Abstract
Removal of cargos from the cell surface via endocytosis is an efficient mechanism to regulate activities of plasma membrane (PM)-resident proteins, such as receptors or transporters. Salicylic acid (SA) is an important plant hormone that is traditionally associated with pathogen defense. Here, we describe an unanticipated effect of SA on subcellular endocytic cycling of proteins. Both exogenous treatments and endogenously enhanced SA levels repressed endocytosis of different PM proteins. The SA effect on endocytosis did not involve transcription or known components of the SA signaling pathway for transcriptional regulation. SA likely targets an endocytic mechanism that involves the coat protein clathrin, because SA interfered with the clathrin incidence at the PM and clathrin-deficient mutants were less sensitive to the impact of SA on the auxin distribution and root bending during the gravitropic response. By contrast, SA did not affect the ligand-induced endocytosis of the FLAGELLIN SENSING2 (FLS2) receptor during pathogen responses. Our data suggest that the established SA impact on transcription in plant immunity and the nontranscriptional effect of SA on clathrin-mediated endocytosis are independent mechanisms by which SA regulates distinct aspects of plant physiology.
Salicylic acid (SA) is an important plant signaling molecule involved in a broad range of biotic and abiotic stress responses, including immunity, defense-related cell death, systemic acquired resistance (1), drought (2), salt stress (3), ozone (4), and chilling (5). Moreover, SA action converges with signaling of several growth regulating hormones, such as jasmonic acid, ethylene, gibberellins, abscisic acid, and auxin, by which SA can impact on plant growth and development (6). A current notion of SA signaling suggests that SA mediates this broad range of physiological processes by regulation of gene transcription in the nucleus (1).
A growing number of studies demonstrate the importance of endocytosis in different physiological processes in plants, including immunity (7, 8). Endocytosis is the mechanism by which plasma membrane (PM) materials (including lipids and proteins) and cargos from the extracellular space are internalized and redirected toward different subcellular destinations. In plants, the most prominent endocytic mechanism is endocytosis that depends on the vesicle coat protein clathrin or clathrin-mediated endocytosis (CME) (9, 10). Besides its involvement in plant immune responses (8), CME also plays an essential role in nutrient uptake (11) and intercellular transport of the plant hormone auxin, specifically in internalization of auxin transporters from the PIN-FORMED (PIN) family (12). Interestingly, multiple endogenous signals, such as auxin (13), cytokinin (14), and GOLVEN peptides (15), have been shown to converge on the regulation of endocytosis via signal transduction pathways that might not require regulation of transcription.
Here, we found that SA acts as an endogenous signal that impairs CME. Whereas SA was found to potentiate secretion by transcriptional up-regulation of secretory pathway genes (16), we found that this CME inhibition by SA neither involves SA-induced transcriptional changes nor known components of the SA-regulated transcriptional signaling. This result opens unsuspected possibilities by which SA regulates different aspects of plant physiology.
Results
SA Interferes with the Endocytic Cycling of PM Proteins.
To identify possible mechanism(s) by which SA can affect the cellular behavior, we tested its effect on the endocytic cycling of PM proteins. We visualized the auxin transporters PIN1 and PIN2 or the aquaporin PLASMA MEMBRANE INTRINSIC PROTEIN2 (PIP2) that constitutively undergo cycles of endocytosis and recycling back to the PM (17). This recycling (18) and, to a lesser extent, endocytosis (19) are inhibited by the trafficking inhibitor brefeldin A (BFA). The imbalance in recycling and endocytosis caused by the BFA treatment results in intracellular accumulation of internalized PM proteins, which end up in BFA-induced aggregations of endosomes, called BFA bodies (20). Therefore, we used the relative amount of PM proteins in BFA bodies as a proxy for internalization rate.
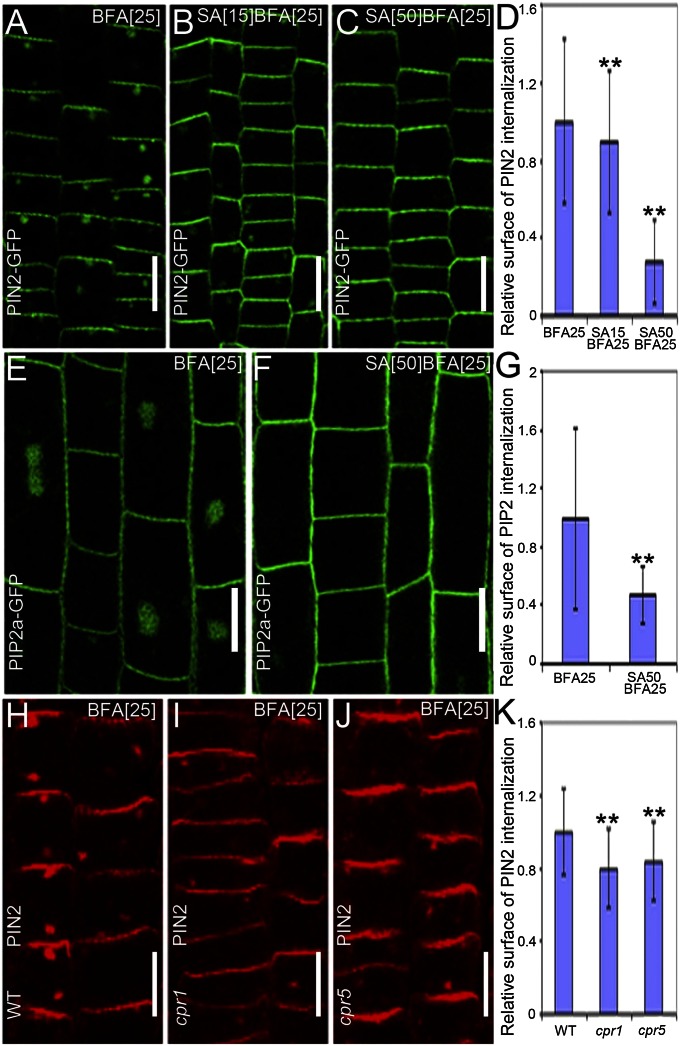
Previous studies had established that concentrations of SA from 0.1 to 1 mM were effective in plant defense (21, 22). When Arabidopsis thaliana seedlings were treated with a range of SA concentrations in combination with BFA, the BFA-induced PIN2 internalization was partially inhibited in root epidermal cells at concentrations as low as 15 µM. At this concentration, the PIN2-positive BFA bodies were smaller than those in the controls (Fig. 1 A, B, and D), whereas at 50 μM, the PIN2-GFP internalization was nearly completely abolished, as reflected by the strongly reduced PIN2-GFP signal in BFA bodies and the reduced number of BFA bodies per cell (Fig. 1 A, C, and D). Similar effects were visible for PIN1 (detected by anti-PIN1 antibodies) (Fig. S1) and PIP2-GFP (Fig. 1 E–G). Because SA is a weak acid, it lowers the pH of the medium from 5.8 to 5.4 when added at a final concentration of 200 µM (Fig. S2N). However, using 0.5× Murashige and Skoog (MS) medium at pH 5.4 did not reduce the BFA body size for PIN1 and PIN2 (Fig. S2 B, D, F, and H). Moreover, the more neutral sodium salicylate also reduced the BFA body size from 50 µM onward (Fig. S2 I–M). These results show that physiological concentrations of exogenous SA interfere with the accumulation of diverse PM proteins in intracellular BFA bodies.
Fig. 1.
SA interferes with the BFA-visualized internalization of PM proteins. (A–C) PIN2-GFP localization in young epidermal root cells treated with 25 μM BFA for 90 min (n = 215 BFA bodies; 12 roots) (A) or cotreated with either 15 μM SA (n = 262 BFA bodies; 14 roots) (B) or 50 μM SA (n = 25 BFA bodies; 16 roots) (C) for 90 min, after a 30-min SA pretreatment. (D) Quantification of the relative surface of the internalized PIN2-GFP in A–C. (E and F) PIP2a-GFP localization in elongated root epidermal cells after treatment with 25 μM BFA for 90 min (n = 257 BFA bodies; 15 roots) (E) or cotreated with 50 μM SA for 90 min after pretreatment with 50 μM SA for 30 min (n = 86 BFA bodies; 27 roots) (F). (G) Quantification of the relative surface of the internalized PIP2a-GFP in E and F. (H–J) Immunolocalization of PIN2 in young epidermal root cells of WT (Col-0) (n = 430 BFA bodies; 23 roots) (H), cpr1 (n = 258 BFA bodies; 14 roots) (I), and cpr5 (n = 237 BFA bodies; 12 roots) (J) mutants after treatment with 25 μM BFA for 90 min. (K) Quantification of the relative surface of the internalized PIN2 in H–J. Values in D, G, and K are mean surface areas normalized to the respective control treatments. Data are means ± SD; **P < 0.01 (Student t test). (Scale bars: 10 µm).
To test whether endogenously increased SA levels had an impact, we examined the mutants cpr1 (an allele of constitutive expressor of PR gene 1) and cpr5, which are known for their higher endogenous SA levels (23, 24). In roots of these mutants, the BFA-induced PIN2 and PIN1 intracellular accumulations were significantly lower than those of controls (Fig. 1 H–K and Fig. S3). Thus, both exogenously and endogenously increased levels of SA intervene with the BFA-visualized endocytic cycling of PM proteins.
SA Interferes with Endocytosis.
To distinguish between an SA effect on the internalization and the recycling step of the endocytic cycling, a washout experiment was carried out. First, seedlings were treated with BFA to internalize PM proteins into BFA bodies, whereafter BFA was washed out. The PIN2-GFP localization at the PM was recovered either in the presence or absence of SA. These experiments at different time points did not reveal any effect of SA on the PIN2-GFP recovery to the PM (Fig. S4), suggesting that SA does not influence exocytosis/recycling.
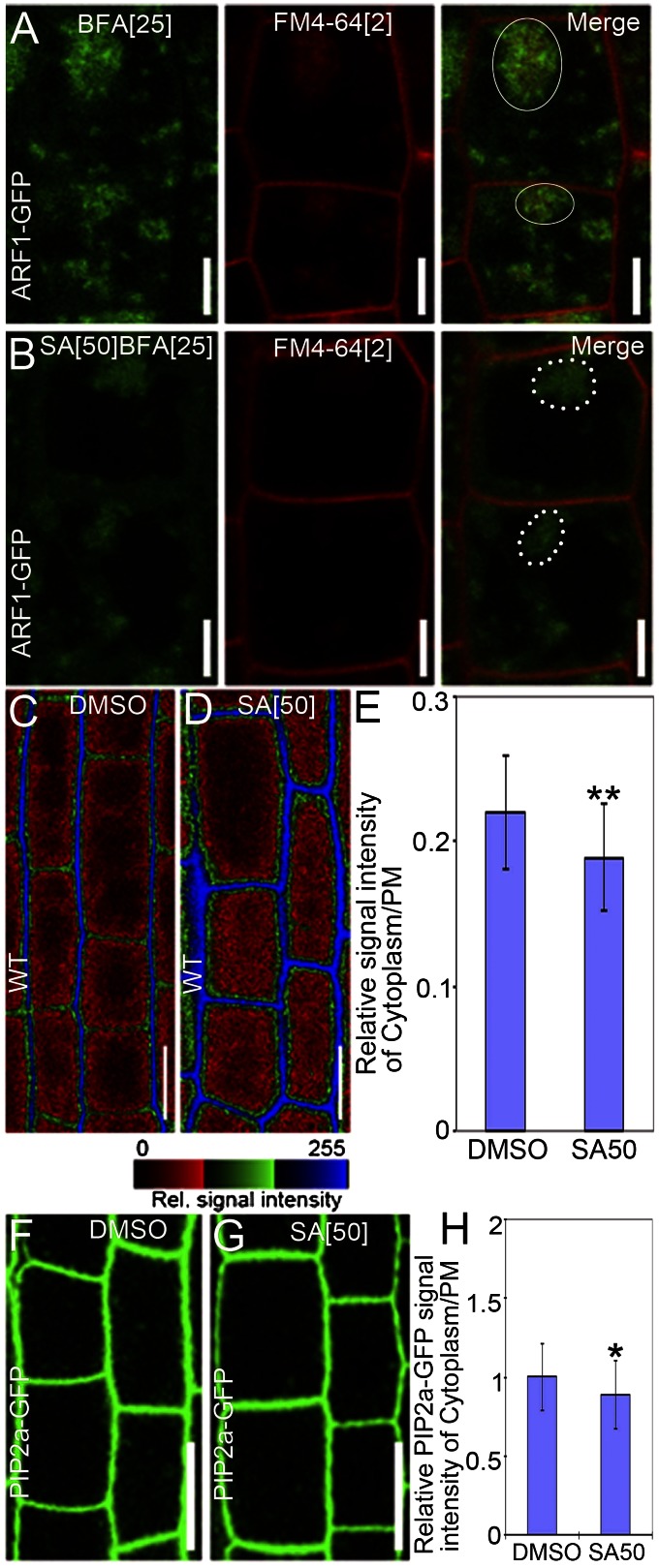
An alternative explanation would be that SA somehow interferes with the BFA action on intracellular dynamics. To test this possibility, we examined the SA effect on BFA-induced aggregations of trans-Golgi network/early endosome (TGN/EE) markers (17, 25). We used the vacuolar H+-ATPase subunit-a1 (VHAa1) or the ADP Ribosylation Factor 1 (ARF1) fused to GFP (VHAa1-GFP or ARF1-GFP) to label TGN/EEs (26, 27). As shown (26, 27), BFA caused aggregations of these markers (Fig. 2A and Fig. S5A), which was also clearly visible after the SA treatment (Fig. 2B and Fig. S5B). Notably, SA also did not markedly affect the mobility of the TGN/EEs labeled by syntaxin of plants 61 (SYP61) (28) or VHAa1-GFP (Fig. S6 D and H), revealing that BFA was still effective in the presence of SA and that SA did not generally interfere with endosomal dynamics.
Fig. 2.
SA reduces endocytosis and promotes protein accumulation at the PM. (A and B) Visualization of endosome aggregations (ARF1-GFP) and uptake of the endocytic tracer FM4-64 (2 µM for 5 min), cotreated with 25 µM BFA and either DMSO (n = 8 roots) (A) or 50 µM SA (n = 14 roots) (B) for 90 min. Closed circles in merged pictures (A) indicate colocalization between BFA-induced aggregations of the endosomal markers (ARF1-GFP) and the internalized FM4-64, whereas dashed circles (B) show the BFA-induced aggregations of endosomal markers (ARF1-GFP) and partially internalized FM4-64. (C and D) FM4-64 uptake of WT seedlings treated with DMSO (n = 37 cells; eight roots) (C) or 50 μM SA (n = 61 cells; 12 roots) (D). After 120 min of treatment, seedlings were stained for 5 min with 2 μM FM4-64. The relative fluorescence intensity is color-coded: red, low; green, medium; and blue, high fluorescence. (E) Quantification of the FM4-64 uptake in C and D as estimated by the ratio of the average signal intensity in the cytosol over that at the PM. (F and G) PIP2a-GFP fluorescence after treatments with DMSO (n = 58 cells; five roots) (F) or 50 µM SA (n = 40 cells; six roots) (G) for 120 min. (H) Relative signal intensities of PIP2a-GFP in the cytoplasm vs. at the PM in F and G. Data are means ± SD; *P < 0.05, **P < 0.01 (Student’s t test). (Scale bars: A and B, 5 µm; C, D, F, and G, 10 µm).
To investigate whether SA affected the PM-to-endosome trafficking, seedlings were stained with the endocytic tracer Fei Mao dye 4-64 (FM4-64) of which the internalization can be used as an endocytosis measure (29). Treatment with BFA alone led to a known coaggregation of FM4-64–stained endosomes and TGN/EE markers (26) (Fig. 2A and Fig. S5A). When seedlings were cotreated with SA, the FM4-64–stained intracellular signal dramatically decreased, but the BFA-induced aggregation of TGN/EEs was still visible (Fig. 2 B and Fig. S5B). These experiments hint at a preferential interference of SA with the cargo flow from the PM rather than with other BFA stimuli on organellar dynamics.
To examine the direct effect of SA on endocytosis, we used FM4-64 without BFA. In this setup, SA reduced the endocytic uptake of FM4-64 as reflected by the higher signal intensity at the PM and weaker FM4-64 internalization than those of untreated controls (Fig. 2 C–E). Treatment with SA in the absence of BFA also increased the incidence of constitutively endocytosed proteins, such as PIP2, at the PM (Fig. 2 F–H). In summary, these observations strongly suggest that SA interferes primarily with endocytosis, thereby increasing levels of constitutively cycling proteins at the PMs.
Impact of SA on Endocytosis Is Related to Clathrin.
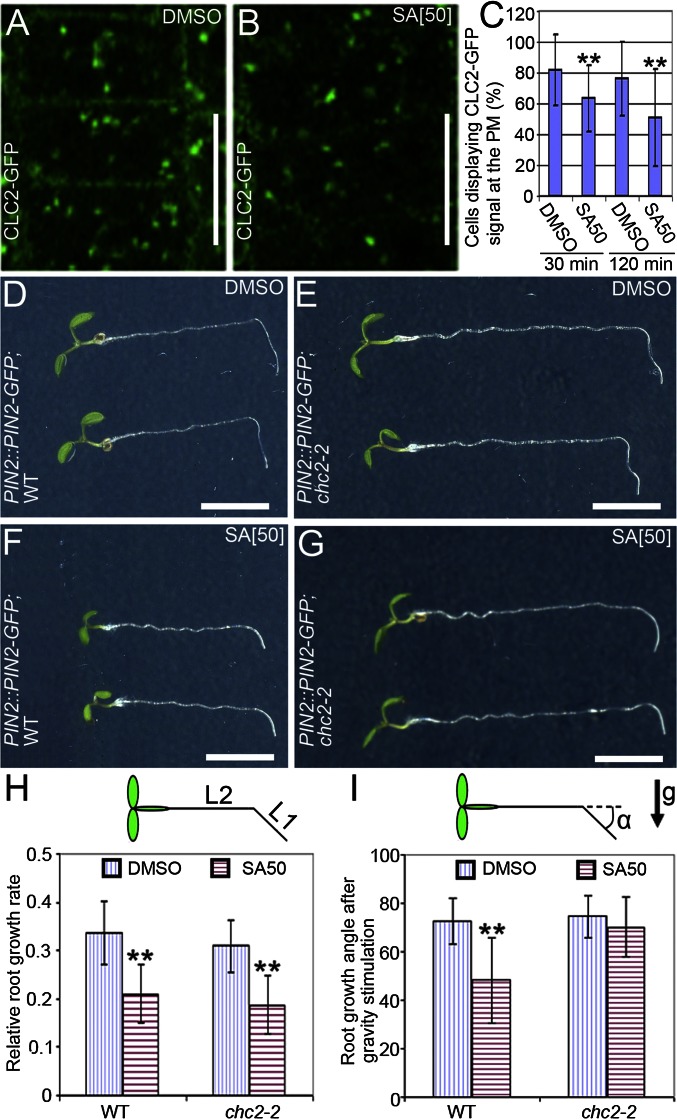
Because the prevalent endocytosis mechanism in plant cells depends on the coat protein clathrin (10, 12), we tested for an effect of SA on the clathrin incidence at the PM. Seedlings expressing clathrin light chain 2 fused to GFP (CLC2-GFP) were treated with SA for 30 min or 120 min. Quantification of the CLC2-GFP signal at the PM revealed that SA decreased the incidence of CLC2-GFP at the PM (Fig. 3 B and C). As a complementary approach, anti-clathrin heavy chain (CHC) antibodies were used to localize CHCs (10). Similarly to CLC2, the SA treatment decreased the occurrence of CHC at the PM (Fig. S5 D and E). In both experiments, the CLC2-GFP or CHC association with TGN/EEs remained seemingly unaffected (Fig. 3B and Fig. S5D). These observations show that SA interferes with the presence of the key component of endocytosis—clathrin—at the PM.
Fig. 3.
SA impairs clathrin-mediated endocytosis. (A and B) Visualization of the CLC2-GFP abundance at the PM after treatment with either DMSO (n = 374 cells; 44 roots) (A) or 50 μM SA (n = 470 cells; 50 roots) (B) for 120 min. (C) Percentage of cells showing the CLC2-GFP signal at the PM after treatment with SA for either 30 min or 120 min. (D–G) Root growth of 5-d-old WT (D and F) and chc2-2 mutants (E and G) expressing PIN2::PIN2-GFP after transfer to medium with DMSO (D and E) or with 50 μM SA (F and G) and a subsequent 20-h gravistimulation. (H) Quantification of the relative root growth of WT and chc2-2 mutants after transfer to medium supplemented with SA or not. Relative root growth rate was calculated as the ratio between the size of the root fragment grown after transfer (L1) and the total root length at the time of the transfer (L2) (nWT;DMSO = 45 roots and nchc2-2;DMSO = 44 roots; nWT;SA = 38 roots; and nchc2-2;SA = 39 roots). (I) Quantification of the root gravitropic bending of WT and chc2-2 mutants in the presence or absence of SA. Gravitropic bending was measured as the root growth angle (α) after gravistimulation (nWT;DMSO = 41 roots; nchc2-2;DMSO = 50 roots; nWT;SA = 34 roots; nchc2-2;SA = 28 roots). Data are means ± SD; **P < 0.01 (Student’s t test). Arrow indicates the gravity direction (g). (Scale bars: A and B, 10 μm; D–G, 5 mm).
The physiological relevance of the SA impact on clathrin-mediated processes was examined in the chc2-2 mutant, which is partially defective in CME (12). Treatment with SA reduced root growth and gravitropic root curvature in the WT (Fig. 3 D, F, H, and I), whereas in the chc2-2 seedlings, the sensitivity to SA was reduced in terms of gravity response, but was normal regarding the root growth (Fig. 3 D–I). Consistently, SA-treated seedlings had a less pronounced gravity-induced asymmetry in the auxin response as monitored by the auxin response reporter DR5rev::GFP (30) (Fig. S7 B and C), similarly to seedlings with inhibited clathrin function (12). By contrast, no SA resistance was found in the BFA-visualized endocytic trafficking 1 (ben1) mutant that is also defective in endocytosis, but not in the clathrin function (31) (Fig. S7D). In summary, the effect of SA on the clathrin incidence at the PM and the resistance of the clathrin mutants to SA suggest that SA affects endocytosis in relation to the clathrin-dependent mechanism of endocytosis.
Rapid Effect of SA on Endocytosis Is Independent of Transcription and Protein Synthesis.
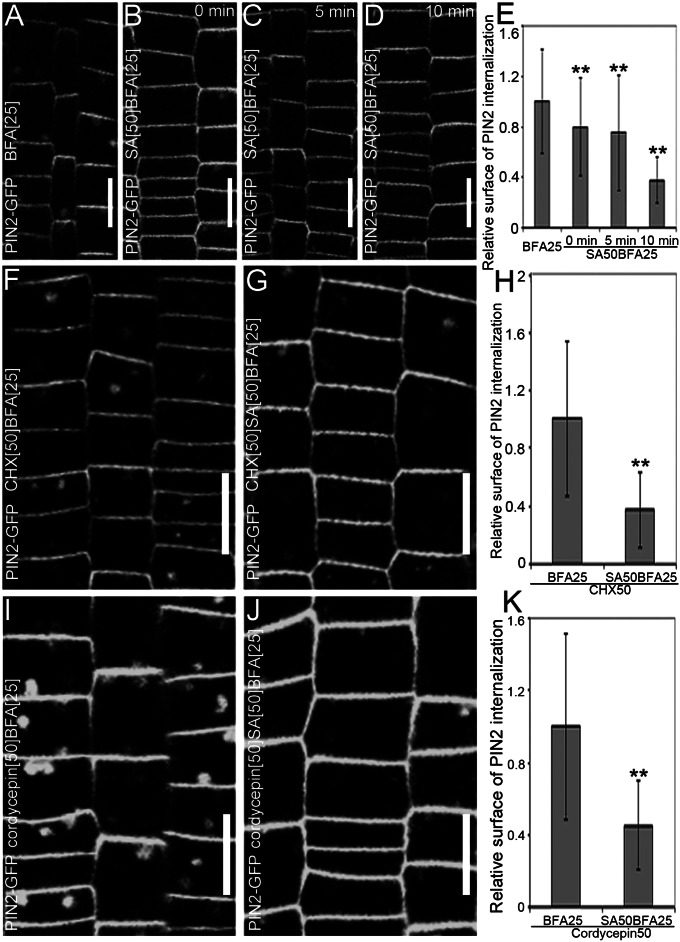
To gain insight into the cellular mechanism of the SA impact on endocytosis, we tested its kinetics of the BFA-induced PIN2-GFP internalization. An almost immediate effect was observed. Whereas 10 min of pretreatment with SA nearly maximally affected the BFA-induced PIN2 internalization, pronounced effects were visible already after the simultaneous application of SA and BFA (Fig. 4 A–E), implying that the effect of SA on endocytosis is fast and, thus, might not involve transcriptional regulation.
Fig. 4.
Transcription- and protein synthesis-independent effect of SA on endocytosis. (A–E) Evaluation of the effect of pretreatment with SA on the BFA-induced internalization of PIN2-GFP. (A) PIN2-GFP internalization treated with 25 µM BFA for 90 min (n = 248 BFA bodies; 18 roots). (B–D) Effect of cotreatment of 25 µM BFA and 50 µM SA after pretreatment with 50 µM SA for 0 min (n = 114 BFA bodies; 20 roots) (B), 5 min (n = 85 BFA bodies; 27 roots) (C), or 10 min (n = 64 BFA bodies; 18 roots) (D). (E) Quantitative evaluation of the effect of the SA pretreatment on the BFA-visualized PIN2-GFP internalization. (F and G) BFA-visualized internalization of PIN2-GFP in the presence of 50 μM CHX (n = 249 BFA bodies; 16 roots) (F) or cotreated with 50 μM SA for 90 min after a 30-min pretreatment with 50 µM SA (n = 241 BFA bodies; 24 roots) (G). (H) Quantification of the SA effect on the BFA-induced PIN2-GFP internalization in the presence of CHX. (I and J) BFA-visualized internalization of PIN2-GFP in the presence of 50 μM cordycepin (n = 303 BFA bodies; 13 roots) (I) or cotreated with 50 μM SA for 90 min after a 30-min pretreatment with 50 µM SA (n = 227 BFA bodies; 23 roots) (J). (K) Quantification of the SA effect on the BFA-induced PIN2-GFP internalization in the presence of cordycepin. Values in E, H, and K represent the mean surface area relative to the corresponding control treatment. Data are means ± SD; **P < 0.01 (Student’s t test). (Scale bars: 10 μm).
To address this hypothesis, the SA effect on the BFA-induced internalization in the presence of the protein synthesis inhibitor cycloheximide (CHX) or transcription inhibitor cordycepin was studied. In both cases, under conditions in which these drugs had been demonstrated to be effective (17), SA still effectively impaired the BFA-induced internalization of PIN2-GFP (Fig. 4 G, H, J, and K), implying that this effect does not involve transcription or de novo protein synthesis. Similarly, when seedlings were pretreated with the proteasome inhibitor MyoGenics132 (MG132) for 30 min and, subsequently, cotreated with SA, SA still affected effectively the BFA-induced PIN2-GFP internalization (Fig. S8 B and C). These results indicate that the SA impact on endocytosis is distinct from its known effect on transcription and that SA might regulate the clathrin endocytic machinery without involvement of transcriptional regulation.
SA Regulation of Endocytosis Does Not Require Known Signaling Components.
The observed effects of SA on CME are reminiscent of the effect of the plant hormone auxin on endocytosis that also targets clathrin and does not involve transcription regulation (17), but the putative auxin receptor AUXIN BINDING PROTEIN 1 (ABP1) (13). Therefore, we tested whether the effect of SA on endocytosis requires the ABP1 function as well. The effect of SA on the PIN2 accumulation in BFA bodies was examined in various conditional abp1 knockdown lines (ABP1AS, abp1-SS12K, and abp1-SS12S) (32), an auxin-insensitive allele (abp1-5), all of which are defective in auxin-induced endocytosis (13), and an ABP1 overexpression line (13). In all these lines, SA still effectively reduced the protein internalization as seen in WT lines (Fig. S9 A–M). Furthermore, although the auxin effect on endocytosis and clathrin occurrence at the PM is only transient (13), the SA effect was more persistent and was still visible after 120 min (Fig. 3C). These observations imply that the effects of SA and auxin on endocytosis are distinct and involve different mechanisms.
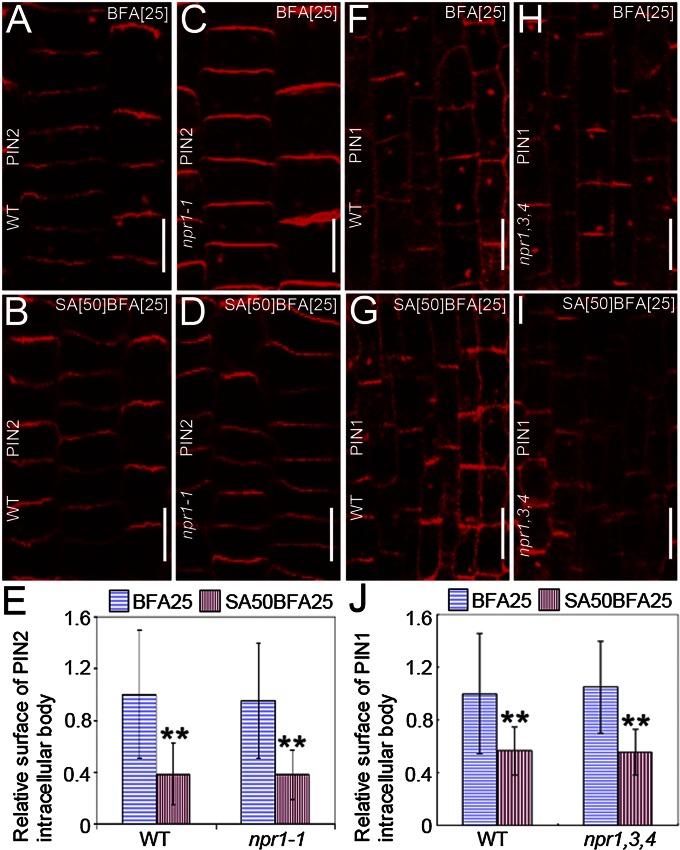
The observations that CME is repressed by SA independently of transcription imply the existence of an SA signaling that does not depend on the known transcription-regulating pathway. This assumption was examined with the nonexpresser of PR genes1-1 (npr1-1) and npr1-2npr3-1npr4-3 mutants that are defective in the SA-mediated transcription regulation (21, 33). In these receptor mutants, SA effectively reduced the PIN1 and PIN2 internalization as observed in WT seedlings (Fig. 5 B, D, E, G, I, and J). Thus, the SA impact on endocytosis and on transcription is mediated by a distinct mechanism and requires a separate set of signaling components. The hypothesis was further supported by the observation that 100 μM of the synthetic SA analog benzothia-diazole S-methylester (BTH), which is known to functionally mimic the effect of SA on transcription (34), was ineffective in inhibiting the BFA-induced PIN2-GFP internalization (Fig. S9O). These results show that the SA effect on endocytosis does not require established SA signaling components, implying the involvement of another, transcription-independent, SA signaling pathway.
Fig. 5.
SA receptors are not required for SA effect on endocytosis. (A–D) Immunolocalization of PIN2 in WT (A and B), and npr1-1 (C and D) treated with 25 μM BFA (A and C) or cotreated with 50 μM SA for 90 min after a 30-min pretreatment with 50 µM SA (B and D). (E) Quantification of the effect of SA on the BFA-induced PIN2 internalization in A–D (nWT;DMSO = 513 BFA bodies from 26 roots; nnpr1-1;DMSO = 169 BFA bodies from 10 roots; nWT;SA = 68 BFA bodies from 34 roots; nnpr1-1;SA = 85 BFA bodies from 33 roots). (F–I) Immunolocalization of PIN1 in WT (F and G), and npr1,3,4 (H and I) treated with 25 μM BFA (F and H) or cotreated with 50 μM SA for 90 min after a 30-min pretreatment with 50 µM SA (G and I). (J) Quantification of the effect of SA on the BFA-induced PIN1 internalization in F–I (nWT;DMSO = 120 BFA bodies from eight roots; nnpr1,3,4;DMSO = 115 BFA bodies from eight roots; nWT;SA = 114 BFA bodies from 13 roots; nnpr1,3,4;SA = 65 BFA bodies from four roots). Values in E and J represent the mean surface area relative to the control. Data are means ± SD; **P < 0.01 (Student’s t test). (Scale bars: 10 μm).
SA Does Not Inhibit the flagellin (flg22)-Triggered Endocytosis of the FLAGELLIN SENSING2 (FLS2) Receptor.
Our observations revealed that SA, a well-established hormone in pathogen defenses (1), interferes with endocytosis. A typical endocytosis-mediated process in plant immunity is the internalization of the FLS2 receptor kinase from the PM in response to the bacterial flagellin or its derived elicitor-active peptide flg22 (35, 36). In contrast to the above studied endocytic trafficking pathways, the ligand-induced endocytosis of FLS2 is insensitive to BFA treatments (37), suggesting at least a partially distinct mechanism of this process.
Therefore, we tested for possible SA effects on the flg22-induced accumulation of FLS2-GFP–containing endosomes. Treatment with 50 µM or 200 µM SA did not block flg22-induced FLS2 endocytosis (Fig. S10 C, F, and I), and only a slight delay was noted in plants treated with 200 µM SA compared with the control (Fig. S10 C and I). When seedlings were treated with unusually high SA concentrations (1 mM), the FLS2 endocytosis was strongly delayed (Fig. S10 J–Q). However, because binding of flg22 to its receptor FLS2 is highly sensitive to pH (38), this delay in FLS2-GFP endocytosis at very high SA concentrations is probably due to low pH levels resulting from the treatment with acidic SA solutions. Thus, at physiological concentrations, SA has no impact on the flg22-induced endocytosis of FLS2-GFP, further supporting the notion that SA affects a BFA-sensitive endocytic pathway, which is distinct from the endocytosis of activated FLS2.
Discussion
SA is a classical plant hormone that plays an important role in plant immunity against pathogens. Recently, the mechanism of SA perception has been identified and the SA action mode has been established to transcriptionally regulate a number of genes, including those related to plant immunity (33, 39–41). Here, we found an effect of SA interference on the BFA-sensitive endocytic recycling of PM proteins that occurs probably at the level of the endocytic coat protein clathrin and does not involve transcriptional regulation.
Physiological concentrations of exogenous SA or mutants with increased endogenous SA levels hamper the constitutive endocytosis of PM proteins, the endocytic uptake of fluorescent tracers, and the incidence of the endocytic coat protein clathrin at the PM. Furthermore, SA interferes with the root gravitropism and the auxin distribution during this process as also observed in clathrin-deficient mutants (12). Furthermore, mutants defective in the clathrin function are less sensitive to the impact of SA on the gravitropic root growth (Fig. 3 G and I). All these observations consistently imply that SA targets CME and that by hindering this process, SA increases the levels of various proteins at the PM, thus providing a mechanism to control their activity.
During pathogen infection, endocytosis plays an important role in at least two instances: (i) perception of a bacterial flagellin and oomycete-derived cryptogein associated with endocytosis of its receptor and activation of downstream immune responses, respectively (35, 42, 43) and (ii) internalization of eukaryotic pathogen effector molecules in the host cell (44). SA is part of the immune response in plants and, therefore, it is plausible that the SA effect on endocytosis is related to its role in these processes. However, because SA had no obvious effects on the flg22-activated internalization of FLS2-GFP, the first hypothesis should be ruled out. The effect of SA on effector uptake remains to be tested. Moreover, given that SA is involved in many more physiological conditions (e.g., stomatal aperture regulation, seed germination, flowering, senescence, thermogenesis, hormonal cross-talk, and abiotic stress) than response to pathogens alone (6), it is plausible that endocytosis is regulated through a mechanism by which SA impacts on them.
The SA impact on CME is rapid and does not require transcription and neither of the known components of the SA signaling for transcription regulation; also it is distinct from an effect of auxin that targets CME via ABP1 and downstream ROP GTPase-mediated signaling (13, 45, 46). Therefore, an alternative SA signaling pathway might regulate clathrin-mediated endocytosis. Identification of the molecular components of this pathway and elucidation of the endocytosis-affecting cross-talk between SA and auxin remain fascinating topics.
Materials and Methods
Plant Material and Growth Conditions.
The transgenic lines PIN2::PIN2-GFP (27), VHAa1::VHAa1-GFP (26), 35S::GFP-PIP2a (47), 35S::CLC2-GFP (48), and SYP61::SYP61-CFP (28), DR5rev::GFP (30), and 35S::ABP1-GFP (13), ARF1::ARF1-GFP (27), p35S:AlcR>pAlcA:ABP1AS (Alc>>ABP1AS), Alc>>abp1-SS12K and Alc>>abp1-SS12S (49), and the mutants abp1-5 (50), cpr1 (24), cpr5 (23), chc2-2 (12) and npr1-1 (51), npr1-2npr3-1npr4-3 (33) have been described. Seeds were grown on solid Arabidopsis medium (AM) (0.5× MS basal salt mixture, 1% sucrose, and 0.8% agar at pH 5.8) in vertically oriented plates at 22 °C under continuous illumination.
Drug Treatments, Confocal Microscopy, Quantitative Analysis of BFA Bodies, and PM Fluorescence.
Five-day-old seedlings were incubated for the indicated time points in liquid AM supplemented with appropriate volumes of 100 mM SA (Sigma-Aldrich), 50 mM BFA (Molecular Probes), 50 mM CHX (Sigma-Aldrich), 50 mM cordycepin (Sigma-Aldrich), 50 mM MG132 (Sigma-Aldrich), or 100 mM BTH (Sigma-Aldrich) DMSO-prepared stock solutions, as described (13), unless mentioned otherwise. Labeling with FM4-64 (stock 2 mM in water; Invitrogen) was carried out as described (13). Confocal images were obtained with Leica SP2 and Carl Zeiss LSM 710 confocal microscopes. The relative surface of the internalized proteins or relative fluorescence intensity at the PM was calculated as the average BFA body area or fluorescence intensity at the PM, normalized to the average value of the control. First, we measured the BFA body sizes or fluorescence intensities for all roots and treatments/genotypes. Subsequently, the individual BFA body sizes/fluorescence intensities were divided by this averaged control body size/fluorescence intensity, yielding a relative surface of internalized protein/relative fluorescence intensity. The ImageJ 1.41 software was used for all of the measurements as described (12).
Immunodetection.
Whole-mount root immunodetection was performed as described (52). The antibody dilutions used were as follows: 1:1,000 for anti-PIN1 (17), 1:1,000 for anti-PIN2 (53), and 1:400 for anti-CHC (10). The secondary antibodies were anti-rabbit IgG Alexa488-conjugated antibody (Invitrogen) (1:600) and anti-rabbit IgG Cyanine Dye3 (Cy3) conjugated antibody (Sigma-Aldrich) (1:600).
Gravity Stimulation.
Five-day-old seedlings were transferred to new plates containing AM supplemented with 50 µM SA or the equivalent amount of DMSO as control. The plates were turned 90° compared with the original vertical position. Seedlings were gravistimulated for 20 h under continuous illumination or 16 h light, followed by 4 h of darkness.
SA Treatment for FLS2 Internalization.
FLS2 endocytosis was imaged by confocal microscopy as described (37) with the Opera microscope (PerkinElmer) equipped with three 1.3-megapixel charge-coupled device cameras with a Nipkow spinning disk. The samples were excited at 488 nm for GFP; the emission spectrum was taken from 502 to 577 nm. Leaves were prepared in 96-well plates with optical glass bottoms (Greiner Bio-One). Detached cotyledons of 2-wk-old Arabidopsis plants (Col-0/FLS2-GFP) (54) were used and preincubated with water or 50 µM, 200 µM, and 1 mM SA for 30 min, before 10 μM flg22 was added. Different time points after flg22 addition were imaged. For the images, a consecutive series of 21 planes with a distance of 1 μm were taken and displayed as a maximum projection by using the Acapella software (PerkinElmer).
Supplementary Material
Acknowledgments
We thank Eva Benková, Alan Jones, Ben Scheres, and Xinnian Dong for kindly sharing materials and the Nottingham Arabidopsis Stock Centre for providing seed stocks. This work was supported by the Deutsche Forschungsgemeinschaft (M.B.), the Gatsby Charitable Foundation (S.R.), the Ghent University Special Research Fund (E.H.), the Odysseus program of the Research Foundation-Flanders, and the Körber European Science Foundation (J.F.). S.V. is a Postdoctoral Fellow of the Research Foundation-Flanders.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220205110/-/DCSupplemental.
References
- 1.Vlot AC, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 2.Chini A, Grant JJ, Seki M, Shinozaki K, Loake GJ. Drought tolerance established by enhanced expression of the CC-NBS-LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 2004;38(5):810–822. doi: 10.1111/j.1365-313X.2004.02086.x. [DOI] [PubMed] [Google Scholar]
- 3.Borsani O, Valpuesta V, Botella MA. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 2001;126(3):1024–1030. doi: 10.1104/pp.126.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch JR, et al. Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiol. 2000;123(2):487–496. doi: 10.1104/pp.123.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott IM, Clarke SM, Wood JE, Mur LAJ. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004;135(2):1040–1049. doi: 10.1104/pp.104.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: Its role in plant growth and development. J Exp Bot. 2011;62(10):3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- 7. Frei dit Frey N, Robatzek S (2009) Trafficking vesicles: Pro or contra pathogens? Curr Opin Plant Biol 12(4):437–443. [DOI] [PubMed]
- 8.Leborgne-Castel N, Adam T, Bouhidel K. Endocytosis in plant-microbe interactions. Protoplasma. 2010;247(3-4):177–193. doi: 10.1007/s00709-010-0195-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Irani NG, Friml J. Clathrin-mediated endocytosis: The gateway into plant cells. Curr Opin Plant Biol. 2011;14(6):674–682. doi: 10.1016/j.pbi.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Dhonukshe P, et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17(6):520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 11. Barberon M, et al. (2011) Monoubiquitin-dependent endocytosis of the IRON-REGULATED TRANSPORTER 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA 108(32):12985–12986, E450–E458. [DOI] [PMC free article] [PubMed]
- 12.Kitakura S, et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23(5):1920–1931. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert S, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143(1):111–121. doi: 10.1016/j.cell.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marhavý P, et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell. 2011;21(4):796–804. doi: 10.1016/j.devcel.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Whitford R, et al. GOLVEN secretory peptides regulate auxin carrier turnover during plant gravitropic responses. Dev Cell. 2012;22(3):678–685. doi: 10.1016/j.devcel.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Weaver ND, Kesarwani M, Dong X. Induction of protein secretory pathway is required for systemic acquired resistance. Science. 2005;308(5724):1036–1040. doi: 10.1126/science.1108791. [DOI] [PubMed] [Google Scholar]
- 17.Paciorek T, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435(7046):1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 18.Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413(6854):425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 19.Naramoto S, et al. ADP-ribosylation factor machinery mediates endocytosis in plant cells. Proc Natl Acad Sci USA. 2010;107(50):21890–21895. doi: 10.1073/pnas.1016260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grebe M, et al. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr Biol. 2003;13(16):1378–1387. doi: 10.1016/s0960-9822(03)00538-4. [DOI] [PubMed] [Google Scholar]
- 21.Spoel SH, et al. Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell. 2009;137(5):860–872. doi: 10.1016/j.cell.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol. 2007;17(20):1784–1790. doi: 10.1016/j.cub.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9(9):1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowling SA, et al. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell. 1994;6(12):1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geldner N, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112(2):219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 26.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18(3):715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Scheres B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell. 2005;17(2):525–536. doi: 10.1105/tpc.104.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert S, et al. Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci USA. 2008;105(24):8464–8469. doi: 10.1073/pnas.0711650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jelínková A, et al. Probing plant membranes with FM dyes: Tracking, dragging or blocking? Plant J. 2010;61(5):883–892. doi: 10.1111/j.1365-313X.2009.04102.x. [DOI] [PubMed] [Google Scholar]
- 30.Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426(6963):147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Kitakura S, De Rycke R, De Groodt R, Friml J. Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Curr Biol. 2009;19(5):391–397. doi: 10.1016/j.cub.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 32.Tromas A, et al. The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS ONE. 2009;4(9):e6648. doi: 10.1371/journal.pone.0006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu ZQ, et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486(7402):228–232. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Görlach J, et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 1996;8(4):629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20(5):537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zipfel C, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428(6984):764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 37.Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell. 2012;24(10):4205–4219. doi: 10.1105/tpc.112.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer Z, Gómez-Gómez L, Boller T, Felix G. Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J Biol Chem. 2001;276(49):45669–45676. doi: 10.1074/jbc.M102390200. [DOI] [PubMed] [Google Scholar]
- 39.Chandran D, et al. Temporal global expression data reveal known and novel salicylate-impacted processes and regulators mediating powdery mildew growth and reproduction on Arabidopsis. Plant Physiol. 2009;149(3):1435–1451. doi: 10.1104/pp.108.132985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenk PM, et al. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA. 2000;97(21):11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, et al. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012;1(6):639–647. doi: 10.1016/j.celrep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Salomon S, Robatzek S. Induced endocytosis of the receptor kinase FLS2. Plant Signal Behav. 2006;1(6):293–295. doi: 10.4161/psb.1.6.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leborgne-Castel N, et al. The plant defense elicitor cryptogein stimulates clathrin-mediated endocytosis correlated with reactive oxygen species production in bright yellow-2 tobacco cells. Plant Physiol. 2008;146(3):1255–1266. doi: 10.1104/pp.107.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafiqi M, Ellis JG, Ludowici VA, Hardham AR, Dodds PN. Challenges and progress towards understanding the role of effectors in plant-fungal interactions. Curr Opin Plant Biol. 2012;15(4):477–482. doi: 10.1016/j.pbi.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, et al. ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr Biol. 2012;22(14):1326–1332. doi: 10.1016/j.cub.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Lin D, et al. A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr Biol. 2012;22(14):1319–1325. doi: 10.1016/j.cub.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR. Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA. 2000;97(7):3718–3723. doi: 10.1073/pnas.97.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konopka CA, Backues SK, Bednarek SY. Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell. 2008;20(5):1363–1380. doi: 10.1105/tpc.108.059428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braun N, et al. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell. 2008;20(10):2746–2762. doi: 10.1105/tpc.108.059048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu T, et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010;143(1):99–110. doi: 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell. 1994;6(11):1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauer M, Paciorek T, Benková E, Friml J. Immunocytochemical techniques for whole-mount in situ protein localization in plants. Nat Protoc. 2006;1(1):98–103. doi: 10.1038/nprot.2006.15. [DOI] [PubMed] [Google Scholar]
- 53.Abas L, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8(3):249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 54.Chinchilla D, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448(7152):497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.