Abstract
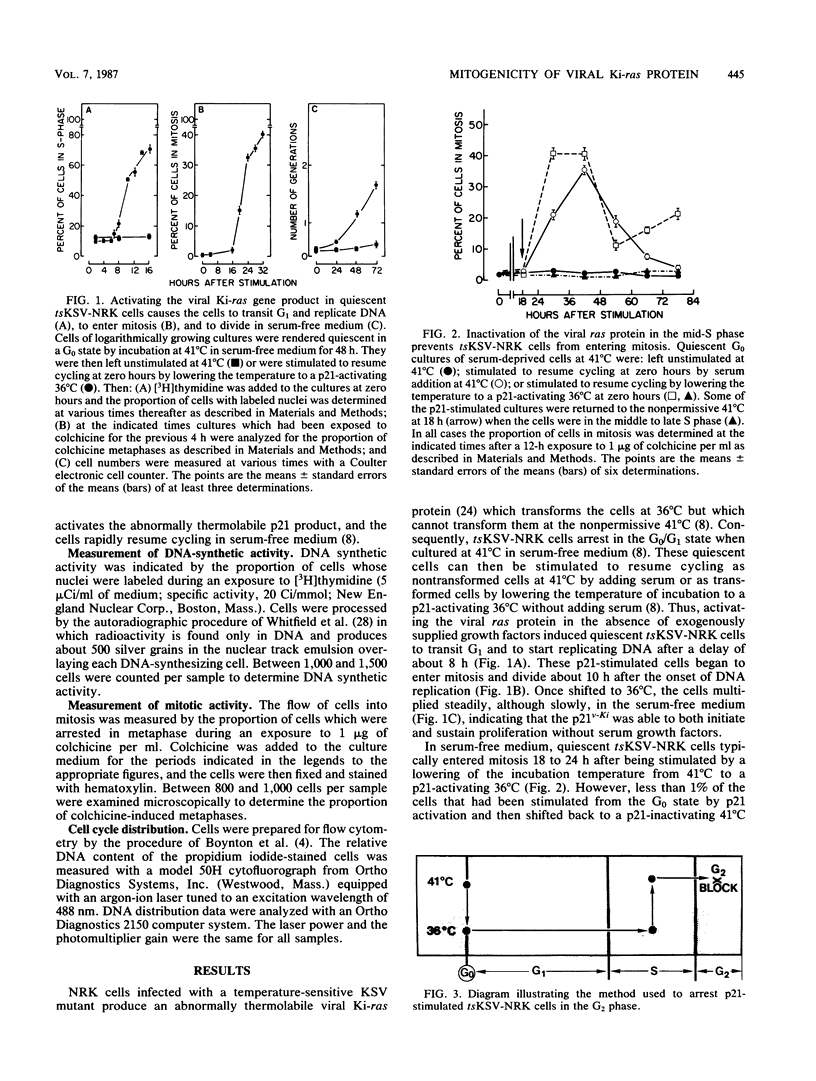
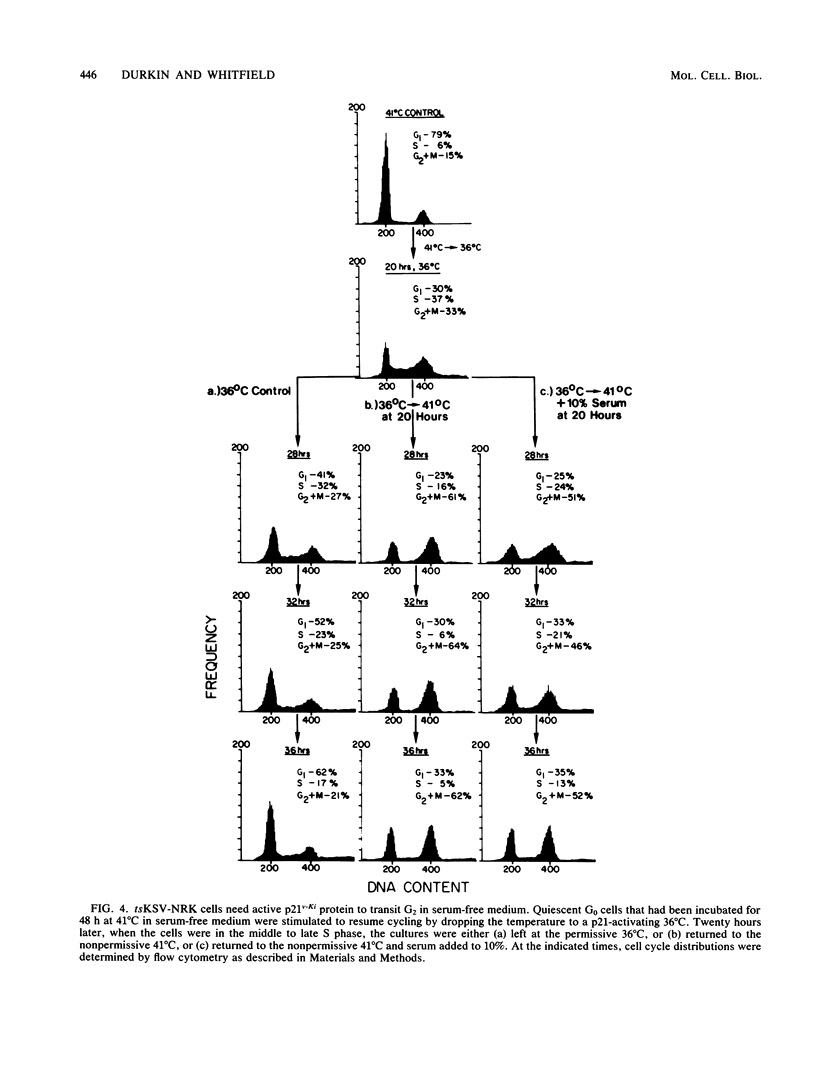
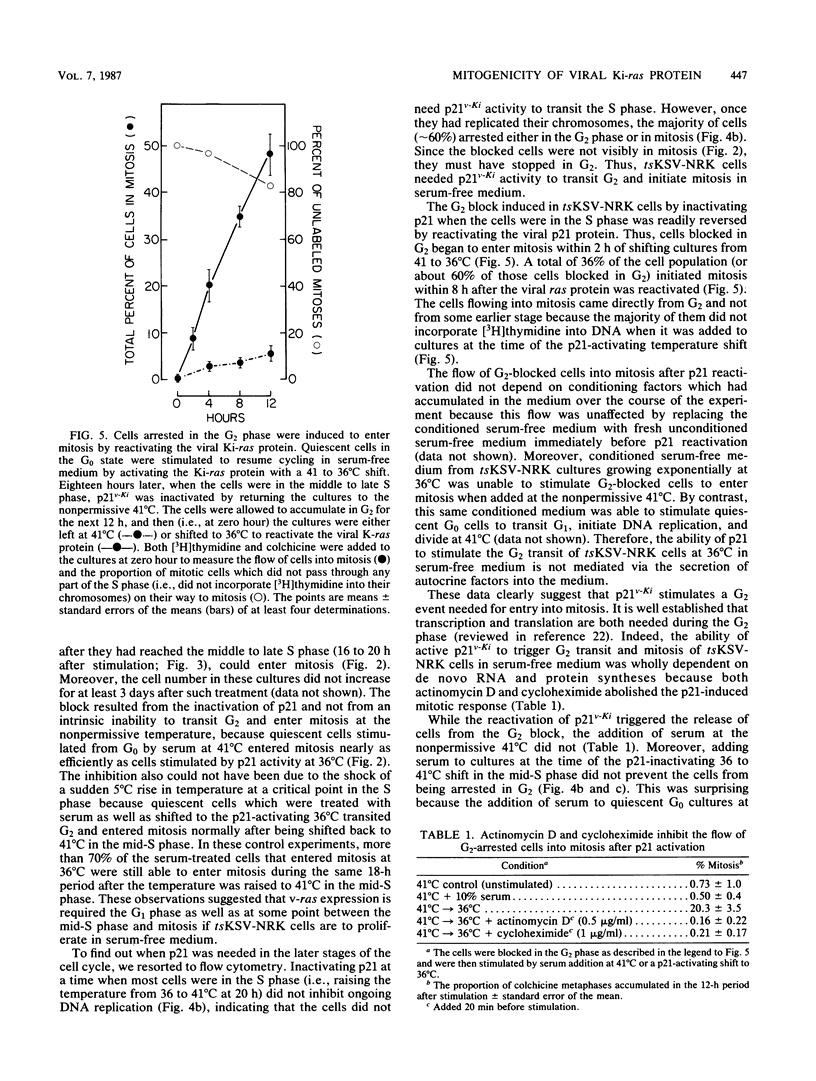
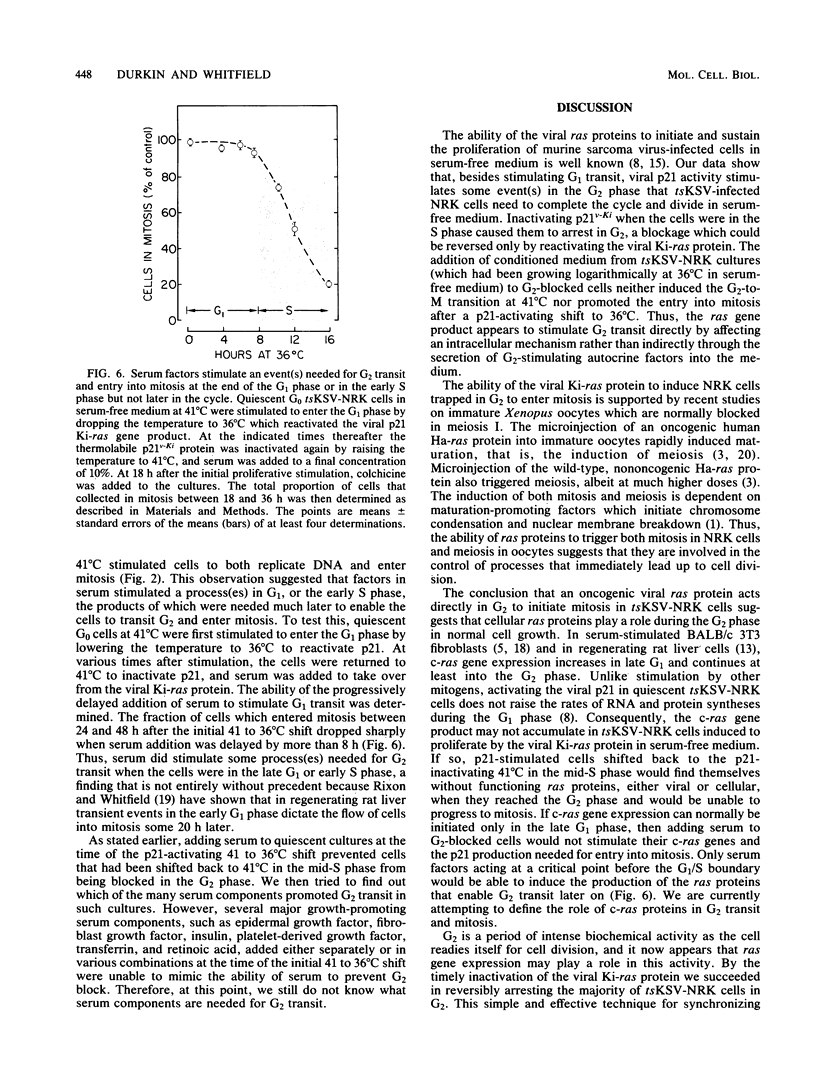
NRK cells infected with a temperature-sensitive Kirsten sarcoma virus (ts371 KSV) are transformed at 36 degrees C, but are untransformed at 41 degrees C which inactivates the abnormally thermolabile oncogenic p21Ki product of the viral Ki-ras gene. At 41 degrees C, tsKSV-infected NRK cells were arrested in G0/G1 when incubated in serum-free medium, but could then be stimulated to transit G1, replicate DNA, and divide by adding serum at 41 degrees C or dropping the temperature to a p21-activating 36 degrees C without adding serum. When quiescent cells at 41 degrees C were stimulated to transit G1 in serum-free medium by activating p21 at 36 degrees C and then shifted back to the p21-inactivating 41 degrees C in the mid-S phase, they continued replicating DNA but could not transit G2. Reactivating p21 in the G2-arrested cells by once again lowering the temperature to 36 degrees C stimulated a rapid entry into mitosis. By contrast, while serum-stimulated quiescent G0 cells at 41 degrees C replicate DNA and divide, serum did not induce G2-arrested cells to enter mitosis, indicating that serum growth factors may trigger events in the G1 phase that ultimately determine G2 transit. These observations made with the viral ras product suggest that cellular ras proto-oncogene products have a role in G2 transit of normal cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargmann C. I., Hung M. C., Weinberg R. A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986 Jan 16;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Kleine L. P. Ca2+/phospholipid-dependent protein kinase activity correlates to the ability of transformed liver cells to proliferate in Ca2+-deficient medium. Biochem Biophys Res Commun. 1983 Aug 30;115(1):383–390. doi: 10.1016/0006-291x(83)91015-x. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. Characterization of G1 transit induced by the mitogenic-oncogenic viral Ki-ras gene product. Mol Cell Biol. 1986 May;6(5):1386–1392. doi: 10.1128/mcb.6.5.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Gelfant S. A new concept of tissue and tumor cell proliferation. Cancer Res. 1977 Nov;37(11):3845–3862. [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P. The role of cellular oncogenes in cancers of non-viral etiology. Pharmacol Ther. 1985;29(2):205–220. doi: 10.1016/0163-7258(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Rixon R. H., Whitfield J. F. The possible cyclic AMP-dependence of an early prereplicative event that determines mitosis in regenerating rat liver. J Cell Physiol. 1985 Sep;124(3):397–402. doi: 10.1002/jcp.1041240307. [DOI] [PubMed] [Google Scholar]
- Sadler S. E., Schechter A. L., Tabin C. J., Maller J. L. Antibodies to the ras gene product inhibit adenylate cyclase and accelerate progesterone-induced cell division in Xenopus laevis oocytes. Mol Cell Biol. 1986 Feb;6(2):719–722. doi: 10.1128/mcb.6.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Stokes P. E., Smythers G. W., Dhar R., Oroszlan S. Characterization of the phosphorylation sites and the surrounding amino acid sequences of the p21 transforming proteins coded for by the Harvey and Kirsten strains of murine sarcoma viruses. J Biol Chem. 1982 Oct 10;257(19):11767–11773. [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O. Oncogenes and cancer: the p21 ras genes. Cancer Invest. 1984;2(2):109–123. doi: 10.3109/07357908409020294. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Youdale T., Franks D. J. The roles of calcium and cyclic AMP in the stimulatory action of parathyroid hormone on thymic lymphocyte proliferation. J Cell Physiol. 1971 Dec;78(3):355–368. doi: 10.1002/jcp.1040780305. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]



