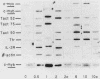
Abstract
Antigen-stimulated human T lymphocytes must bind the immunoregulatory hormone interleukin 2 (IL-2) if they are to transit from the G1 to the S phase of the cell cycle. Indirect methods, such as the measurement of thymidine uptake rates, were previously the only means available for exploring the mechanism of action of IL-2. Several cDNA clones have been isolated which are expressed subsequent to IL-2 binding, and the expression of two of these genes. Tact52 and Tact75, is regulated directly at the level of transcription; expression of the proto-oncogene c-myb is also regulated directly by IL-2 binding. These genes thus constitute a set which is coordinately regulated in the course of the transition from G1 to S phase of human T lymphocytes, and their expression depends on IL-2 binding.
Full text
PDF

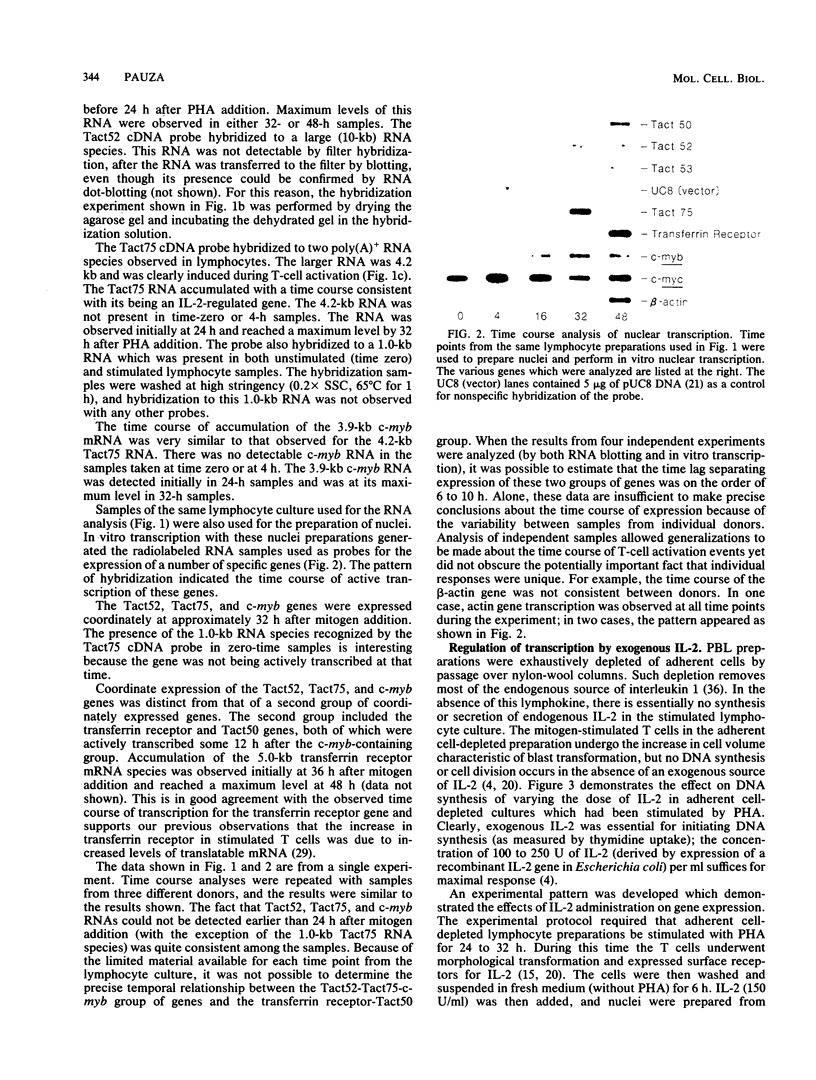
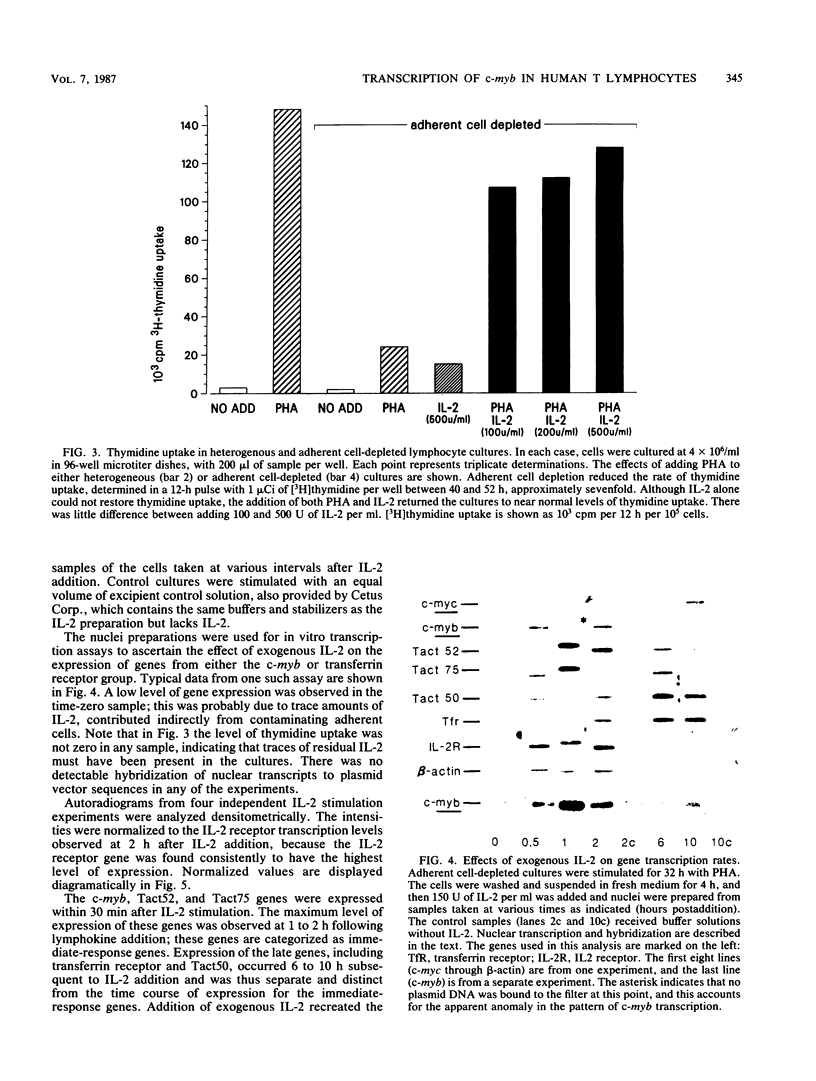
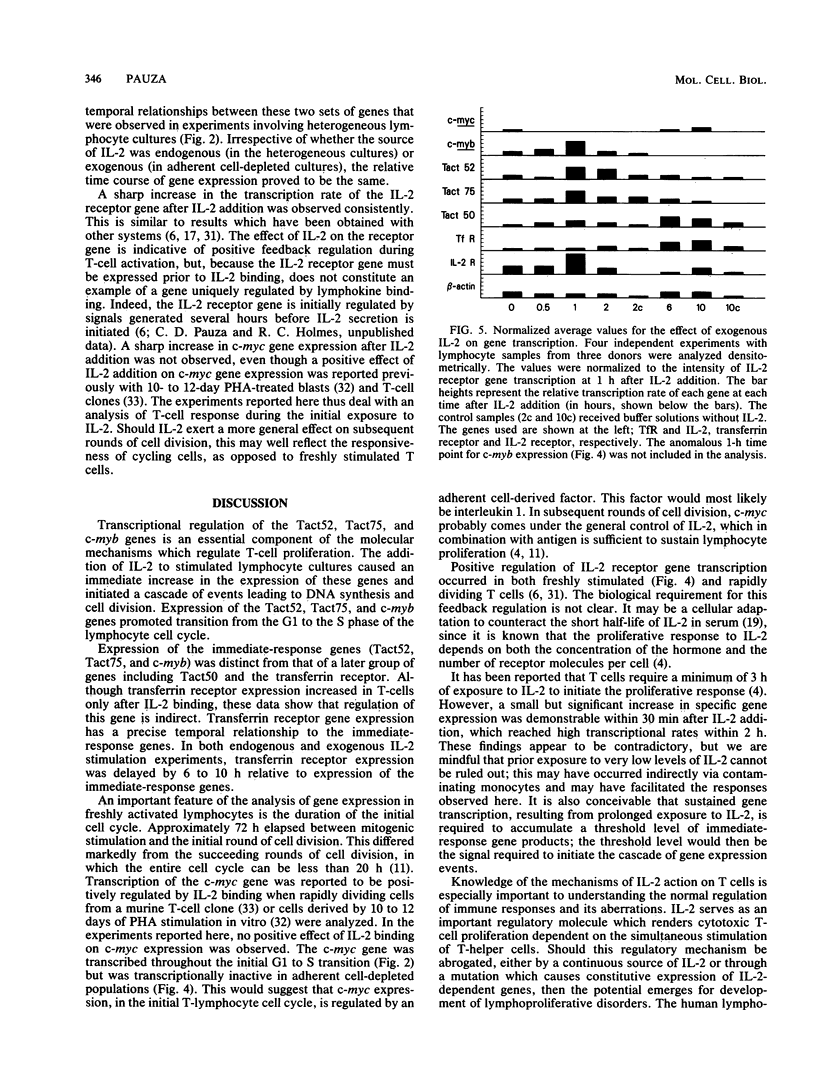


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Brincker H., Birkeland S. A. The relationship between disease activity, treatment response, and immunologic reactivity in immunoblastic lymphadenopathy: a longitudinal study of treatment with levamisole and cytostatics. Cancer. 1981 Jan 15;47(2):266–271. doi: 10.1002/1097-0142(19810115)47:2<266::aid-cncr2820470210>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Chang A. E., Hyatt C. L., Rosenberg S. A. Systemic administration of recombinant human interleukin-2 in mice. J Biol Response Mod. 1984 Oct;3(5):561–572. [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Flomenberg N., Welte K., Mertelsmann R., Kernan N., Ciobanu N., Venuta S., Feldman S., Kruger G., Kirkpatrick D., Dupont B. Immunologic effects of interleukin 2 in primary immunodeficiency diseases. J Immunol. 1983 Jun;130(6):2644–2650. [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Baluda M. A., Lengel C., Tronick S. R. Structural organization and expression of human DNA sequences related to the transforming gene of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7385–7389. doi: 10.1073/pnas.80.24.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzera G., Moran E. M., Rappaport H. Angio-immunoblastic lymphadenopathy. Diagnosis and clinical course. Am J Med. 1975 Dec;59(6):803–818. doi: 10.1016/0002-9343(75)90466-0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Brenner M. B., McLean J. M., Strominger J. L. Antigenic stimulation regulates the level of expression of interleukin 2 receptor on human T cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2172–2175. doi: 10.1073/pnas.81.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K. Synthesis of RNA containing polyadenylic acid in resting and stimulated human lymphocytes. Exp Cell Res. 1975 Apr;92(1):191–200. doi: 10.1016/0014-4827(75)90652-7. [DOI] [PubMed] [Google Scholar]
- Katzen D., Chu E., Terhost C., Leung D. Y., Gesner M., Miller R. A., Geha R. S. Mechanisms of human T cell response to mitogens: IL 2 induces IL 2 receptor expression and proliferation but not IL 2 synthesis in PHA-stimulated T cells. J Immunol. 1985 Sep;135(3):1840–1845. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Robb R. J., Sharrow S. O., Frana L. W., Rosenberg S. A. Systemic administration of interleukin-2 in humans. J Biol Response Mod. 1984 Oct;3(5):475–482. [PubMed] [Google Scholar]
- Maizel A. L., Mehta S. R., Hauft S., Franzini D., Lachman L. B., Ford R. J. Human T lymphocyte/monocyte interaction in response to lectin: kinetics of entry into the S-phase. J Immunol. 1981 Sep;127(3):1058–1064. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mountz J. D., Mushinski J. F., Smith H. R., Klinman D. M., Steinberg A. D. Modulation of c-myb transcription in autoimmune disease by cyclophosphamide. J Immunol. 1985 Oct;135(4):2417–2422. [PubMed] [Google Scholar]
- Mountz J. D., Steinberg A. D., Klinman D. M., Smith H. R., Mushinski J. F. Autoimmunity and increased c-myb transcription. Science. 1984 Nov 30;226(4678):1087–1089. doi: 10.1126/science.6494925. [DOI] [PubMed] [Google Scholar]
- Müller R., Verma I. M. Expression of cellular oncogenes. Curr Top Microbiol Immunol. 1984;112:73–115. doi: 10.1007/978-3-642-69677-0_4. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Henry P. H. An analysis of the rapidly synthesized ribonucleic acid of the normal human lymphocyte by agarose-polyacrylamide gel electrophoresis. Biochemistry. 1971 Apr 27;10(9):1733–1740. doi: 10.1021/bi00785a035. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Pauza C. D., Bleil J. D., Lennox E. S. The control of transferrin receptor synthesis in mitogen-stimulated human lymphocytes. Exp Cell Res. 1984 Oct;154(2):510–520. doi: 10.1016/0014-4827(84)90175-7. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Hamlyn P. H., Baer R. Altered nucleotide sequences of a translocated c-myc gene in Burkitt lymphoma. Nature. 1983 Dec 22;306(5945):760–765. doi: 10.1038/306760a0. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Greene W. C., Hoover R. G., Nowell P. C. Monoclonal antibody OKT11A inhibits and recombinant interleukin 2 (IL 2) augments expression of IL 2 receptors at a pretranslational level. J Immunol. 1985 Oct;135(4):2478–2482. [PubMed] [Google Scholar]
- Reed J. C., Nowell P. C., Hoover R. G. Regulation of c-myc mRNA levels in normal human lymphocytes by modulators of cell proliferation. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4221–4224. doi: 10.1073/pnas.82.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Sabath D. E., Hoover R. G., Prystowsky M. B. Recombinant interleukin 2 regulates levels of c-myc mRNA in a cloned murine T lymphocyte. Mol Cell Biol. 1985 Dec;5(12):3361–3368. doi: 10.1128/mcb.5.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scheurich P., Ucer U., Wrann M., Pfizenmaier K. Early events during primary activation of T cells: antigen receptor cross-linking and interleukin 1 initiate proliferative response of human T cells. Eur J Immunol. 1985 Nov;15(11):1091–1095. doi: 10.1002/eji.1830151105. [DOI] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Beldotti L. Malignant lymphomas following allogenic disease: transition from an immunological to a neoplastic disorder. Science. 1965 Sep 24;149(3691):1511–1514. doi: 10.1126/science.149.3691.1511. [DOI] [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Warren H. S., Atkinson K., Pembrey R. G., Biggs J. C. Human bone marrow allograft recipients: production of, and responsiveness to, interleukin 2. J Immunol. 1983 Oct;131(4):1771–1775. [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]