Abstract
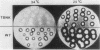
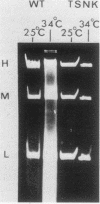
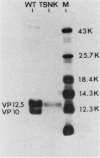
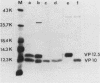
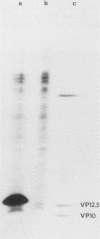
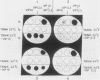
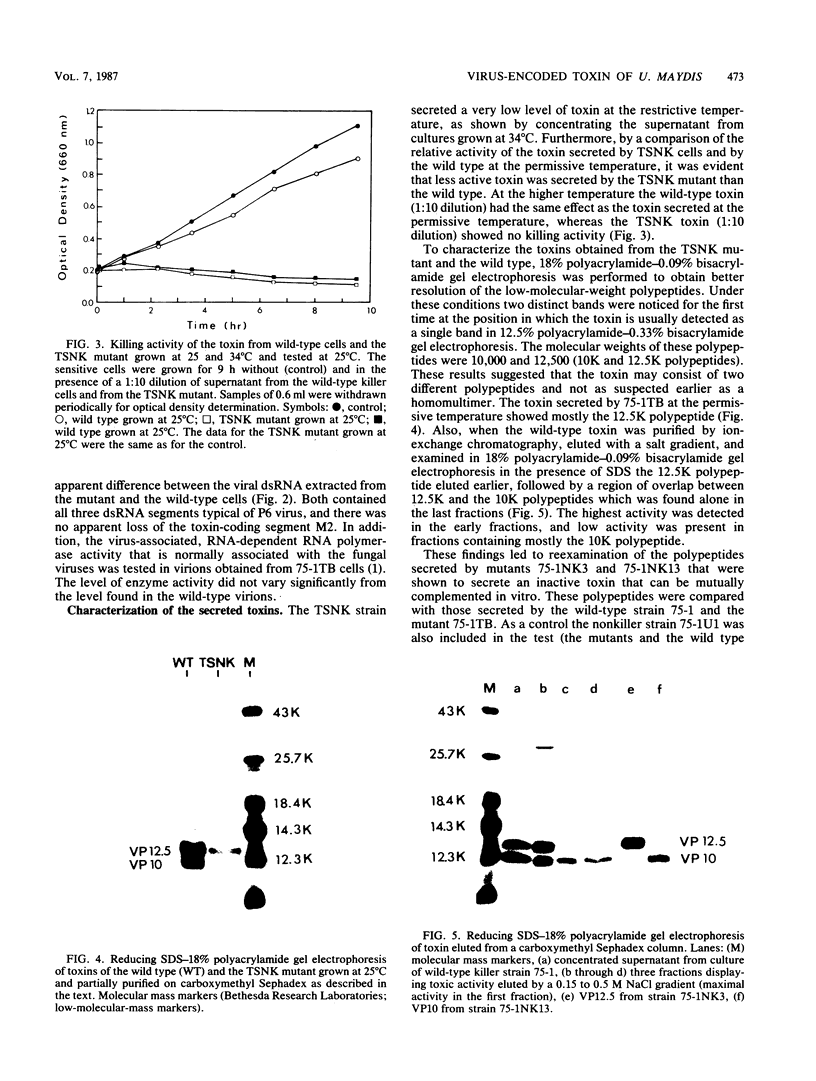
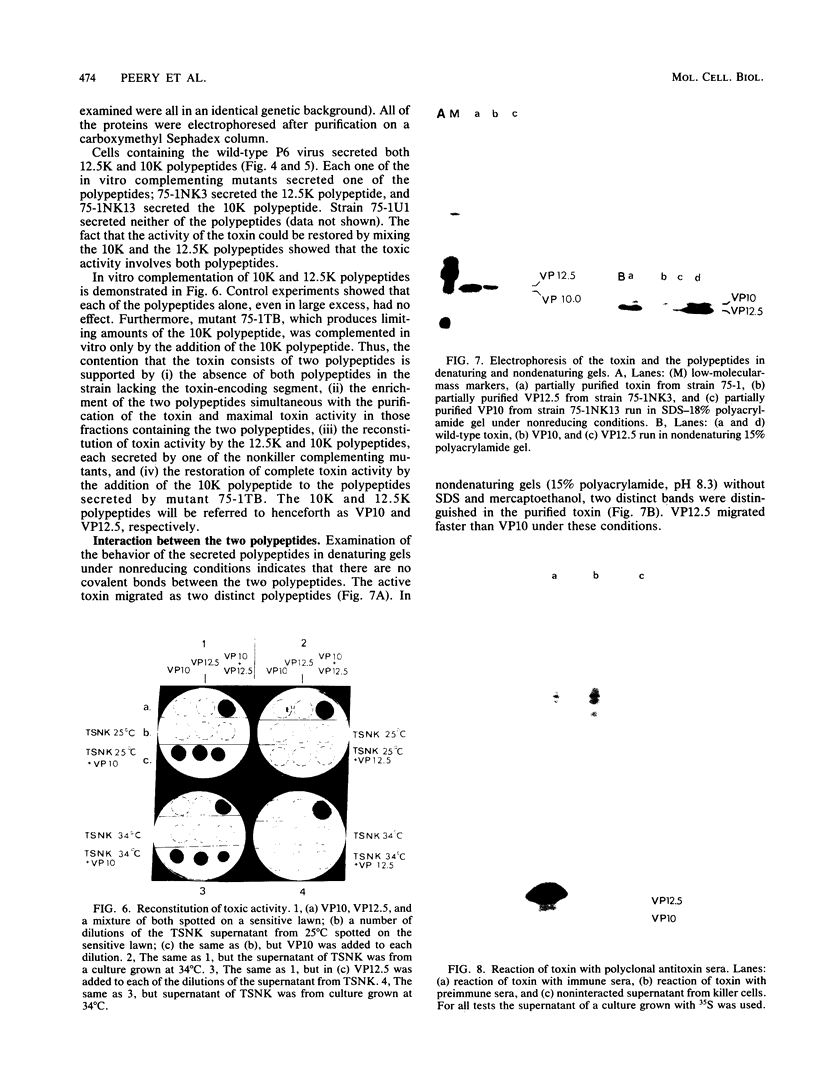
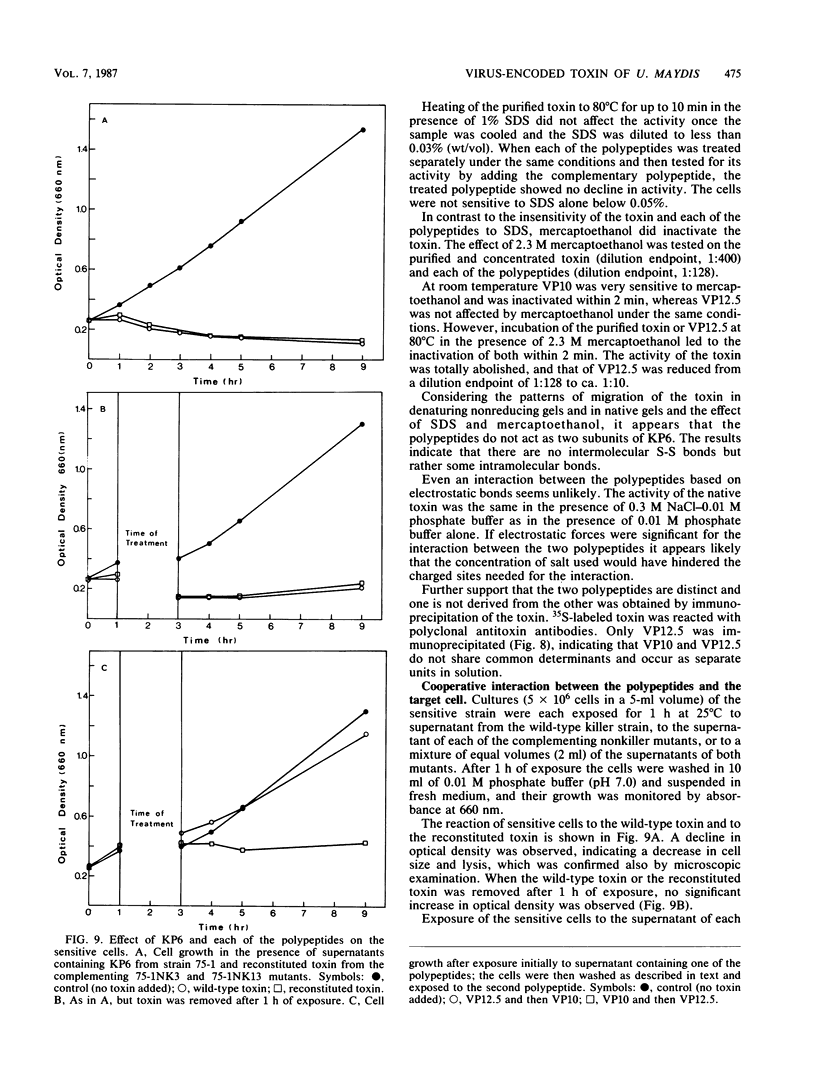
Cells of Ustilago maydis containing double-stranded RNA viruses secrete a virus-encoded toxin to which other cells of the same species and related species are sensitive. Mutants affected in the expression of the KP6 toxin were characterized, and all were viral mutants. A temperature-sensitive nonkiller mutant indicated that the toxin consists of two polypeptides, 12.5K and 10K, that are essential for the toxic activity. The temperature-sensitive nonkiller mutant was affected in the expression of the 10K polypeptide, and its toxic activity was restored by the addition of the 10K polypeptide to its secreted inactive toxin. These results led to the reexamination of other mutants that were known to complement in vitro. Each was found to secrete one of the two polypeptides. Here we show for the first time that P6 toxin consists of two polypeptides that do not interact in solution, but both are essential for the toxic effect. Studies on the interaction between the two polypeptides indicated that there are no covalent or hydrogen bonds between the polypeptides. Toxin activity is not affected by the presence of 0.3 M NaCl in the toxin preparations and in the medium, suggesting that no electrostatic forces are involved in this interaction. Also, the two polypeptides do not share common antigenic determinants. The activity of the two polypeptides appears to be dependent on a sequential interaction with the target cell, and it is the 10K polypeptide that initiates the toxic effect. The similarity of the U. maydis virus-encoded toxin to that of Saccharomyces cerevisiae is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Tzvi B. S., Koltin Y., Mevarech M., Tamarkin A. RNA polymerase activity in virions from Ustilago maydis. Mol Cell Biol. 1984 Jan;4(1):188–194. doi: 10.1128/mcb.4.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dalton R. E., Podila G. K., Flurkey W. H., Bozarth R. F. In vitro translation of the major capsid polypeptide from Ustilago maydis virus stain P1. Virus Res. 1985 Sep;3(2):153–163. doi: 10.1016/0168-1702(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Day P. R., Anagnostakis S. L. Corn smut dikaryon in culture. Nat New Biol. 1971 May 5;231(18):19–20. doi: 10.1038/newbio231019a0. [DOI] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J., Bruenn J. A., Chang T. H., Pinhasi O., Koltin Y. Two Ustilago maydis viral dsRNAs of different size code for the same product. Nucleic Acids Res. 1983 May 11;11(9):2765–2778. doi: 10.1093/nar/11.9.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Breithaupt H. Mini-review. The crotoxin complex--an example of biochemical and pharmacological protein complementation. Toxicon. 1978;16(1):19–30. doi: 10.1016/0041-0101(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Day P. R. Specificity of Ustilago maydis killer proteins. Appl Microbiol. 1975 Oct;30(4):694–696. doi: 10.1128/am.30.4.694-696.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Kandel J. S. Killer Phenomenon in USTILAGO MAYDIS: The Organization of the Viral Genome. Genetics. 1978 Feb;88(2):267–276. doi: 10.1093/genetics/88.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Levine R., Peery T. Assignment of functions to segments of the DsRNA genome of the Ustilago virus. Mol Gen Genet. 1980 Apr;178(1):173–178. doi: 10.1007/BF00267226. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Mayer I., Steinlauf R. Killer phenomenon in Ustilago maydis: mapping viral functions. Mol Gen Genet. 1978 Oct 30;166(2):181–186. doi: 10.1007/BF00285920. [DOI] [PubMed] [Google Scholar]
- Koltin Y. Virus-like particles in Ustilago maydis: mutants with partial genomes. Genetics. 1977 Jul;86(3):527–534. doi: 10.1093/genetics/86.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Levine R., Koltin Y., Kandel J. Nuclease activity associated with the Ustilago maydis virus induced killer proteins. Nucleic Acids Res. 1979 Aug 24;6(12):3717–3731. doi: 10.1093/nar/6.12.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peery T., Koltin Y., Tamarkin A. Mapping the immunity function of the ustilago maydis P1 virus. Plasmid. 1982 Jan;7(1):52–58. doi: 10.1016/0147-619x(82)90026-9. [DOI] [PubMed] [Google Scholar]
- Philliskirk G., Young T. W. The occurrence of killer character in yeasts of various genera. Antonie Van Leeuwenhoek. 1975;41(2):147–151. doi: 10.1007/BF02565046. [DOI] [PubMed] [Google Scholar]
- Rogers D. T., Saville D., Bussey H. Saccharomyces cerevisiae killer expression mutant kex2 has altered secretory proteins and glycoproteins. Biochem Biophys Res Commun. 1979 Sep 12;90(1):187–193. doi: 10.1016/0006-291x(79)91607-3. [DOI] [PubMed] [Google Scholar]
- Stumm C., Hermans J. M., Middelbeek E. J., Croes A. F., de Vries G. J. Killer-sensitive relationships in yeasts from natural habitats. Antonie Van Leeuwenhoek. 1977;43(2):125–128. doi: 10.1007/BF00395667. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Bostian K. A. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol Rev. 1984 Jun;48(2):125–156. doi: 10.1128/mr.48.2.125-156.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]