Abstract
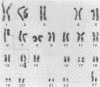
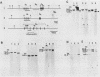
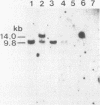
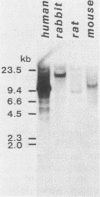
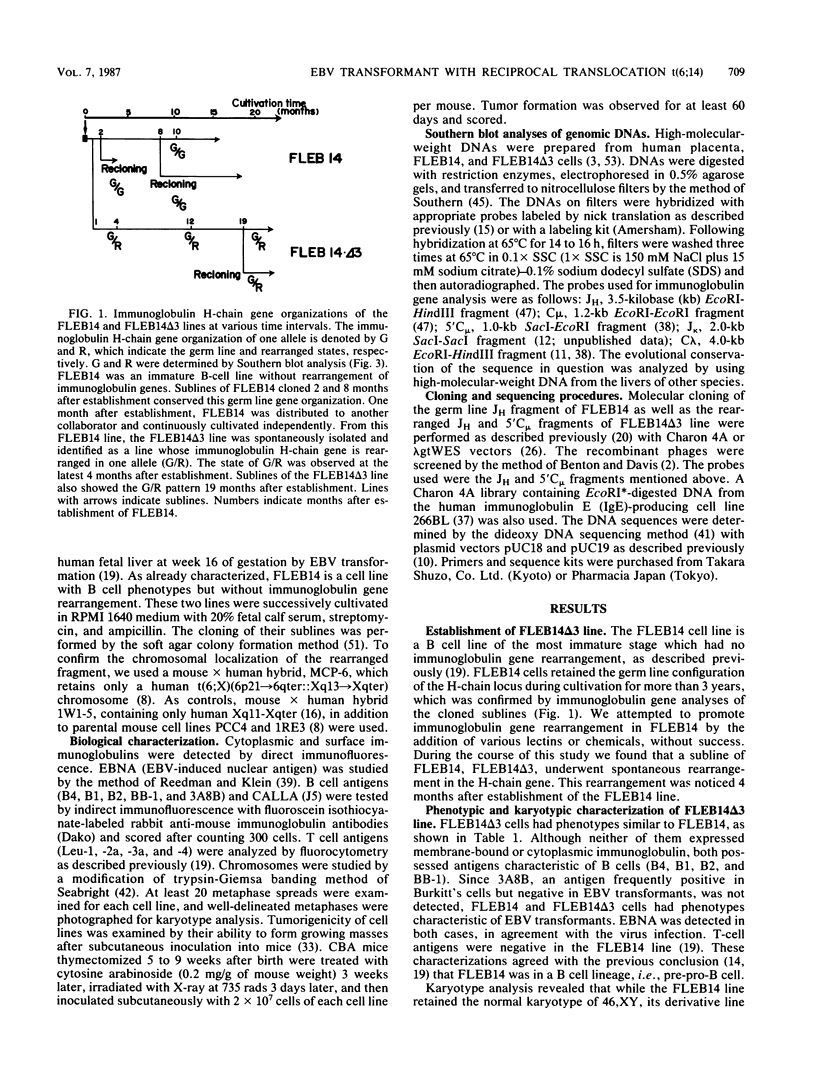
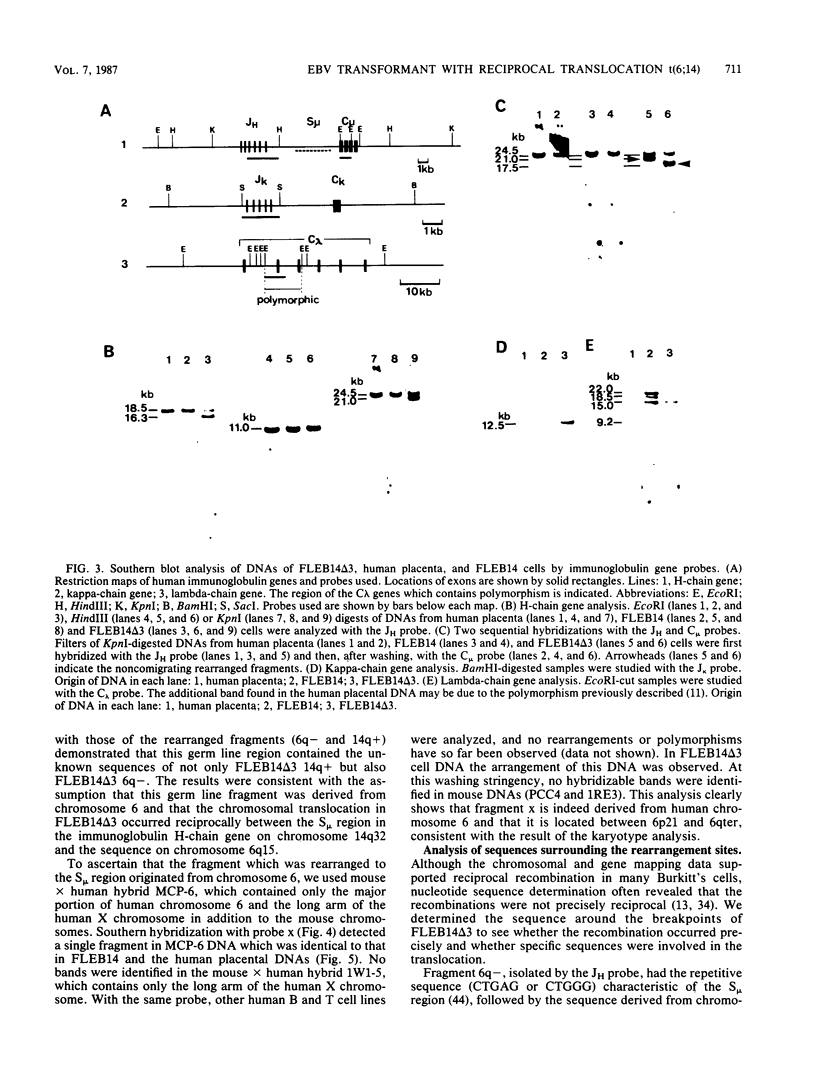
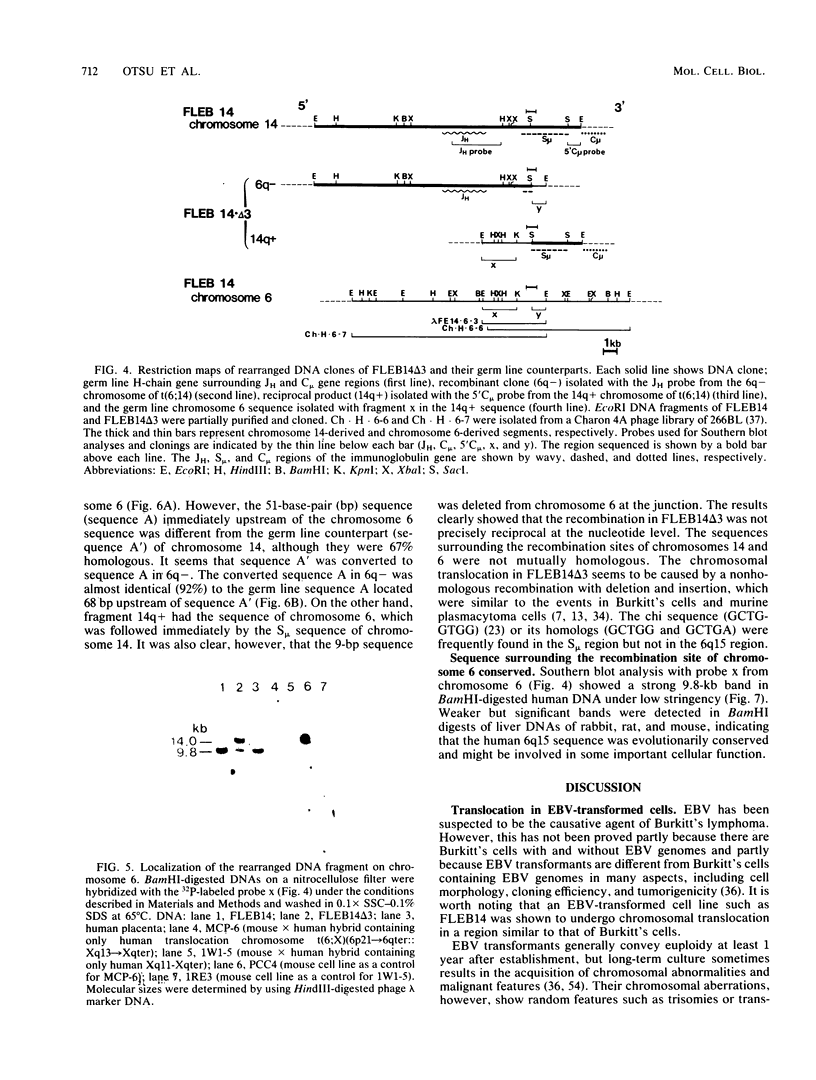
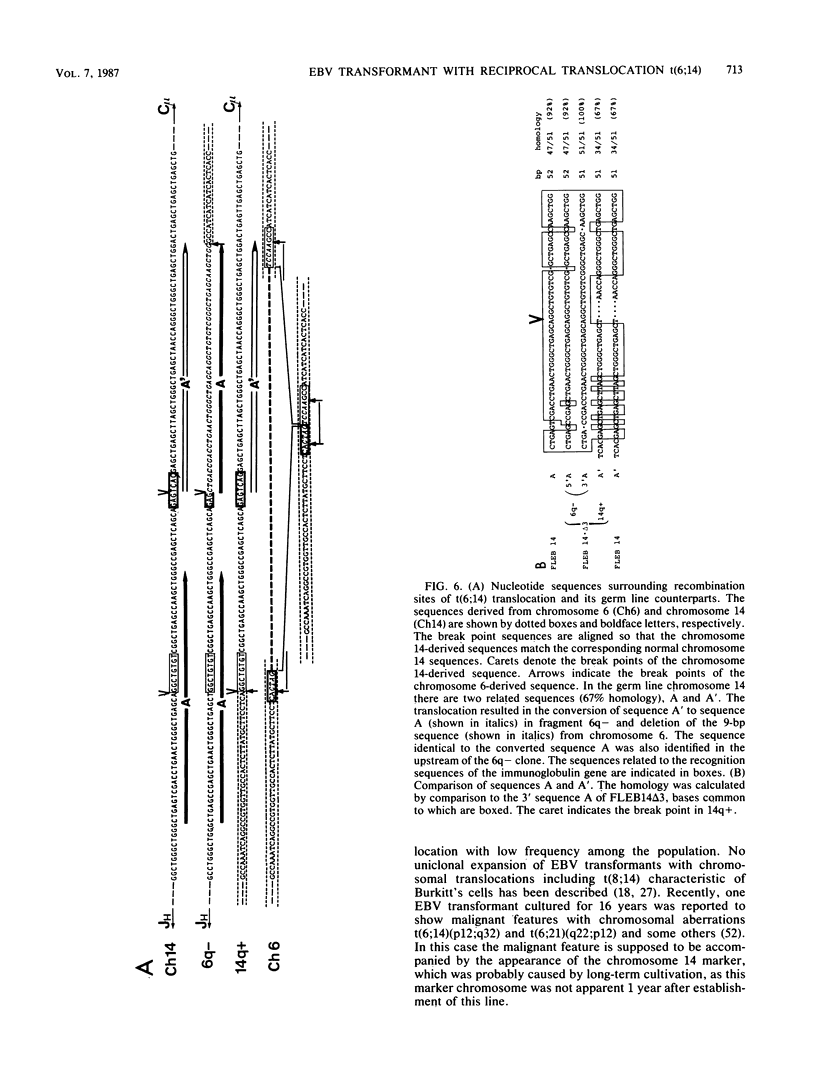
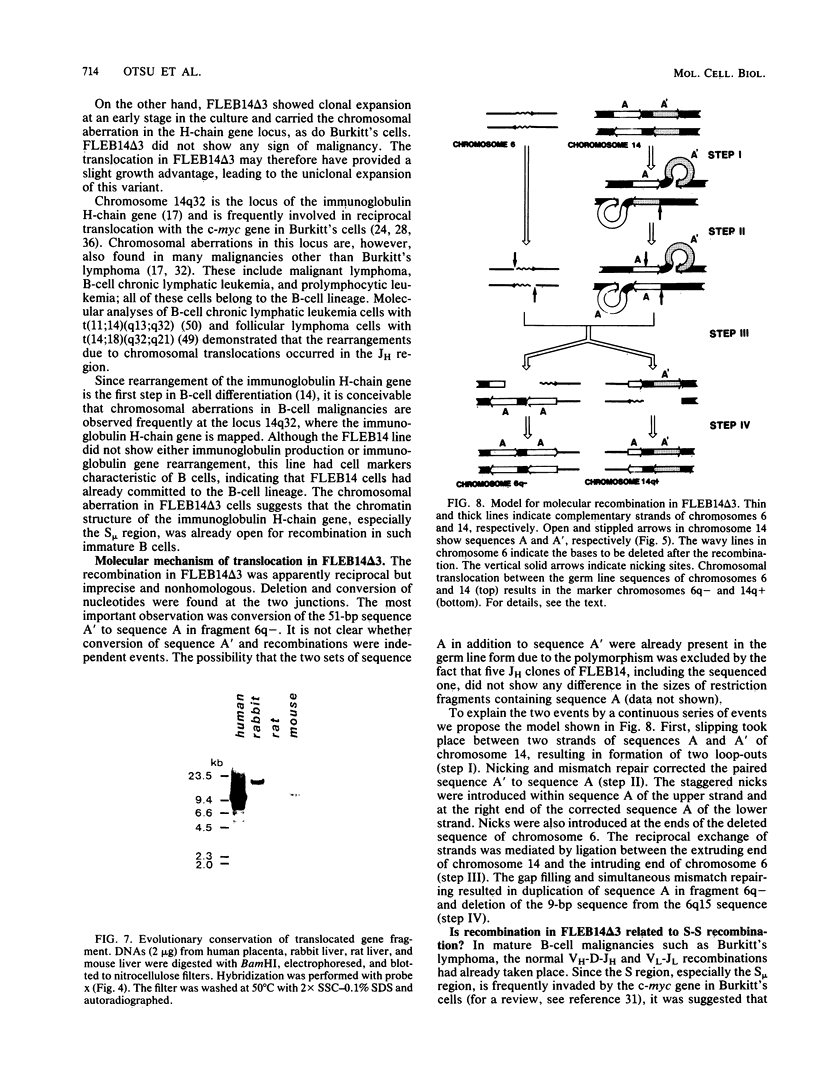
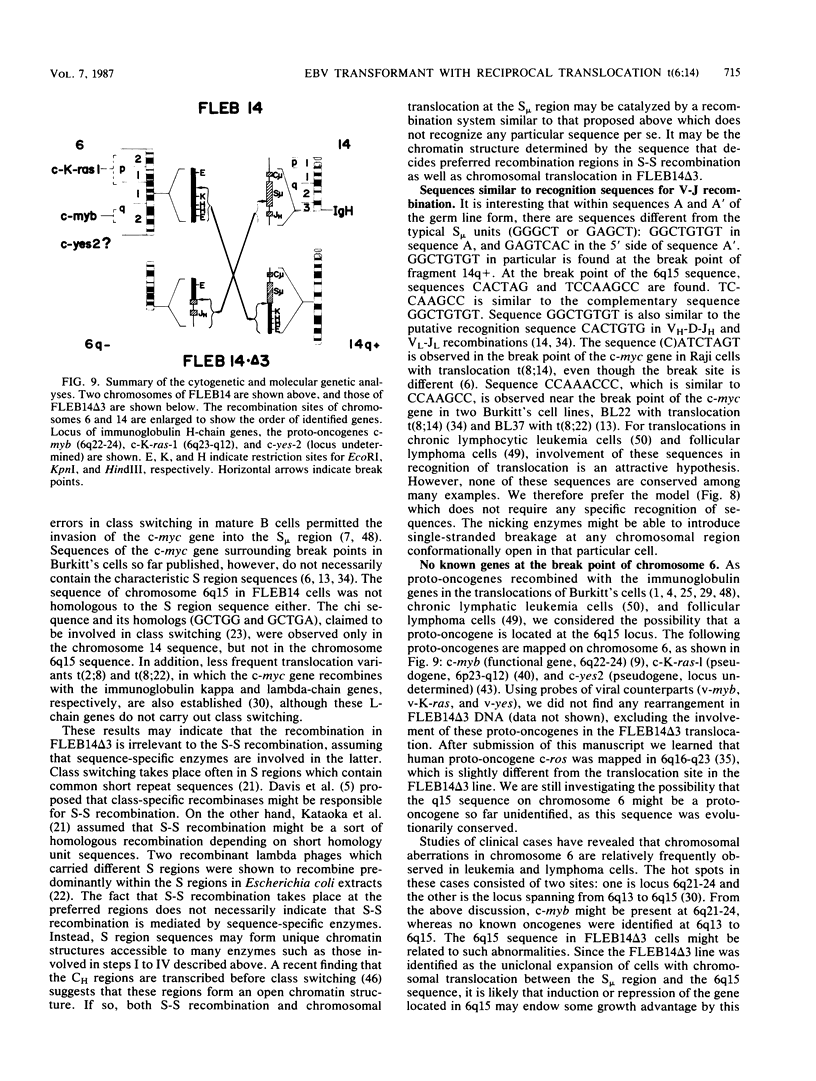
An in vitro culture of FLEB14 cells, an Epstein-Barr virus-transformed B cell precursor containing the germ line immunoglobulin genes, gave rise to a uniclonally expanded variant, FLEB14 delta 3, which was rearranged at the immunoglobulin heavy-chain gene locus. Cytogenetic analysis showed that FLEB14 delta 3 had a novel reciprocal translocation, t(6;14)(q15;q32). Molecular cloning of the rearranged DNA fragments and determination of their nucleotide sequence revealed that the recombination event was reciprocal, imprecise, and nonhomologous and took place in the S mu region, like those found in Burkitt's lymphoma cells. We propose a molecular model to explain this genetic event which may be relevant to class switch recombination. The translocated sequence of chromosome 6 did not contain any known oncogenes, although the sequence is conserved among mammals. FLEB14 delta 3 did not show tumorigenicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Corcoran L. M., Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Martinotti S., Gallo R. C., Erikson J., Croce C. M. Translocation and rearrangements of the c-myc oncogene locus in human undifferentiated B-cell lymphomas. Science. 1983 Feb 25;219(4587):963–967. doi: 10.1126/science.6401867. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Dyson P. J., Rabbitts T. H. Chromatin structure around the c-myc gene in Burkitt lymphomas with upstream and downstream translocation points. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1984–1988. doi: 10.1073/pnas.82.7.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S., Cory S., Adams J. M. Translocation of the myc cellular oncogene to the immunoglobulin heavy chain locus in murine plasmacytomas is an imprecise reciprocal exchange. Cell. 1984 Apr;36(4):973–982. doi: 10.1016/0092-8674(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Trowsdale J., Chambers S., Solomon E. Introduction of a human X-6 translocation chromosome into a mouse teratocarcinoma: investigation of control of HLA-A, B, C expression. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1190–1194. doi: 10.1073/pnas.79.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Franchini G., Love J., Simon M. I., Gallo R. C., Wong-Staal F. Chromosomal sublocalization of human c-myb and c-fes cellular onc genes. Nature. 1983 Jul 14;304(5922):169–171. doi: 10.1038/304169a0. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Hollis G. F., Mitchell K. F., Battey J., Potter H., Taub R., Lenoir G. M., Leder P. A variant translocation places the lambda immunoglobulin genes 3' to the c-myc oncogene in Burkitt's lymphoma. Nature. 1984 Feb 23;307(5953):752–755. doi: 10.1038/307752a0. [DOI] [PubMed] [Google Scholar]
- Honjo T., Habu S. Origin of immune diversity: genetic variation and selection. Annu Rev Biochem. 1985;54:803–830. doi: 10.1146/annurev.bi.54.070185.004103. [DOI] [PubMed] [Google Scholar]
- Honjo T., Obata M., Yamawaki-Katoaka Y., Kataoka T., Kawakami T., Takahashi N., Mano Y. Cloning and complete nucleotide sequence of mouse immunoglobulin gamma 1 chain gene. Cell. 1979 Oct;18(2):559–568. doi: 10.1016/0092-8674(79)90072-2. [DOI] [PubMed] [Google Scholar]
- Hope R. M., Goodfellow P. N., Solomon E., Bodmer W. F. Identification of MIC5, a human X-linked gene controlling expression of a cell surface antigen: definition by a monoclonal antibody raised against a human X mouse somatic cell hybrid. Cytogenet Cell Genet. 1982;33(3):204–212. doi: 10.1159/000131756. [DOI] [PubMed] [Google Scholar]
- Jarvis J. E., Ball G., Rickison A. B., Epstein M. A. Cytogenetic studies on human lymphoblastoid cell lines from Burkitt's lymphomas and other sources. Int J Cancer. 1974 Dec 15;14(6):716–721. doi: 10.1002/ijc.2910140604. [DOI] [PubMed] [Google Scholar]
- Katamine S., Otsu M., Tada K., Tsuchiya S., Sato T., Ishida N., Honjo T., Ono Y. Epstein-Barr virus transforms precursor B cells even before immunoglobulin gene rearrangements. Nature. 1984 May 24;309(5966):369–372. doi: 10.1038/309369a0. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Kawakami T., Takahashi N., Honjo T. Rearrangement of immunoglobulin gamma 1-chain gene and mechanism for heavy-chain class switch. Proc Natl Acad Sci U S A. 1980 Feb;77(2):919–923. doi: 10.1073/pnas.77.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Miyata T., Honjo T. Repetitive sequences in class-switch recombination regions of immunoglobulin heavy chain genes. Cell. 1981 Feb;23(2):357–368. doi: 10.1016/0092-8674(81)90131-8. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Takeda S., Honjo T. Escherichia coli extract-catalyzed recombination in switch regions of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 1983 May;80(9):2666–2670. doi: 10.1073/pnas.80.9.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenter A. L., Birshtein B. K. Chi, a promoter of generalized recombination in lambda phage, is present in immunoglobulin genes. Nature. 1981 Oct 1;293(5831):402–404. doi: 10.1038/293402a0. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Manolov G., Manolova Y., Klein G., Levan A., Kieler J. Chromosome #14 markers in two Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines of normal origin differ from the Burkitt lymphoma (BL)-associated 14q+ marker. Cancer Genet Cytogenet. 1981 Oct;4(2):179–184. doi: 10.1016/0165-4608(81)90082-0. [DOI] [PubMed] [Google Scholar]
- Manolova Y., Manolov G., Kieler J., Levan A., Klein G. Genesis of the 14q+ marker in Burkitt's lymphoma. Hereditas. 1979;90(1):5–10. doi: 10.1111/j.1601-5223.1979.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Harris L. J., Stanton L. W., Erikson J., Watt R., Croce C. M. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman F., Levan G. Clustering of aberrations to specific chromosomes in human neoplasms. IV. A survey of 1,871 cases. Hereditas. 1981;95(1):79–139. doi: 10.1111/j.1601-5223.1981.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Morten J. E., Hay J. H., Steel C. M., Foster M. E., De Angelis C. L., Busuttil A. Tumorigenicity of human lymphoblastoid cell lines, acquired during in vitro culture and associated with chromosome gains. Int J Cancer. 1984 Oct 15;34(4):463–470. doi: 10.1002/ijc.2910340406. [DOI] [PubMed] [Google Scholar]
- Moulding C., Rapoport A., Goldman P., Battey J., Lenoir G. M., Leder P. Structural analysis of both products of a reciprocal translocation between c-myc and immunoglobulin loci in Burkitt lymphoma. Nucleic Acids Res. 1985 Mar 25;13(6):2141–2152. doi: 10.1093/nar/13.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan L., Louie E., Tsujimoto Y., Balduzzi P. C., Huebner K., Croce C. M. The human c-ros gene (ROS) is located at chromosome region 6q16----6q22. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6568–6572. doi: 10.1073/pnas.83.17.6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Miki T., Hisajima H., Honjo T. Cloning of human immunoglobulin epsilon chain genes: evidence for multiple C epsilon genes. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3833–3837. doi: 10.1073/pnas.79.12.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y., Yaoita Y., Matsunami N., Rosén A., Klein G., Honjo T. Immunoglobulin gene organization of ultraviolet-illuminated human lymphoblastoid cell lines producing both IgM and IgG. Mol Biol Med. 1984 Oct;2(5):337–350. [PubMed] [Google Scholar]
- Sakaguchi A. Y., Zabel B. U., Grzeschik K. H., Law M. L., Ellis R. W., Scolnick E. M., Naylor S. L. Regional localization of two human cellular Kirsten ras genes on chromosomes 6 and 12. Mol Cell Biol. 1984 May;4(5):989–993. doi: 10.1128/mcb.4.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Semba K., Yamanashi Y., Nishizawa M., Sukegawa J., Yoshida M., Sasaki M., Yamamoto T., Toyoshima K. Location of the c-yes gene on the human chromosome and its expression in various tissues. Science. 1985 Mar 1;227(4690):1038–1040. doi: 10.1126/science.2983418. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Honjo T. Immunoglobulin class switching. Cell. 1984 Apr;36(4):801–803. doi: 10.1016/0092-8674(84)90029-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Nakai S., Honjo T. Cloning of human immunoglobulin mu gene and comparison with mouse mu gene. Nucleic Acids Res. 1980 Dec 20;8(24):5983–5991. doi: 10.1093/nar/8.24.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Hinuma Y. Clonal transformation of human leukocytes by Epstein-Barr virus in soft agar. Int J Cancer. 1976 Feb 15;17(2):191–196. doi: 10.1002/ijc.2910170207. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Little J. B. Chromosome 14 marker appearance in a human B lymphoblastoid cell line of nonmalignant origin. Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):231–239. doi: 10.1016/0165-4608(86)90078-6. [DOI] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]