Abstract
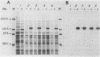
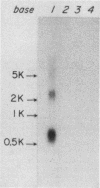
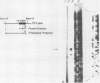
A rat platelet factor 4 (PF4) cDNA has been isolated by immunoscreening a g lambda 11 rat megakaryocyte cDNA expression library. Sequence analysis of the rat PF4 cDNA revealed that this megakaryocyte protein is composed of a leader sequence of 29 amino acid residues and a mature protein sequence of 76 amino acid residues. The structure of rat PF4 derived from its cDNA shows a marked homology with the amino acid sequence of human PF4 obtained by classical protein chemistry techniques. This observation is particularly striking with regard to the carboxy-terminal region of rat and human PF4, where 28 of the last 31 C-terminal residues are identical. The rat PF4 gene was obtained from a rat genomic library by using rat PF4 cDNA as a hybridization probe. Sequence analysis showed that the gene is constructed of three exons and two short introns. The transcriptional start site is located 73 base pairs upstream of the translational start codon as judged by S1 nuclease mapping and primer extension. The 5' noncoding region of the gene also exhibited a sequence homologous to the TATA box at -31, as well as a series of direct and inverted repeat sequences and a cluster of 26 T residues at -155 to -218. This latter domain may be involved in regulating PF4 gene expression during megakaryocytopoiesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. J., Käser-Glanzmann R., Jakábová M., Lüscher E. F. Characterization of a chondroitin 4 -sulfate proteoglycan carrier for heparin neutralizing activity (platelet factor 4 ) released from human blood platelets. Biochim Biophys Acta. 1972 Dec 29;286(2):312–329. [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman M. J., Handin R. I., Cohen P. Distribution of fibrinogen, and platelet factors 4 and XIII in subcellular fractions of human platelets. Br J Haematol. 1975 Sep;31(1):51–55. doi: 10.1111/j.1365-2141.1975.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Busch C., Dawes J., Pepper D. S., Wasteson A. Binding of platelet factor 4 to cultured human umbilical vein endothelial cells. Thromb Res. 1980 Jul 1;19(1-2):129–137. doi: 10.1016/0049-3848(80)90412-0. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Keim P. S., Farmer M., Heinrikson R. L. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2256–2258. doi: 10.1073/pnas.74.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont H., Dupont M. A., Bricaud H., Boisseau M. R. Megakaryocytes separation in homogeneous classes by unit gravity sedimentation: physico-chemical, ultrastructural and cytophotometric characterizations. Biol Cell. 1983;49(2):137–144. doi: 10.1111/j.1768-322x.1984.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- EBBE S., STOHLMAN F., Jr MEGAKARYOCYTOPOIESIS IN THE RAT. Blood. 1965 Jul;26:20–35. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Go M. Modular structural units, exons, and function in chicken lysozyme. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1964–1968. doi: 10.1073/pnas.80.7.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hermodson M., Schmer G., Kurachi K. Isolation, crystallization, and primary amino acid sequence of human platelet factor 4. J Biol Chem. 1977 Sep 25;252(18):6276–6279. [PubMed] [Google Scholar]
- Hiti-Harper J., Wohl H., Harper E. Platelet factor 4: an inhibitor of collagenase. Science. 1978 Mar 3;199(4332):991–992. doi: 10.1126/science.203038. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S., Deuel T. F. Proteoglycan carrier of human platelet factor 4. Isolation and characterization. J Biol Chem. 1982 Oct 10;257(19):11546–11550. [PubMed] [Google Scholar]
- Hull B. E., Sher S. E., Rosen S., Church D., Bell E. Structural integration of skin equivalents grafted to Lewis and Sprague-Dawley rats. J Invest Dermatol. 1983 Nov;81(5):429–436. doi: 10.1111/1523-1747.ep12522599. [DOI] [PubMed] [Google Scholar]
- Kaplan K. L., Broekman M. J., Chernoff A., Lesznik G. R., Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979 Apr;53(4):604–618. [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Katz I. R., Thorbecke G. J., Bell M. K., Yin J. Z., Clarke D., Zucker M. B. Protease-induced immunoregulatory activity of platelet factor 4. Proc Natl Acad Sci U S A. 1986 May;83(10):3491–3495. doi: 10.1073/pnas.83.10.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D., Hyldig-Nielsen J. J., Servenius B., Andersson G., Rask L., Peterson P. A. Exon-intron organization and complete nucleotide sequence of a human major histocompatibility antigen DC beta gene. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7313–7317. doi: 10.1073/pnas.80.23.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonky S. A., Marsh J., Wohl H. Stimulation of human granulocyte elastase by platelet factor 4 and heparin. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1113–1118. doi: 10.1016/0006-291x(78)90657-5. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Mandel T. E. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979 Feb;98(2):401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W., Friday T. J., Charsha D. E. Effects of thrombocytopenia on megakaryocytopoiesis. Br J Haematol. 1969 Jul;17(1):91–101. doi: 10.1111/j.1365-2141.1969.tb05667.x. [DOI] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W. Polyploidy and maturation of rat megakaryocytes. Blood. 1968 Jul;32(1):102–110. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus J. M. DNA metabolism and development of organelles in guinea-pig megakaryocytes: a combined ultrastructural, autoradiographic and cytophotometric study. Blood. 1970 Mar;35(3):298–311. [PubMed] [Google Scholar]
- Penington D. G., Streatfield K. Heterogeneity of megakaryocytes and platelets. Ser Haematol. 1975;8(1):22–48. [PubMed] [Google Scholar]
- Rabellino E. M., Levene R. B., Leung L. L., Nachman R. L. Human megakaryocytes. II. Expression of platelet proteins in early marrow megakaryocytes. J Exp Med. 1981 Jul 1;154(1):88–100. doi: 10.1084/jem.154.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo R., Nakeff A., Huang S. S., Ginsberg M., Deuel T. F. New synthesis of a platelet-specific protein: platelet factor 4 synthesis in a megakaryocyte-enriched rabbit bone marrow culture system. J Cell Biol. 1983 Feb;96(2):515–520. doi: 10.1083/jcb.96.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun J. W., Schwarzbauer J. E., Hynes R. O. A single rat fibronectin gene generates three different mRNAs by alternative splicing of a complex exon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5140–5144. doi: 10.1073/pnas.81.16.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci G., Tabilio A., Deschamps J. F., Van Haeke D., Henri A., Guichard J., Tetteroo P., Lansdorp P. M., Hercend T., Vainchenker W. Immunological study of in vitro maturation of human megakaryocytes. Br J Haematol. 1984 Apr;56(4):589–605. doi: 10.1111/j.1365-2141.1984.tb02184.x. [DOI] [PubMed] [Google Scholar]
- Wagner R. C., Kreiner P., Barrnett R. J., Bitensky M. W. Biochemical characterization and cytochemical localization of a catecholamine-sensitive adenylate cyclase in isolated capillary endothelium. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3175–3179. doi: 10.1073/pnas.69.11.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz D. A., Wu V. Y., de Lamo R., Dene H., McCoy L. E. Primary structure of human platelet factor 4. Thromb Res. 1977 Dec;11(6):893–898. doi: 10.1016/0049-3848(77)90117-7. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]