients is its type III secretion system (TTSS) (Roy-Burman et al., 2001; Hauser et al., 2002). The TTSS is a specialized protein secretion apparatus that allows bacterial proteins to be translocated directly into the cyto-sol of host cells. In P. aeruginosa the TTSS apparatus is encoded by more than 25 genes that lie within four operons (pscNOPQRSTU, popNpcr1234DR, pcrGVHpopBD and exsDpscBCDEFGHIJK) (Fig. 1). The translocated bacterial proteins called effectors subsequently modulate the host cell to create an environment that favours bacterial survival and spread within the host (Cornelis and Van Gijsegem, 2000). Four effector proteins have been described for P. aeruginosa, namely ExoS, ExoT, ExoU and ExoY (Yahr et al., 1996; 1997; 1998; Finck-Barbancon et al., 1997; Hauser et al., 1998). ExoS (49 kDa) and ExoT (53 kDa) are related bifunctional proteins that act as GTPase activating proteins for Rho, Rac and Cdc42 (Goehring et al., 1999; Kazmierczak and Engel, 2002). ExoS and ExoT inhibit bacterial uptake by macrophages and epithelial cells, which allows P. aeruginosa to exist primarily as an extracellular pathogen (Pederson et al., 1999; Cowell et al., 2000; Garrity-Ryan et al., 2000). ExoS and ExoT also possess ADP-ribosyltransferase activity (Radke et al., 1999; Garrity-Ryan et al., 2004). ExoS ADP-ribosylates Ras signalling molecules and disrupts Ras-mediated signalling by host cells (Ganesan et al., 1999). ExoT ADP-ribosylates and inactivates Crk-I and Crk-II, two proteins that function in focal adhesion formation and phagocytosis (Sun and Barbieri, 2003). The effector ExoU is a 77 kDa protein that rapidly kills eukaryotic cells (Finck-Barbancon et al., 1997; Hauser et al., 1998). Recent work has shown that ExoU is a phospho-lipase whose translocation leads to perturbation of host cell membranes and consequent necrosis (Phillips et al., 2003; Sato et al., 2003). ExoY is a 42 kDa adenylate cyclase whose role in virulence is not clearly understood (Yahr et al., 1998). Together these effectors cause extensive tissue damage and host cell killing and evoke a host inflammatory response. It is worth pointing out that different clinical and laboratory strains express different combinations of these effectors; the strain characterized here, PA103, secretes ExoT and ExoU (Feltman et al., 2001). Thus, a TTSS-deficient mutant of PA103 is avirulent (because it cannot secrete the cytotoxin ExoU) and invasive (because it does not secrete the anti-internalization factor ExoT).
Fig. 1.

Regulation of TTSS expression in P. aeruginosa. The expression of the operons encoding the P.aeruginosa TTSS (which encode the TTSS apparatus, regulators and effectors) is under the control of the known regulators ExsA, ExsD, ExsC, CyaB and Vfr. ExsA is an AraC-like transcriptional activator that directly binds to a conserved sequence upstream of the genes it regulates, while ExsD is an antiactivator that binds to and sequesters ExsA. When bacteria are grown in low-calcium media, ExsC binds to and sequesters ExsD, which releases active ExsA. A second input into the system is provided by CyaB, an adenylate cyclase that is induced under low-calcium conditions. Increased cAMP levels produced by CyaB in turn activate the cAMP binding transcription factor, Vfr; both proteins are required for TTSS gene transcription. The CyaB–Vfr pathway may work upstream of or in parallel to ExsA.
Secretion of P. aeruginosa effector proteins is stimulated by contact with host cells, but it can also be elicited in vitro by growing bacteria at 37°C in media containing a chelator, such as nitriloacetate (NTA) or EGTA (Frank, 1997; Hornef et al., 2000). Such a TTSS-inducing media is conventionally referred to as ‘low-calcium’ media, even though NTA and EGTA are capable of chelating other divalent cations. Expression of the P. aeruginosa TTSS and its effectors is controlled at the transcriptional level by an AraC-like transcriptional activator, ExsA. ExsA binds to a consensus sequence (TNAAAANA) approximately 50 base pairs upstream of the transcriptional start site of TTSS genes (Yahr and Frank, 1994; Hovey and Frank, 1995; Yahr et al., 1995). ExsA also binds a negative regulator, ExsD, whose overexpression inhibits TTSS gene transcription (McCaw et al., 2002). A third regulator, ExsC, has recently been shown to interact with ExsD and is thought to act as an ‘anti-anti-activator’ that releases ExsA from inhibition by ExsD (Dasgupta et al., 2004). It is not clear, however, how the activity of these three proteins is related to environmental signals that activate type III secretion. Yahr and colleagues propose that an as yet unidentified ‘protein X’ sequesters ExsC when the TTSS channel is closed, but is secreted when the channel is activated by a signal such as low calcium, leaving ExsC free to interact with ExsD (Dasgupta et al., 2004). Such a coupling between gene expression and TTSS secretion is observed in other pathogens (Miller, 2002).
Wolfgang et al. recently identified an adenylate cyclase, CyaB, whose transcription is induced by growth in low-calcium media (Wolfgang et al., 2003). CyaB and a cAMP-dependent transcription factor, Vfr, are also required for expression of TTSS effectors in response to calcium depletion, and for full virulence of P. aeruginosa strain PAK in a murine model of acute pneumonia (Wolfgang et al., 2003; Smith et al., 2004). Overexpression of ExsA suppresses the TTSS expression defect of CyaB– and Vfr–mutants, suggesting that CyaB and Vfr may work upstream of ExsA (Wolfgang et al., 2003). However, the mechanism by which the CyaB–Vfr pathway responds to extracellular signals is still unclear.
Other pathogenic bacteria, notably Bordetella spp., Salmonella typhimurium and Vibrio spp., employ two-component signal transduction pathways to integrate environmental sensing with activation of their TTSS pathways (Yuk et al., 1998; Groisman, 2001; Henke and Bassler, 2004). We describe a novel regulator of type III secretion, RtsM, which is required for transcription of the genes encoding the effectors and the apparatus of the P. aeruginosa TTSS. RtsM is predicted to reside in the inner membrane and has a periplasmic domain coupled to cytoplasmic histidine kinase and response regulator domains characteristic of two-component signalling proteins. Thus we propose that RtsM may serve to link environmental sensing with activation of type III secretion in P. aeruginosa.
Results
RtsM is required for P. aeruginosa cytotoxicity in vitro
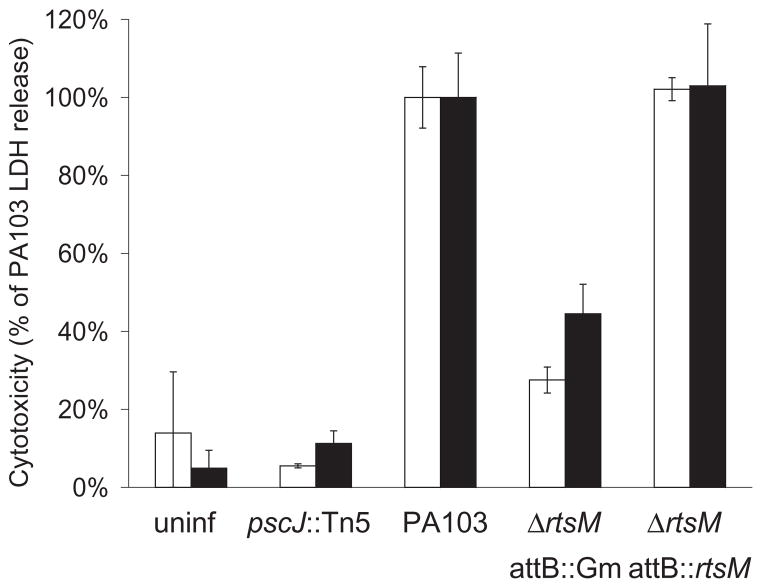
The gene rtsM (Regulator of Type III Secretion) (PA4856) was originally identified as the site of a transposon insertion that rendered the TTSS-deficient strain PA103ΔpscJ non-invasive toward epithelial cells (B. I. Kazmierczak et al., in preparation). As type III secretion in P. aeruginosa is induced by host cell contact (Vallis et al., 1999; Hornef et al., 2000), we reasoned that a mutant incapable of interactions required to promote bacterial internalization might also be defective in type III-mediated cytotoxicity toward epithelial cells. Therefore, we constructed an in-frame deletion of rtsM in P. aeruginosa strain PA103, a well characterized clinical isolate that causes rapid necrosis of cultured epithelial cells (Liu, 1966). Such cell damage, which can be quantified by measuring LDH release, has been primarily attributed to the cytotoxic effects of the TTSS effector ExoU (Finck-Barbancon et al., 1997; Hauser et al., 1998; Garrity-Ryan et al., 2000). ΔrtsM was markedly less cytotoxic than PA103, causing only 27.5% ± 3.3% and 44.5% ± 7.6% as much LDH release at 2 and 4 hpi respectively (Fig. 2). This defect could be complemented by reintroducing a single copy of the rtsM gene under control of its own promoter at the attB site of ΔrtsM (ΔrtsM attB::rtsM). ΔrtsM bound epithelial cells as well as PA103 (data not shown), suggesting that decreased adhesion is not responsible for the reduced cytotoxicity of this mutant. Likewise, ΔrtsM showed no significant growth defects as compared to PA103 under any of our assay conditions, arguing that impaired growth did not account for the reduction in cytotoxicity that we observed (data not shown).
Fig. 2.
Cytotoxicity toward HeLa cells as measured by LDH release. HeLa cells were infected with bacteria at an MOI of 10–15 in triplicate and sampled at 2 hpi (white bars) and 4 hpi (black bars). Values are normalized to cytotoxicity caused by PA103 at each time point. Negative controls include uninfected HeLa cells and cells infected with the TTSS– mutant, pscJ::Tn5. ΔrtsM attB::rtsM carries a copy of RtsM under control of its own promoter integrated into the chromosomal attB site, while ΔrtsM attB::Gm has a gentamicin resistance cassette inserted at the attB locus. Bars show the mean ± SD of an assay which is representative of three independent experiments.
RtsM is required for virulence in a murine model of acute pneumonia
The decreased cytotoxicity of ΔrtsM in vitro suggested that this mutant might be attenuated for virulence in vivo. Therefore, we tested whether ΔrtsM is less virulent than PA103 in a murine model of acute pneumonia (Schultz et al., 2002). We included ΔexsA as a negative control, as this mutant fails to express any TTSS effectors because of the deletion of the positive transcriptional activator ExsA (Frank, 1997; Schulert et al., 2003). Approximately 6 × 105 cfu of PA103, ΔrtsM or ΔexsA was used to infect C57Bl/6 mice intranasally. Mice were euthanized at 18 hpi, and the number of viable bacteria in the lungs, liver or spleen was determined. As seen in Fig. 3, PA103 was recovered from the lungs in about 10-fold greater numbers than the infecting dose, and was reproducibly isolated from the liver and spleen, indicating that the organism could disseminate systemically. Almost all PA103-infected animals appeared ill at the time of the sacrifice. In marked contrast, all ΔrtsM or ΔexsA-infected animals appeared healthy at 18 hpi; bacteria were recovered from the lungs in numbers equal to approximately 1% of the inoculum dose, and could not be detected in the liver or spleen. Thus, the ΔrtsM mutant appeared to be as attenuated in vivo as ΔexsA.
Fig. 3.
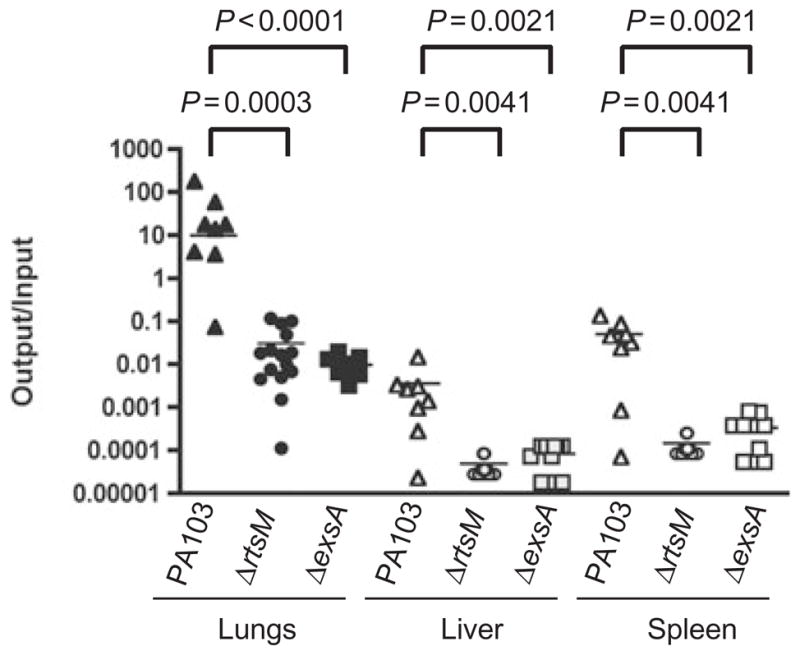
RtsM is required for virulence in a murine model of acute pneumonia. Eight to ten week old female C57Bl/6 mice were infected with 0.4–1.0 × 106 cfu of PA103 (n = 8), ΔrtsM (n = 15) or ΔexsA (n = 10). Mice were euthanized at 18 hpi and the number of bacteria present in lungs, liver and spleen were determined as described in Experimental procedures. Results are expressed as the ratio of cfu recovered/g tissue (output) to cfu present in the inoculum (input); each animal is represented by a data point, while the bar shows the geometric mean for each group. The Mann–Whitney test was used to calculate P-values (two-tailed) for each pair-wise comparison indicated. No colonies of ΔrtsM or ΔexsA were recovered from spleen or liver in any of the animals; the values shown indicate the limit of detection of the assay.
Production and secretion of the type III effectors is decreased in ΔrtsM
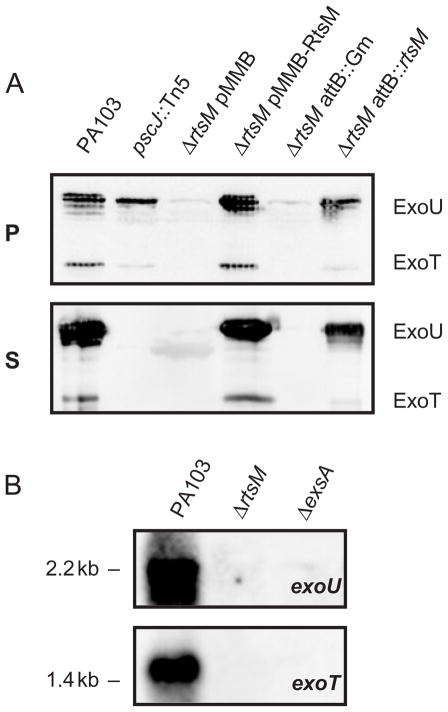
Expression of TTSS effectors is strongly correlated with P. aeruginosa virulence both in animal models and in studies of human disease (Roy-Burman et al., 2001; Hauser et al., 2002; Smith et al., 2004). The decreased cytotoxicity and virulence of ΔrtsM could result from decreased synthesis, secretion or translocation of the TTSS effectors. Therefore, we assayed whether ΔrtsM appropriately produced and secreted the effectors ExoU and ExoT when grown under inducing conditions for TTSS. As seen in Fig. 4A, ΔrtsM showed markedly diminished secretion of these effectors into culture supernatant. By analysing samples of secreted proteins produced by increasing numbers of ΔrtsM bacteria, we estimated that wild-type PA103 secretes 30- to 40-fold more ExoU than the ΔrtsM mutant (data not shown). pscJ::Tn5, a mutant bearing a transposon insertion in the operon encoding the structural components of the TTSS, also failed to secrete ExoU and ExoT. Appreciable amounts of ExoU and ExoT were detected in the pscJ::Tn5 cell pellet, indicating that this mutant still produces these effectors; ΔrtsM, however, revealed minimal cell-associated ExoU or ExoT. Thus, RtsM is required for either expression or stabilization of the TTSS effectors. Expression of RtsM from a low copy number plasmid (pMMB-RtsM) or from a single copy of the gene (ΔrtsM attB::rtsM) restored production and secretion of ExoU and ExoT.
Fig. 4.
A. Western immunoblot analysis of whole-cell pellet lysates (P) and culture supernatants (S) of PA103 and mutant derivatives grown under TTSS-inducing conditions. Sample loading was normalized to total protein (15 μg lane−1) or bacterial counts (5 × 108 cfu lane−1) for pellet and supernatant samples respectively. Polypeptides were separated by SDS-PAGE, transferred to PVDF and probed with polyclonal antiserum that recognizes TTSS effectors. The absence of ExoU or ExoT bands in supernants indicates a defect in secretion, while the absence of bands in the pellet indicates a defect in production. ΔrtsM pMMB (empty vector) and ΔrtsM attB::Gm serve as controls for the plasmid complemented strain ΔrtsM pMMB-RtsM and the strain carrying an ectopic copy of RtsM integrated at the attB site of ΔrtsM respectively.
B. Northern blot analysis of RNA prepared from PA103, ΔrtsM or ΔexsA bacteria grown under TTSS-inducing conditions. Five microgram total RNA was separated by electrophoresis, blotted to nitrocellulose and probed with riboprobes specific for exoU or exoT mRNA. Equal loading was confirmed by methylene blue staining of rRNA (not shown).
Ectopic expression of RtsM cannot overcome the requirement for a TTSS-inducing signal, such as calcium depletion, as demonstrated by the absence of ExoU and ExoT secretion when RtsM was expressed from an inducible promoter (e.g. pMMB-RtsM) during growth in non-inducing conditions (data not shown). We also observed that expression of RtsM from a high copy number plasmid under its own promoter (pMLD4) resulted in decreased production and secretion of the TTSS effectors in PA103 grown under inducing conditions (data not shown). Inhibition of normal signalling through two-component phosphorelays upon overexpression of a sensor kinase has been previously described, as for the PilS/PilR pathway, which governs transcription of the pilA gene in P. aeruginosa (Boyd and Lory, 1996).
RtsM is required for the transcription of the TTSS apparatus and effectors
Increased production of the TTSS effectors under inducing conditions is the result of increased transcription, which requires the transcriptional activator ExsA (Hovey and Frank, 1995). We tested whether RtsM was necessary for transcription of several TTSS effectors in response to calcium depletion by constructing transcriptional fusions of the exoS, exoT and exoU promoters to the luxCDABE reporter, which were integrated into the chromosome of PA103, ΔrtsM or ΔexsA. In PA103, exoS, exoT and exoU, transcription increased ≥30-fold over 4 h of growth in inducing conditions (Table 1). In contrast, only a twofold to threefold increase in transcription of these reporters was observed in ΔrtsM during growth under inducing conditions. This defect in transcriptional induction was indistinguishable from that observed for ΔexsA. These results were confirmed by Northern blotting RNA isolated from PA103, ΔrtsM or ΔexsA bacteria grown under inducing conditions. mRNA for exoT and exoU could only be detected in PA103, again suggesting that RtsM is required for increased TTSS effector transcription in response to calcium limitation (Fig. 4B).
Table 1.
Activation of TTSS transcription by calcium depletion requires RtsM.
| Fusion | PA103 | ΔrtsM | ΔexsA | |
|---|---|---|---|---|
| exoS::lux | 0 h | 3.8 ± 0.8a | 1.6 ± 0.3 | 2.0 ± 0.4 |
| 4 h | 118.2 ± 39.1 | 4.5 ± 0.6 | 12.4 ± 3.8 | |
| Fold increase | 30.9 ± 3.2 | 2.7 ± 0.2 | 6.5 ± 3.1 | |
| exoT::lux | 0 h | 3.9 ± 1.9 | 17.2 ± 21.9 | 3.5 ± 2.3 |
| 4 h | 403. ± 60.7 | 11.2 ± 3.8 | 8.9 ± 2.0 | |
| Fold increase | 76.9 ± 48.8 | 3.4 ± 2.8 | 3.1 ± 1.5 | |
| exoU::lux | 0 h | 3.2 ± 0.4 | 1.7 ± 0.1 | 2.7 ± 0.6 |
| 4 h | 93.7 ± 5.9 | 3.5 ± 1.5 | 7.5 ± 1.0 | |
| Fold increase | 29.7 ± 5.7 | 2.1 ± 1.0 | 2.8 ± 0.3 | |
| pscN::lux | 0 h | 3.8 ± 1.7 | 1.4 ± 0.5 | 1.6 ± 0.2 |
| 4 h | 327.0 ± 134.5 | 5.7 ± 3.2 | 5.5 ± 3.2 | |
| Fold increase | 92.8 ± 37.1 | 3.9 ± 1.6 | 3.4 ± 1.1 | |
| popN::lux | 0 h | 9.1 ± 3.2 | 1.1 ± 0.3 | 1.7 ± 0.7 |
| 4 h | 366.8 ± 21.6 | 9.4 ± 4.3 | 4.9 ± 1.9 | |
| Fold increase | 44.4 ± 18.2 | 8.4 ± 1.5 | 3.0 ± 0.4 | |
| pcrG::lux | 0 h | 19.5 ± 4.7 | 2.6 ± 1.3 | 3.2 ± 1.1 |
| 4 h | 960.9 ± 389.3 | 10.3 ± 3.1 | 12.0 ± 5.6 | |
| Fold increase | 59.5 ± 19.1 | 6.0 ± 0.8 | 5.8 ± 1.9 | |
| exsD::lux | 0 h | 11.1 ± 3.2 | 2.6 ± 1.0 | 1.3 ± 0.7 |
| 4 h | 822.0 ± 409.5 | 63.5 ± 28.8 | 7.4 ± 3.5 | |
| Fold increase | 77.7 ± 38.9 | 24.9 ± 6.5 | 6.0 ± 1.9 | |
| exsC::lux | 0 h | 30.8 ± 12.6 | 1.3 ± 0.1 | 0.8 ± 0.4 |
| 4 h | 934.6 ± 511.5 | 24.4 ± 6.5 | 9.6 ± 5.0 | |
| Fold increase | 33.9 ± 24.9 | 18.2 ± 4.2 | 15.3 ± 9.9 | |
| vfr::lux | 0 h | 2.5 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 |
| 4 h | 32.6 ± 20.3 | 23.7 ± 9.2 | 23.8 ± 9.1 | |
| Fold increase | 13.7 ± 9.5 | 13.1 ± 5.5 | 13.0 ± 6.1 | |
| cyaB::lux | 0 h | 2.5 ± 0.9 | 1.4 ± 0.4 | 2.0 ± 0.4 |
| 4 h | 9.6 ± 2.4 | 7.1 ± 1.5 | 12.9 ± 4.3 | |
| Fold increase | 4.4 ± 2.3 | 5.1 ± 0.6 | 6.4 ± 1.0 | |
| zwf::lux | Fold increase | 6.4 | 4.5 | 8.3 |
| ::luxb | Fold increase | 3.2 | 0.5 | 2.0 |
RLU/mg protein were measured for each promoter::lux transcriptional fusion at t = 0 (when bacteria were subcultured from non-inducing to inducing media conditions) and after 4 h growth in inducing media. Values are expressed as RLU/mg of protein at 0 h and 4 h, and fold increase at 4 h (ratio of t = 4 h/t = 0 h).
Mean ± SD of 2–5 independent experiments.
Promoterless construct.
The genes encoding the structural components of the TTSS are organized into several operons (Fig. 1). We used the same approach as described above to construct transcriptional reporters for the promoters upstream of these operons, and found that RtsM was also required for increased transcription of the pscNOPQRSTU, popNpcr1234DR and pcrGVHpopBD operons in response to low calcium (Table 1). This defect in transcriptional induction was indistinguishable from that observed for ΔexsA. As a control, we constructed a transcriptional reporter for a gene not involved in TTSS, zwf, which encodes glucose-6-phosphate dehydrogenase. Transcription of zwf did not vary between PA103, ΔrtsM and ΔexsA. Thus, RtsM, like ExsA, appeared to be required for transcriptional induction of the TTSS effectors and structural genes under in vitro inducing conditions.
RtsM is required for induction of ExoT transcription upon host cell contact
Production of TTSS effectors by P. aeruginosa following contact with tissue culture cells has been previously reported (Vallis et al., 1999; Hornef et al., 2000). Therefore, we asked whether RtsM is required for such induction by measuring transcription of the exoT::lux reporter construct in wild-type or ΔrtsM bacteria following infection of HeLa cells. We controlled for exposure to tissue culture media components by inoculating tissue culture dishes that lacked HeLa cells in parallel, and by washing all samples vigorously after allowing bacteria to bind for 45 min, so that luciferase production would be measured primarily from bacteria attached to confluent HeLa cells or to tissue culture plastic. Our washes routinely removed c. 95–99% of the initial inoculum. As seen in Fig. 5, exoT transcription increased c. 12-fold in PA103 in the presence of HeLa cells; a similar increase was not observed with the ΔrtsM mutant. As all washes and incubations were carried out using serum-free tissue culture media, our results suggest that RtsM is required to respond to a signal either present on the surface of HeLa cells or actively produced and secreted by them.
Fig. 5.
Transcriptional activation of exoT during exposure of bacteria to HeLa cells requires RtsM. Luminescence (RLU) produced by PA103 or ΔrtsM carrying a single copy of the exoT::lux transcriptional reporter was measured over 4 h following bacterial binding to HeLa cell monolayers or tissue culture plastic (media control). Results are reported as the fold increase in RLU relative to the reading obtained at t = 0 h. Bars represent the mean ± SD of triplicate samples, and are representative of two independent experiments.
RtsM affects transcription of ExsA and ExsD, but not of CyaB or Vfr
TTSS gene transcription is under the control of several positive (ExsA, ExsC, CyaB, Vfr) and negative (ExsD) regulators (McCaw et al., 2002; Wolfgang et al., 2003; Dasgupta et al., 2004). To examine whether RtsM is required for the expression of any of these regulators, we used the luxCDABE reporter fused to the promoters of exsCBA, exsDpscBCDEFGHIJK, cyaB and vfr. Transcription of cyaB and vfr did not require either ExsA or RtsM (Table 1). In contrast, transcription from the exsCBA and exsD promoters was altered in the absence of RtsM or ExsA. Wolfgang et al. have reported that the exsCBA promoter is not strongly induced by low calcium, which we also observed (data not shown, Wolfgang et al., 2003). The fold increase in transcription from exsC::lux that we measured during the 4 h growth in low-calcium media did not differ significantly between PA103, ΔrtsM or ΔexsA (Table 1). However, PA103 bacteria showed much higher amounts of basal transcription from the exsCBA promoter (30.8 ± 12.6 RLU/mg protein, measured when bacteria were subcultured from non-inducing to inducing conditions) than either PA103ΔrtsM (1.3 ± 0.1 RLU/mg protein) or PA103ΔexsA (0.75 ± 0.39 RLU/mg protein) (Table 1).
The exsD operon encodes both a negative regulator of type III secretion (ExsD) and many of the structural components of the TTSS ‘needle complex’ and is transcribed under non-inducing conditions (McCaw et al., 2002; Wolf-gang et al., 2003). Growth in low-calcium media did increase transcription of these genes in PA103 (Table 1). This increase was absent in ΔexsA and diminished in ΔrtsM, suggesting that RtsM is necessary for maximal activation of transcription. Basal transcription from the exsD promoter was somewhat higher in PA103 than in ΔrtsM or ΔexsA; a similar level of basal transcription was observed for the pcrG::lux and popN::lux reporters (Table 1). Thus, it is possible to subdivide the TTSS promoters into three groups. One group (exoS, exoT, exoU and pscN), which includes all the effectors, showed minimal basal transcription in the PA103, ΔrtsM and ΔexsA backgrounds and required both RtsM and ExsA for transcriptional activation in response to low calcium, as described above. A second group consists of the exsCBA promoter, which did not show strong transcriptional activation in response to calcium depletion in wild-type or mutant backgrounds, but which required RtsM and ExsA for its relatively high basal level of transcription. The rest of the promoters fall into a third group, which showed intermediate levels of basal transcription in PA103 and required ExsA and RtsM for maximal activation in response to calcium limitation.
Defects in TTSS effector production and secretion in ΔrtsM are complemented by overexpression of Vfr or ExsA
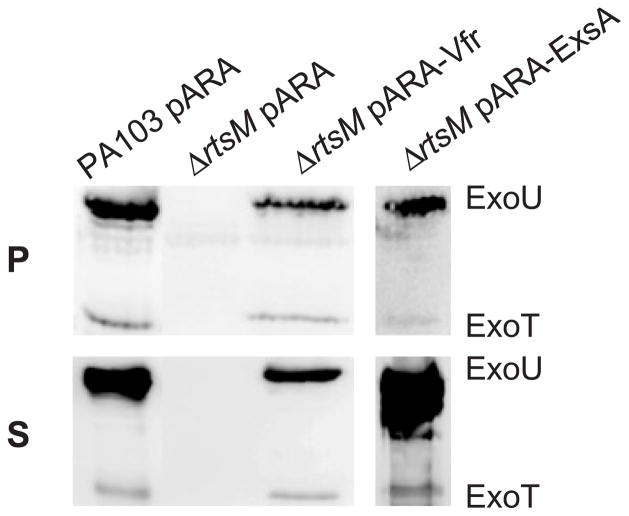
TTSS expression defects observed in CyaB− or Vfr− mutants can be completely suppressed by ectopic expression of the transcriptional activator ExsA (Wolfgang et al., 2003). Because RtsM is required for basal exsCBA transcription, we tested whether overexpressing ExsA would restore TTSS effector production and secretion in ΔrtsM bacteria. As seen in Fig. 6, ExsA overexpression complemented these defects in the ΔrtsM mutant. Likewise, over-expression of Vfr restored TTSS effector production and secretion in ΔrtsM. The amount of secreted ExoU and ExoT appeared to be greater when ExsA was overex-pressed than when Vfr was overexpressed. We cannot tell whether this simply reflects the synthesis of greater amounts of ExsA in the strain carrying the pARA-ExsA construct or whether more complex differences between the sets of proteins upregulated as a consequence of Vfr or ExsA overexpression account for this observation (Wolfgang et al., 2003).
Fig. 6.
Western immunoblot analysis of whole-cell pellet lysates (P) and culture supernatants (S) of PA103 and mutant derivatives grown under TTSS-inducing conditions in the presence of carbenicillin (200 μg ml−1) and 0.2% L-arabinose. Samples were normalized to total protein (15 μg lane−1) or bacterial counts (5 × 108 cfu lane−1) for pellet and supernatant samples respectively. ExoU and ExoT were detected by Western blotting with polyclonal antiserum (as described for Fig. 2A). PA103 and ΔrtsM carry the empty vector pARA, allowing all strains to be grown under identical conditions.
Expression and sequence analysis of RtsM
The rtsM gene is predicted to encode a 101 kDa protein. We confirmed that RtsM is indeed expressed in PA103 by constructing an epitope-tagged version of RtsM (RtsM-BB2) in which the BB2 tag (EVHTNQDPLD) was introduced immediately prior to the stop codon of rtsM by PCR (Brookman et al., 1995). The RtsM-BB2 construct was expressed under control of its own promoter from pUCP-SK (pRtsM-BB2) in bacteria grown in MinS media supplemented with 2.5 mM CaCl2 (non-inducing) or with 10 mM NTA (inducing) and detected in bacterial lysates by Western blotting with an anti-BB2 antiserum. As seen in Fig. 7, RtsM-BB2 was detected in similar amounts under both growth conditions.
Fig. 7.
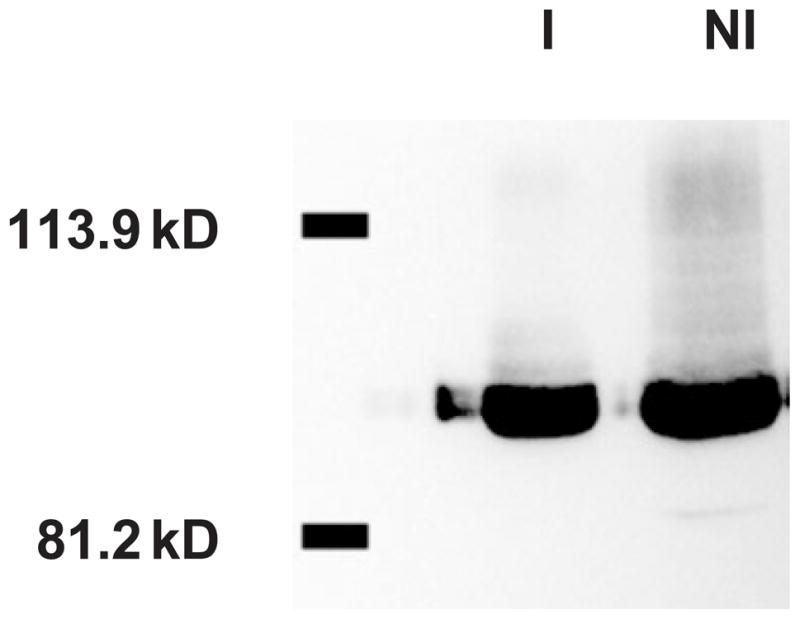
Detection of RtsM-BB2 in bacterial lysates grown under TTSS non-inducing and inducing conditions. ΔrtsM pRtsM-BB2 was grown in MinS media supplemented with 2.5 mM CaCl2 (non-inducing, NI) or 10 mM NTA (inducing, I). Bacterial pellets were lysed, and 5 μg of total protein was separated by SDS-PAGE. Proteins were transferred to PVDF and detected by Western immunoblotting using an anti-BB2 antibody. The positions of the 81.2 and 113.9 kDa molecular weight markers are indicated.
Pfam analysis indicated that RtsM possesses both sensor kinase and response regulator domains characteristic of two-component signalling proteins (Bateman et al., 2002) (Fig. 8). RtsM contains a histidine kinase domain (aa 418–621) [subtype 1B in the classification scheme of (Kim and Forst, 2001)] in which His424 is predicted to be autophosphorylated (Hoch and Silhavy, 1995). Two CheY-like response regulator domains follow in tandem (aa 654–786 and aa 803–929); each possesses the conserved residues and folds characteristic of such domains, including the invariant aspartates that are predicted sites of phosphorylation (Asp713 and Asp858) (Hoch and Silhavy, 1995). Using Pfam, we searched the PA01 genome for other proteins containing both histidine kinase and response regulator domains and found 16 proteins in addition to RtsM which are predicted to have such a hybrid structure (Stover et al., 2000). These are PA0928 (lemA/gacS), PA1243, PA1396, PA1611, PA1976, PA1992, PA2177, PA2583, PA2824, PA3044, PA3271, PA3462, PA3946, PA3974, PA4112 and PA4982. None of these proteins possess two response regulator domains in tandem.
Fig. 8.

Predicted domain structure of RtsM. rtsM (PA4856) is predicted to encode a 942 aa protein, with a cleaved signal sequence, large periplasmic domain and seven transmembrane regions followed by a sensor kinase domain and two response regulator domains in tandem. Numbers in parentheses indicate the predicted boundaries of each domain (aa), while predicted sites of phosphorylation (H424, D713 and D858) are indicated for each domain.
Phosphoryl transfer usually occurs from His to Asp to His to Asp within two-component phosphorelays, with an Hpt domain providing the intermediate His phosphoacceptor (Appleby et al., 1996). Although several of the sensor kinase/response regulator hybrids found in the PA01 genome also possess an Hpt domain (PA0928, PA3044, PA3946 and PA4112), giving them an overall domain structure similar to that of BvgS (Uhl and Miller, 1996), no HPt domain is present in RtsM. Moreover, although many response regulator proteins also possess DNA-binding domains that allow them to activate transcription directly once phosphorylated, RtsM has no domains characteristic of DNA-binding proteins or of transcriptional activators (Hoch and Silhavy, 1995).
The SignalP 3.0 algorithm predicts that RtsM has an N-terminal, cleaved signal sequence (aa 1–27) and seven transmembrane domains (aa 189–387) (Bendtsen et al., 2004). Thus, RtsM may be anchored in the inner membrane with a periplasmic domain of 160 amino acids. BLAST analysis reveals that homologues of RtsM (Evalue = 0.0) are present in several related organisms, including P. fluorescens (Pflu4318), P. putida (PP4824), Azotobacter vinelandii (Avin2657) and P. syringae pv syringae (Psyr2376) (Altschul et al., 1997). The functions of none of these homologues are known at this time; however, all possess a predicted large periplasmic region followed by multiple predicted transmembrane domains. RtsM lies between purD, which encodes phosphoribosy-lamine-glycine ligase, and PA4857, which encodes a hypothetical protein with no obvious structural features. Although the reading frames for all three genes are oriented in the same direction, the genes are widely spaced and not obviously part of an operon.
Discussion
Type III secretion systems are thought to be tightly controlled so that production and secretion of effector proteins occur primarily when bacteria come in contact with host cells. The basis for this contact-dependent regulation is not completely understood, although many environmental signals specifying presence within a host (temperature, osmolarity, altered carbon or nitrogen sources) or close interaction with mammalian cells (divalent cations, quorum sensing analogues, ATP) have been shown to be capable of altering expression or secretion of TTSS effectors (Francis et al., 2002; Sperandio et al., 2003; Tran Van Nhieu et al., 2003; Rietsch et al., 2004). In this work, we describe a novel P. aeruginosa protein, RtsM, that is required for induction of TTSS gene expression in response to both low calcium and host cell contact, and for virulence in a murine pneumonia model.
Our results establish that RtsM is necessary for transcription of genes encoding the TTSS effectors, structural components and regulators. RtsM possesses canonical histidine kinase and response regulator domains characteristic of two-component signalling proteins, which have all the catalytic residues and structural folds characteristic of bona fide sensor–regulator proteins. Experiments to prove that RtsM participates in a phosphorelay are currently underway. The structure of RtsM is unusual in one respect, namely that it possesses two CheY-like response regulator domains in tandem. We found no other protein with a similar domain structure in the PA01 genome database (http://www.pseudomonas.com), confirming a prior survey of putative two-component regulators in P. aeruginosa (Rodrigue et al., 2000), although other proteins with tandem response regulator domains are described, e.g. PleD of Caulobacter crescentus (Hecht and Newton, 1995). Both response regulator domains of RtsM have consensus sequences required for phosphorylation, unlike PleD, in which the second response regulator domain lacks a phosphorylatable Asp and a catalytic Lys residue (Hecht and Newton, 1995). We are currently examining what roles these two response regulator domains play in RtsM function.
RtsM lacks DNA-binding motifs, suggesting that it regulates transcription indirectly. None of the other known TTSS transcriptional regulators in P. aeruginosa—ExsA, ExsC, Vfr, CyaB or ExsD—have Asp or His residues that can serve as phosphate acceptors in a phosphorelay. Therefore, RtsM may work through an as yet unidentified intermediary, or it may activate one of the known TTSS regulators through a mechanism other than phosphorylation. Although two-component systems are known to regulate TTSS in other pathogens (Yuk et al., 1998; Groisman, 2001; Henke and Bassler, 2004), to our knowledge, this is the first example of a sensor kinase/response regulator protein involved in TTSS regulation in P. aeruginosa.
As demonstrated above, overexpression of ExsA can overcome the requirement for RtsM; however, overex-pressing RtsM in a ΔexsA mutant does not restore expression of TTSS during growth in low-calcium media (data not shown). Thus, RtsM may act upstream of ExsA. ΔrtsM bacteria show no significant difference from PA103 or ΔexsA bacteria when fold induction of the exsCBA promoter is measured under inducing conditions; indeed, transcription of the exsCBA promoter is relatively insensitive to calcium concentration in wild-type PA103 (Wolf-gang et al., 2003). What is striking, however, is the ≥20-fold difference in transcription under non-inducing conditions of exsCBA in PA103 versus either the ΔrtsM or ΔexsA mutant. If this difference in transcription translates into much lower protein levels of ExsA and ExsC, the two positive regulators of TTSS, and a relative excess of the negative regulators (ExsD and the postulated ‘Protein X’), the resulting ‘tone’ or responsiveness of the TTSS system may be very different in the ΔrtsM mutant.
We identified RtsM in a screen for P. aeruginosa mutants that had lost the ability to invade epithelial cells. Unlike many of the mutants recovered in that screen, RtsM− bacteria showed no defect in adherence to epithelial cells. It is unlikely that the effects of RtsM on the TTSS account for its non-invasive phenotype. The original screen was carried out in a TTSS− background (PA103pscJ::Tn5), as bacteria that translocate ExoT effectively inhibit internalization by both professional phagocytes and epithelial cells (Garrity-Ryan et al., 2000). Therefore, we hypothesize that RtsM is required for the expression of proteins in addition to those involved in TTSS, which we will identify using microarray-based techniques.
Wolfgang et al. recently used microarrays to identify gene products whose transcription was altered in response to calcium limitation (Wolfgang et al., 2003). RtsM (PA4856) was not identified in this screen, as it was expressed at similar levels in low-calcium medium versus LB, nor was it found among genes that are regulated by Vfr or CyaB (Wolfgang et al., 2003). We detect expression of an epitope-tagged allele of RtsM (expressed from its own promoter) in bacteria grown either in medium plus NTA or in medium plus calcium. Nonetheless, although RtsM transcription and expression do not appear to be regulated by calcium, RtsM may nonetheless be activated post-translationally by changes in calcium concentration.
At this time, we do not know the nature of the signal(s) sensed by RtsM. As shown above, RtsM is required for TTSS transcriptional induction both in response to low calcium and to contact with tissue culture cells. It clearly plays an important role during infection, as ΔrtsM bacteria cannot replicate in the lung or disseminate to spleen and liver in a murine acute pneumonia model. The presence of RtsM homologues in other pathogenic bacteria that employ a TTSS, such as P. syringae, raises the possibility that these homologues also play roles in regulating TTSS. If this proves to be the case, it will be interesting to ask whether the signal(s) transduced by RtsM and its homologues are also conserved between plant and human pathogens.
Experimental procedures
Bacterial strains and plasmids
All bacterial strains and plasmids used in this study are listed in Tables 2 and 3 respectively. P. aeruginosa strains were maintained on Vogel–Bonner minimal (VBM) media or cultured in Luria–Broth (LB) with antibiotics as required (200 μg ml−1 carbenicillin, 100 μg ml−1 gentamicin, 100 μg ml−1 tetracycline) (Vogel and Bonner, 1956). Escherichia coli strains were cultured in LB with antibiotics as required (100 μg ml−1 ampicillin, 15 μg ml−1 gentamicin, 20 μg ml−1 tetracycline). All strains were maintained at −80°C as 15% glycerol stocks. To induce type III secretion, P. aeruginosa strains were grown in MinS, a minimal media containing the calcium chelator NTA (Nicas and Iglewski, 1984), at 37°C with aeration. This media was rendered non-inducing by the omission of nitriloacetate and the addition of 2.5 mM CaCl2.
Table 2.
Bacterial strains used in this study.
| Strain | Description | Source |
|---|---|---|
| E. coli XL1 blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tcr)] | Stratagene |
| E. coli XL2 blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tcr) Amy Cmr] | Stratagene |
| E. coli S17-1 | Used for mating constructs into P. aeruginosa; thi pro hsdR recA RP4-2 (Tc::Mu) (Km::Tn7) | Simon et al. (1983) |
| PA103 | Virulent lung isolate of P. aeruginosa, known type III-secreted effector proteins are ExoT and ExoU | Liu (1966) |
| PA103pscJ::Tn5 | Non-cytotoxic and defective in type III secretion; Tcr | Kang et al. (1997) |
| ΔrtsM | PA103 strain containing an in-frame deletion of amino acids 37–924 of rtsM ORF | This study |
| ΔexsA | PA103 strain containing an in-frame deletion of exsA; defective in expression of type III secretion genes | Schulert et al. (2003) |
| PA103 attB::Gm | Gmr cassette integrated at the attB site of the PA103 chromosome; Gmr | This study |
| PA103 pARA | PA103 carrying empty pARA vector; Cbr | This study |
| ΔrtsM attB::Gm | Gmr cassette integrated at the attB site of the ΔrtsM chromosome; Gmr | This study |
| ΔrtsM attB::rtsM | RtsM under control of its own promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔrtsM pMMB67EH | ΔrtsM carrying empty pMMB67EH vector; Cbr | This study |
| ΔrtsM pMMB-RtsM | ΔrtsM carrying pMMB-RtsM expressing RtsM from IPTG inducible tac promoter; Cbr | This study |
| ΔrtsM pRtsM-BB2 | ΔrtsM carrying pRtsM-BB2 expressing RtsM with a C-terminal BB2 tag under the control of its own promoter; Cbr | This study |
| ΔrtsM pARA | ΔrtsM carrying empty pARA vector; Cbr | This study |
| ΔrtsM pARA-Vfr | ΔrtsM carrying pARA-Vfr expressing Vfr from an arabinose inducible promoter; Cbr | This study |
| ΔrtsM pARA-ExsA | ΔrtsM carring pARA-ExsA expressing ExsA from an arabinose inducible promoter; Cbr | This study |
| PA103 attB::lux | Promoterless lux fusion integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM attB::lux | Promoterless lux fusion integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA attB::lux | Promoterless lux fusion integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 exoS::lux | lux fusion with exoS promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM exoS::lux | lux fusion with exoS promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA exoS::lux | lux fusion with exoS promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 exoT::lux | lux fusion with exoT promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM exoT::lux | lux fusion with exoT promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA exoT::lux | lux fusion with exoT promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 exoU::lux | lux fusion with exoU promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM exoU::lux | lux fusion with exoU promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA exoU::lux | lux fusion with exoU promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 pscN::lux | lux fusion with pscN operon promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM pscN::lux | lux fusion with pscN operon promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA pscN::lux | lux fusion with pscN operon promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 popN::lux | lux fusion with popN operon promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM popN::lux | lux fusion with popN operon promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA popN::lux | lux fusion with popN operon promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 pcrG::lux | lux fusion with pcrG operon promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM pcrG::lux | lux fusion with pcrG operon promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA pcrG::lux | lux fusion with pcrG operon promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 exsD::lux | lux fusion with exsD operon promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM exsD::lux | lux fusion with exsD operon promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA exsD::lux | lux fusion with exsD operon promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 exsC::lux | lux fusion with exsC operon promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM exsC::lux | lux fusion with exsC operon promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA exsC::lux | lux fusion with exsC operon promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 cyaB::lux | lux fusion with cyaB promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM cyaB::lux | lux fusion with cyaB promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA cyaB::lux | lux fusion with cyaB promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 vfr::lux | lux fusion with vfr promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM vfr::lux | lux fusion with vfr promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA vfr::lux | lux fusion with vfr promoter integrated at the attB site of the ΔexsA chromosome | This study |
| PA103 zwf::lux | lux fusion with zwf promoter integrated at the attB site of the PA103 chromosome | This study |
| ΔrtsM zwf::lux | lux fusion with zwf promoter integrated at the attB site of the ΔrtsM chromosome | This study |
| ΔexsA zwf::lux | lux fusion with zwf promoter integrated at the attB site of the ΔexsA chromosome | This study |
Table 3.
Plasmids used in this study.
| Plasmid | Description | Source |
|---|---|---|
| pGEM-T | Cloning vector; Apr | Promega |
| pTopo | Blunt end cloning vector; Apr | Invitrogen |
| pUCP-SK | P. aeruginosa – E. coli shuttle vector; Apr(Cbr) | Watson et al. (1996) |
| pMMB67EH | P. aeruginosa expression vector with an IPTG inducible tac promoter; Apr(Cbr) | Furste et al. (1986) |
| pBAD30 | E. coli expression vector encoding araC and an arabinose inducible promoter (pBAD); Apr | Guzman et al. (1995) |
| pARA | 1.3 kb ClaI-HindIII fragment from pBAD30 containing araC and the arabinose inducible promoter (pBAD) into ClaI-HindIII of pUCP-SK; Apr(Cbr) | This study |
| pEX100T | Allelic replacement suicide plasmid; Apr (Cbr) sacB oriT | Schweizer and Hoang (1995) |
| pKO-RtsM | 624 bp BamHI-EcoRI N-terminus fragment and 835 bp EcoRI-HindIII C-terminus fragment of RtsM cloned in tandem in BamHI-HindIII of pEX100T; Apr(Cbr) | This study |
| mini-CTX-2 | Contains attP site for integration at the attB site of P. aerugionosa chromosome; Tcr | Hoang et al. (1998) |
| mini-CTX-lux | Vector used for construction of transcriptional fusions with the luxCDABE operon from Xenorhabdus luminescens; contains attP site for integration at the attB site of P. aerugionosa chromosome; Tcr | Becher and Schweizer (2000) |
| pFLP2 | Source of inducible Flp recombinase; Apr(Cbr) | Hoang et al. (1998) |
| p0013207 | Cosmid clone containing rtsM in pLA2917, Tcr | Croft et al. (2000) |
| pMLD4 | 3.5 kb NotI-NsiI fragment containing rtsM under control of its own promoter from cosmid pM0013207 into NotI-PstI of pUCP-SK; Apr(Cbr) | This study |
| pRtsM-BB2 | pMLD4 derivative containing C-terminal BB2 tagged rtsM; Apr(Cbr) | This study |
| pMMB-RtsM | 3.3 kb BstBI-HindIII fragment from pMLD4 in SmaI-HindIII of pMMB67EH under IPTG inducible tac promoter; Apr(Cbr) | This study |
| mini-CTX-2-RtsM | 3.5 kb NotI-EcoRI fragment from pMLD4 in NotI-EcoRI of mini-CTX-2; Tcr | This study |
| pX1918G | Source of aacC1 gentamicin resistance cassette; Gmr | Schweizer and Hoang (1995) |
| mini-CTX-2-Gm | aacC1 gene from pX1918G cloned in EcoRI of mini-CTX-2; Tcr, Gmr | This study |
| mini-CTX-exoSp-lux | 303 bp EcoRI-BamHI PCR amplified fragment of exoS promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-exoTp-lux | 310 bp EcoRI-BamHI PCR amplified fragment of exoT promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-exoUp-lux | 310 bp EcoRI-BamHI PCR amplified fragment of exoU promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-pscNp-lux | 330 bp EcoRI-BamHI PCR amplified fragment of pscN operon promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-popNp-lux | 313 bp EcoRI-BamHI PCR amplified fragment of popN operon promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-pcrGp-lux | 290 bp EcoRI-BamHI PCR amplified fragment of pcrG operon promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-exsDp-lux | 309 bp EcoRI-BamHI PCR amplified fragment of exsD operon promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-exsCp-lux | 300 bp EcoRI-BamHI PCR amplified fragment of exsC operon promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-cyaBp-lux | 300 bp EcoRI-PstI PCR amplified fragment of cyaB promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-vfrp-lux | 300 bp EcoRI-BamHI PCR amplified fragment of vfr promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| mini-CTX-zwfp-lux | 304 bp EcoRI-BamHI PCR amplified fragment of zwf promoter region in EcoRI-BamHI of mini-CTX-lux; Tcr | This study |
| pARA-ExsA | 988 bp EcoRI fragment of exsA ORF in EcoRI of pARA; Apr(Cbr) | This study |
| pARA-Vfr | 741 bp EcoRI-XbaI PCR amplified fragment of vfr ORF in EcoRI-XbaI of pARA; Apr(Cbr) | This study |
Ap, ampicillin; Cb, carbenicillin; Cm, chloramphenicol; Gm, gentamicin; Km, kanamycin; Tc, tetracycline.
Strain construction
All PCR primers employed in this study are listed in Table S1 (Supplementary material), and are based on the PA01 genome sequence (http://www.pseudomonas.com) (Stover et al., 2000). All amplifications were carried out with Pfu Turbo polymerase, using PA103 genomic DNA as template. PCR products were subcloned into pGEM-T or pTOPO and sequenced to confirm that no mutations were introduced during amplification. Plasmids were introduced into E. coli strains by electroporation (Sambrook et al., 1989), and transformed into chemically competent P. aeruginosa (Mattick et al., 1987), unless otherwise specified.
An unmarked, in-frame deletion of aa 37–924 of RtsM in PA103 was constructed by allelic exchange (Schweizer and Hoang, 1995). Briefly, N-terminal (624 bp) and C-terminal (835 bp) regions flanking rtsM were amplified using the primer pairs rtsM-N1/rtsM-N2 and rtsM-C1/rtsM-C2 respectively. The amplified regions were subcloned in tandem into the gene replacement vector pEX100T to generate pKO-RtsM. This construct was transformed into E. coli S17-1 and mobilized into PA103 by mating. Carbenicillin-resistant exconjugants (merodiploids) were then resolved by growth on VBM plus 5% sucrose as described previously (Garrity-Ryan et al., 2000). Potential mutants were screened by PCR and confirmed by Southern blot (data not shown).
For the generation of complementation constructs, a 3.5 kb NotI-NsiI fragment containing the RtsM ORF, as well as 259 bp up and 457 bp downstream, was subcloned from cosmid pMO013207 into pUCP-SK (NotI-PstI) to generate pMLD4 (Croft et al., 2000). In this construct, RtsM is under the transcriptional control of its own promoter. All other RtsM expression constructs were derived from pMLD4, as described in Tables 2 and 3.
A BB2 epitope-tagged version of rtsM was constructed by PCR amplifying a 416 bp fragment of rtsM from pMLD4 between the BamHI site internal to rtsM and the EcoRI site 3′ to rtsM. The 3′ primer used in this amplification incorporated the BB2 epitope sequence (GTCGAGCGGGTCCTG GTTGGTGTGCACCTC) into rtsM immediately before the stop codon. The PCR product was verified by sequencing and was cloned into the BamHI-EcoRI of pMLD4 (replacing the wild-type sequence) to generate pRtsM-BB2.
A high copy number expression vector containing an arabinose-inducible promoter (pARA) was constructed by cloning a 1.3 kb ClaI-HindIII fragment containing araC and the arabinose inducible promoter pBAD from pBAD30 into pUCP-SK (ClaI-HindIII). pARA-ExsA was constructed by PCR amplifying the open reading frame (ORF) for ExsA from PA103 with primer pairs ExsA-N/ExsA-C and subcloning the PCR product into pARA (EcoRI) under the arabinose inducible pBAD promoter. Likewise, pARA-Vfr was constructed by PCR amplifying with primer pairs Vfr-N/VfrA-C and subcloning the vfr ORF into pARA (EcoRI-XbaI) under the arabinose inducible pBAD promoter.
Promoter regions for transcriptional reporters were amplified using the P1/P2 primer pairs listed in Table S1 and sub-cloned into mini-CTX-lux (Becher and Schweizer, 2000). All amplifications were carried out using PA103 genomic DNA as template with the exception of the exoS promoter, which was amplified from PA01 genomic DNA. Plasmids derived from mini-CTX were mobilized into PA103, ΔrtsM or ΔexsA by mating. After integration into the attB site, vector backbone sequences were excised by Flp recombinase as previously described (Hoang et al., 1998). Each reporter construct is therefore present as a single, unmarked copy integrated at the chromosomal attB site.
Lactate dehydrogenase (LDH) assay
HeLa cells (ATCC) were cultured in DMEM (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (Gemini Bioproducts) and 10 mM L-glutamate in a 6% CO2 humidified incubator at 37°C. For LDH assays, 5 × 104 cells/well were seeded 48 h prior to infection into 24-well tissue culture plates. Bacterial strains were grown in LB without aeration for 16–18 h, diluted into MEM-etc, and used to infect HeLa cells in triplicate at a multiplicity of infection (MOI) of 10–15 (Garrity-Ryan et al., 2000). The infected cells were incubated at 37°C. At 2 and 4 hpi, we measured LDH released into culture media according to manufacturer’s instructions (Takara Bio).
SDS-PAGE and immunoblot analysis
Bacteria were grown overnight with aeration at 37°C in MinS, then centrifuged to separate cell-associated (pellet) and secreted (supernatant) proteins. Samples were prepared as previously described (Hauser et al., 1998) and normalized to total protein (15 μg lane−1) for cell-associated proteins (determined using the Pierce BCA Assay) and to colony counts (5 × 108 cfu lane−1) for secreted proteins. Proteins were separated by SDS-PAGE, transferred to PVDF membrane (Millipore) and probed for the TTSS effectors ExoT and ExoU with polyclonal rabbit antiserum (1:1000, from A. Hauser and J. Engel). A goat antirabbit IgG horseradish peroxidase conjugate was used as a secondary antibody (1:2000, Bio-Rad). Detection was performed using ECL substrate (100 mM Tris-HCl pH 8.5, 250 mM luminol, 90 mM coumarate, 0.009% H2O2), and blots were visualized and analysed using an Image Station 2000R running 1D Image Analysis Software version 3.6 (Kodak).
To detect RtsM-BB2 expression, bacteria were grown overnight at 37°C with aeration in MinS media containing NTA or in MinS media prepared without NTA and supplemented with 2.5 mM CaCl2. Bacteria were pelleted, lysed in 4% SDS, and normalized to total protein (5 μg lane−1). SDS-PAGE and immunoblotting were carried out as above. Blots were probed with a monoclonal antibody against BB2 (1:2000, kindly provided by C. Tschudi), followed by a goat antimouse IgG horseradish peroxidase conjugate (1:2000, Bio-Rad). Detection and analysis were carried out as above.
mRNA analysis
PA103, ΔrtsM and ΔexsA were grown in LB at 37°C for 14–16 h with aeration and then subcultured into MinS media. Cultures were grown at 37°C with aeration to late exponential phase (4–6 h). Approximately 1 × 108−1 × 109 bacteria were used for isolation of RNA following manufacturer’s recommendations (Qiagen RNeasy™). Five micrograms of RNA from each strain was fractionated on a 1.2% formaldehyde gel and transferred to nitrocellulose. The membrane was probed for exoT or exoU mRNA using [α-32P]UTP labelled riboprobes (Strip-EZ™ RNA, Ambion) and washed in 0.1X SSC, 0.1% SDS for 20 min at 75°C for exoT riboprobe and 84°C for exoU riboprobe. Bands were visualized using a Cyclone PhosphoImager (Packard).
Measurement of luciferase and calculation of transcription
Bacterial strains were grown in LB with aeration at 37°C for 14–16 h. Cultures were centrifuged and bacterial pellets were resuspended and washed twice in MinS. Samples were subcultured 1:100 into 10 ml of MinS and incubated at 37°C with shaking. Relative luciferase units (RLU) of 1 ml of culture were measured at 1 h intervals starting at time zero using a luminometer (Turner Designs, Sunnyvale, CA); protein concentration at each timepoint was determined by the Pierce BCA assay. Transcription is expressed as the fold increase of RLU per mg of protein at a given time point compared to the initial value obtained when that strain was subcultured from non-inducing to inducing media at t = 0.
To measure transcription in response to host cell contact, bacterial strains were grown in LB at 37°C without aeration for 14–16 h. HeLa cells plated at 3 × 105 cells/30 mm dish 24 h prior to assay were washed twice with MEM-etc., then infected with PA103 exoT::lux or ΔrtsM exoT::lux at an MOI of 100. Control infections were set up under identical conditions in the absence of HeLa cells. Samples were centrifuged 10 min at 1500 r.p.m., incubated for 45 min at 37°C to allow bacterial binding, then washed 4x with MEM-etc. to remove unbound bacteria. Based on RLU measurements before and after washing, we estimate that c. 1–5% of the original inoculum remained bound (data not shown). RLU readings from the entire culture dish were taken immediately after washing (t = 0) and at 1 h intervals; transcription is expressed as the fold increase of RLU relative to the initial reading (t = 0) for that sample.
Mouse model of acute pneumonia
Eight to ten week old female C57Bl/6 mice were obtained from NCI and housed under specific pathogen-free conditions. All studies were approved by the Yale University Institutional Animal Care and Use Committee. PA103, ΔrtsM or ΔexsA were cultured in LB at 37°C with aeration for 14–16 h and then subcultured 1:50 into fresh LB and grown at 37°C with aeration to OD600 = 0.2–0.3. Bacteria were washed twice with sterile PBS, then adjusted to a final concentration of c. 2.5 × 108 cfu ml−1. The actual dose administered was determined by plating serial dilutions of the inoculum and counting cfu. Mice were lightly anesthetized with methoxyflurane (2–4%), then infected intranasally with 40 μl of bacterial suspension. Mice were euthanized at 18 hpi, and the liver, lungs and spleen were removed, homogenized and resuspended in 2 ml g−1, 4 ml g−1 and 6 ml g−1 of Hank’s Buffered Salt Solution plus 0.25% Triton-X100 respectively. The suspensions were passed through a sterile screen to obtain a uniform suspension and serial dilutions were plated on VBM to determine recovery. In this model, the LD50 of PA103 is c. 6 × 105 cfu (M. Lebron and B. I. Kazmierczak, unpubl. data) (Schultz et al., 2002).
Supplementary Material
Acknowledgments
We thank J. Engel, A. Hauser, H. Schweizer and C. Tschudi for kindly providing bacterial strains, plasmids and antiserum used in this study. We thank R. Alligood of the Pseudomonas Genetic Stock Center, East Carolina University School of Medicine for kindly providing cosmid pMO013207. We thank Michel Ledizet for constructing pMLD4 and Maria Lebron for the excellent technical assistance. We thank Krystyna Kazmierczak and Christian Tschudi for the critical reading of this manuscript. This work was supported by start-up funds from Yale University School of Medicine, and NIH grants R01 AI054920 (BIK) and T32G007223 (MAL).
Footnotes
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/mmi/mmi4331/mmi4331sm.htm
Table S1. Primer sequences used in this study.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, et al. The Pfam protein families database. Nucleic Acids Res. 2002;30:276–280. doi: 10.1093/nar/30.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher A, Schweizer HP. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques. 2000;29:948–952. doi: 10.2144/00295bm04. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides – SignalP 3.0. J Mol Biol. 2004 doi: 10.1016/j.jmb.2004.05.028. (in press) [DOI] [PubMed] [Google Scholar]
- Boyd JM, Lory S. Dual function of PilS during transcriptional activation of the Pseudomonas aeruginosa pilin subunit gene. J Bacteriol. 1996;178:831–839. doi: 10.1128/jb.178.3.831-839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookman JL, Stott AJ, Cheeseman PJ, Burns NR, Adams SE, Kingsman AJ, Gull K. An immunological analysis of Ty1 virus-like particle structure. Virology. 1995;207:59–67. doi: 10.1006/viro.1995.1051. [DOI] [PubMed] [Google Scholar]
- Cornelis G, Van Gijsegem F. Assembly and function of type III secretory systems. Annu Rev Microbiol. 2000;54:735–774. doi: 10.1146/annurev.micro.54.1.735. [DOI] [PubMed] [Google Scholar]
- Cowell BA, Chen DY, Frank DW, Vallis AJ, Fleiszig SMJ. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect Immun. 2000;68:403–406. doi: 10.1128/iai.68.1.403-406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft L, Beatson SA, Whitchurch CB, Huang B, Blakeley RL, Mattick JS. An interactive web-based Pseudomonas aeruginosa genome database: discovery of new genes, pathways and structures. Microbiology. 2000;146:2351–2364. doi: 10.1099/00221287-146-10-2351. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Lykken GL, Wolfgang MC, Yahr TL. A novel anti-anti-activator mechanism regulates expression of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol. 2004;53:297–308. doi: 10.1111/j.1365-2958.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- Feltman H, Schulert GS, Khan S, Jain M, Peterson L, Hauser A. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SMJ, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- Francis MS, Wolf-Watz H, Forsberg A. Regulation of type III secretion systems. Curr Opin Microbiol. 2002;5:166–172. doi: 10.1016/s1369-5274(02)00301-6. [DOI] [PubMed] [Google Scholar]
- Frank DW. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;4:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- Furste JP, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Ganesan AK, Vincent TS, Olson JC, Barbieri JT. Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor-catalyzed nucleotide exchange. J Biol Chem. 1999;274:21823–21829. doi: 10.1074/jbc.274.31.21823. [DOI] [PubMed] [Google Scholar]
- Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel J. The arginine finger domain of ExoT is required for actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun. 2000;68:7100–7113. doi: 10.1128/iai.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity-Ryan L, Shafikhani S, Balachandran P, Nguyen L, Oza J, Jakobsen T, et al. The ADP ribosyl-transferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect Immun. 2004;72:546–558. doi: 10.1128/IAI.72.1.546-558.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem. 1999;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L, Belin D, Carlson MJ, Beckwith J. Tight regulation, modulation and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AR, Kang PJ, Engel J. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- Hauser A, Cobb E, Bodi M, Mariscal D, Valles J, Engel J, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Hecht GB, Newton A. Identification of a novel response regulator required for the swarmer-to-stalked cell transition in Caulobacter crescentus. J Bacteriol. 1995;21:6223–6229. doi: 10.1128/jb.177.21.6223-6229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JM, Bassler BL. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio para-haemolyticus. J Bacteriol. 2004;186:3794–3805. doi: 10.1128/JB.186.12.3794-3805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Hoch JA, Silhavy TJ, editors. Two-Component Signal Transduction. Washington, DC: American Society for Microbiology Press; 1995. [Google Scholar]
- Hornef MW, Roggenkamp A, Geiger AM, Hogardt M, Jacobi CA, Heesemann J. Triggering the ExoS regulon of Pseudomonas aeruginosa: a GFP-reporter analysis of exoenzyme (Exo) S, ExoT and ExoU synthesis. Microb Pathog. 2000;29:329–343. doi: 10.1006/mpat.2000.0398. [DOI] [PubMed] [Google Scholar]
- Hovey AK, Frank DW. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1995;177:4427–4436. doi: 10.1128/jb.177.15.4427-4436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PJ, Hauser AR, Apodaca G, Fleiszig S, Wiener-Kronish J, Mostov K, Engel JN. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- Kazmierczak B, Engel J. Pseudomonas aeruginosa ExoT acts in vivo as a GTPase activating protein for RhoA, Rac1, and Cdc42. Infect Immun. 2002;70:2198–2205. doi: 10.1128/IAI.70.4.2198-2205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Forst S. Genomic analysis of the histidine kinase family in bacteria and archaea. Microbiology. 2001;147:1197–1212. doi: 10.1099/00221287-147-5-1197. [DOI] [PubMed] [Google Scholar]
- Liu PV. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis: Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966;116:481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Mattick JS, Bills MM, Anderson BJ, Dalrymple B, Mott MR, Egerton JR. Morphogenetic expression of Bacteroides nodosus fimbriae in Pseudomonas aeruginosa. J Bacteriol. 1987;169:33–41. doi: 10.1128/jb.169.1.33-41.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaw ML, Lykken GL, Singh PK, Yahr TL. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol Microbiol. 2002;46:1123–1133. doi: 10.1046/j.1365-2958.2002.03228.x. [DOI] [PubMed] [Google Scholar]
- Miller VL. Connections between transcriptional regulation and type III secretion? Curr Opin Microbiol. 2002;5:211–215. doi: 10.1016/s1369-5274(02)00303-x. [DOI] [PubMed] [Google Scholar]
- Nicas TI, Iglewski BH. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect Immun. 1984;45:470–474. doi: 10.1128/iai.45.2.470-474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson KJ, Vallis AJ, Aktories K, Frank DW, Barbieri JT. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol. 1999;32:393–401. doi: 10.1046/j.1365-2958.1999.01359.x. [DOI] [PubMed] [Google Scholar]
- Phillips RM, Six DA, Dennis EA, Ghosh P. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J Biol Chem. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- Radke J, Pederson KJ, Barbieri JT. Pseudomonas aeruginosa exoenzyme S is a biglutamic acid ADP-ribosyltransferase. Infect Immun. 1999;67:1508–1510. doi: 10.1128/iai.67.3.1508-1510.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A, Wolfgang MC, Mekalanos JJ. Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect Immun. 2004;72:1383–1890. doi: 10.1128/IAI.72.3.1383-1390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue A, Quentin Y, Lazdunski A, Méjean V, Foglino M. Two-component systems in Pseudomonas aeruginosa: why so many? Trends Microbiol. 2000;8:498–504. doi: 10.1016/s0966-842x(00)01833-3. [DOI] [PubMed] [Google Scholar]
- Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, et al. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, et al. The mechanism of action of the Pseudomonas aeruginosa encoded type III cytotoxin, ExoU. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulert GS, Feltman H, Rabin SDP, Martin CG, Battle SE, Rello J, Hauser AR. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188:1695–1706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- Schultz MJ, Rijneveld AW, Florquin S, Edwards CK, Dinarello CA, van der Poll T. Role of inter-leukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L285–L290. doi: 10.1152/ajplung.00461.2000. [DOI] [PubMed] [Google Scholar]
- Schweizer HP, Hoang TT. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology. 1983;1:784–791. [Google Scholar]
- Smith RS, Wolfgang MC, Lory S. An adenylate cyclase-controlled signaling network regulates Pseudomonas aeruginosa virulence in a mouse model of acute pneumonia. Infect Immun. 2004;72:1677–1684. doi: 10.1128/IAI.72.3.1677-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria-host communication: the language of hormones. Proc Natl Acad Sci USA. 2003;100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C, Pham X, Erwin A, Mizoguchi S, Warrener P, Hickey M, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Sun J, Barbieri JT. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10-regulator of kinase (Crk) proteins. J Biol Chem. 2003;278:32794–32800. doi: 10.1074/jbc.M304290200. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti PJ, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- Uhl MA, Miller JF. Integration of multiple domains in a two-component sensor protein: the Bordatella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- Vallis AJ, Yahr TL, Barbieri JT, Frank DW. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun. 1999;67:914–920. doi: 10.1128/iai.67.2.914-920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel HJ, Bonner DM. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- Watson AA, Alm RA, Mattick JS. Construction of improved vectors for protein production in Pseudomonas aeruginosa. Gene. 1996;172:163–164. doi: 10.1016/0378-1119(96)00026-1. [DOI] [PubMed] [Google Scholar]
- Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial genes by a novel adenylate cyclase signaling pathway. Dev Cell. 2003;4:253–263. doi: 10.1016/s1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Yahr T, Frank D. Transcriptional organization of the trans-regulatory locus which controls exoenzyme S synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994;176:3832–3838. doi: 10.1128/jb.176.13.3832-3838.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Goranson J, Frank DW. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III secretion pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- Yahr TL, Hovey AK, Kulich SM, Frank DW. Transcriptional analysis of the Pseudomonas aeruginosa exoenzyme S structural gene. J Bacteriol. 1995;177:1169–1178. doi: 10.1128/jb.177.5.1169-1178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr T, Mende-Mueller LM, Friese MB, Frank DW. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk MH, Harvill ET, Miller JF. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.