Abstract
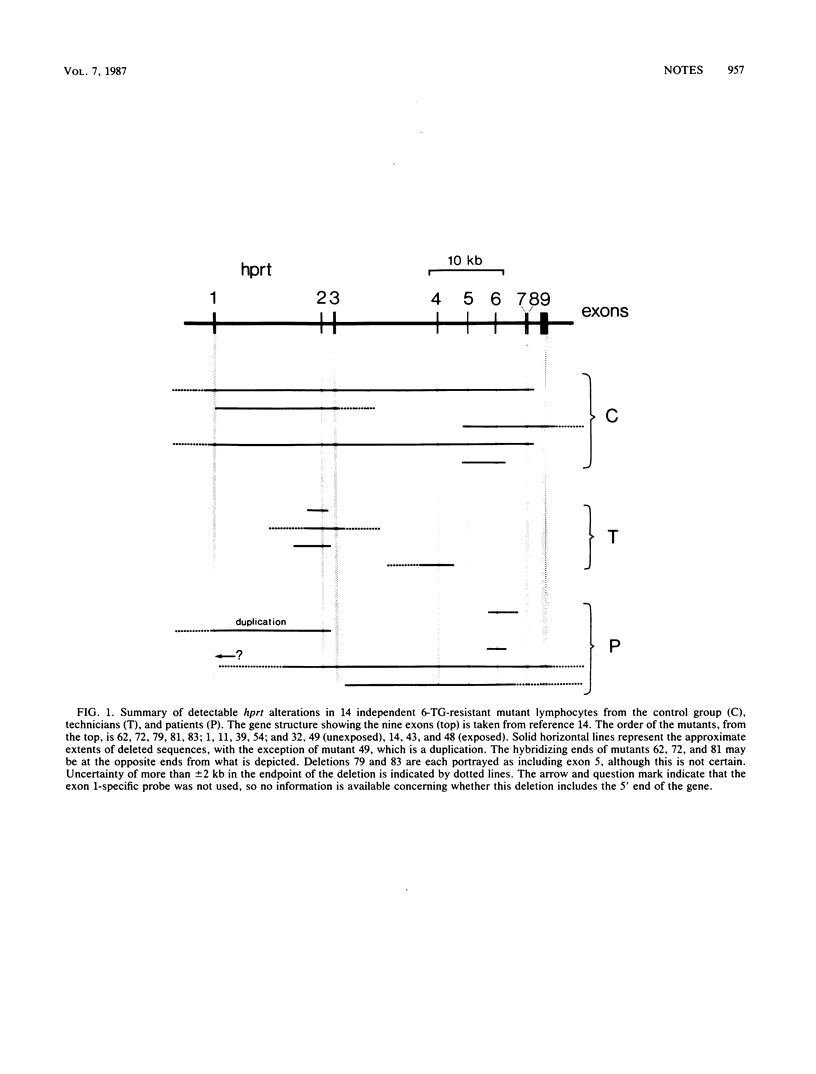
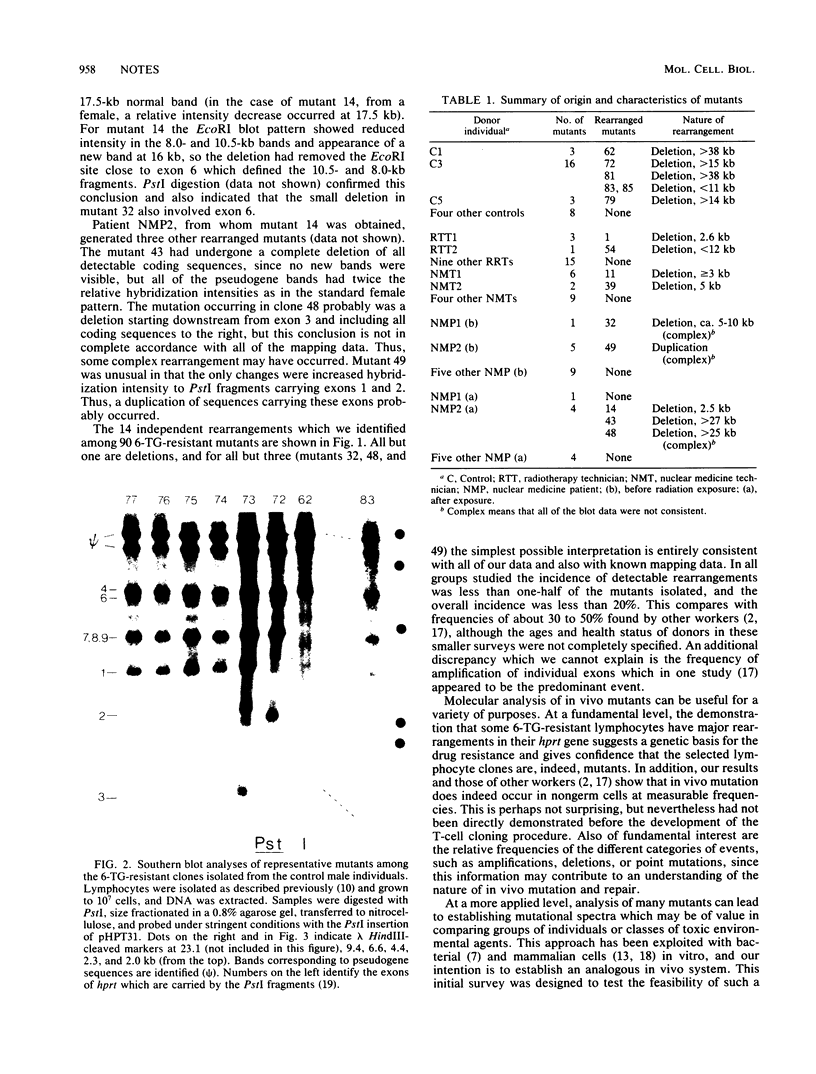
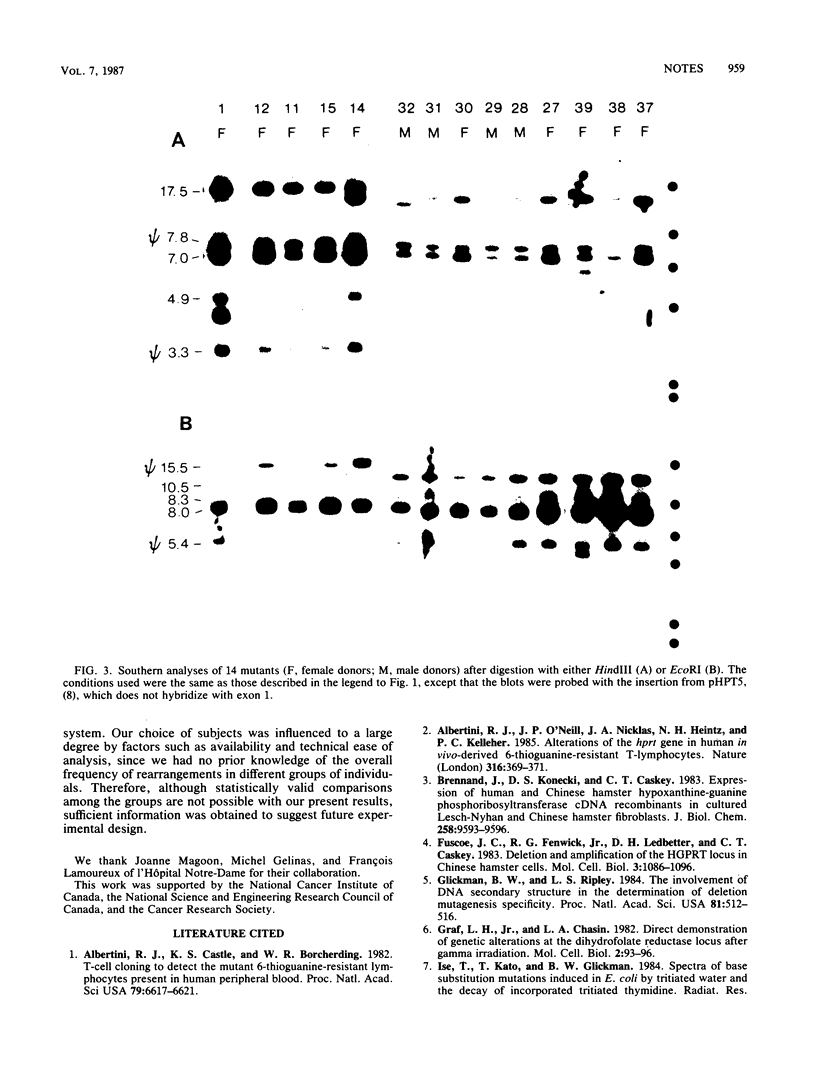
Ninety hypoxanthine phosphoribosyltransferase-deficient mutants were isolated from lymphocytes of 31 individuals drawn from both control populations and populations exposed to low doses of ionizing radiation. Southern analysis of the DNA revealed altered hybridization patterns in 15 mutants. Of these, 14 changes consisted of deletions of 2 to 40 kilobases or more.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., Castle K. L., Borcherding W. R. T-cell cloning to detect the mutant 6-thioguanine-resistant lymphocytes present in human peripheral blood. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6617–6621. doi: 10.1073/pnas.79.21.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Brennand J., Konecki D. S., Caskey C. T. Expression of human and Chinese hamster hypoxanthine-guanine phosphoribosyltransferase cDNA recombinants in cultured Lesch-Nyhan and Chinese hamster fibroblasts. J Biol Chem. 1983 Aug 25;258(16):9593–9596. [PubMed] [Google Scholar]
- Fuscoe J. C., Fenwick R. G., Jr, Ledbetter D. H., Caskey C. T. Deletion and amplification of the HGPRT locus in Chinese hamster cells. Mol Cell Biol. 1983 Jun;3(6):1086–1096. doi: 10.1128/mcb.3.6.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman B. W., Ripley L. S. Structural intermediates of deletion mutagenesis: a role for palindromic DNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):512–516. doi: 10.1073/pnas.81.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf L. H., Jr, Chasin L. A. Direct demonstration of genetic alterations at the dihydrofolate reductase locus after gamma irradiation. Mol Cell Biol. 1982 Jan;2(1):93–96. doi: 10.1128/mcb.2.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing K., Bradley W. E. In vivo mutant frequency rises among breast cancer patients after exposure to high doses of gamma-radiation. Mutat Res. 1985 Oct;152(1):107–112. doi: 10.1016/0027-5107(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Messing K., Seifert A. M., Bradley W. E. In vivo mutant frequency of technicians professionally exposed to ionizing radiation. Prog Clin Biol Res. 1986;207:87–97. [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R., Ryall R. G. Measurement of in vivo mutations in human lymphocytes. Nature. 1983 Mar 10;302(5904):155–156. doi: 10.1038/302155a0. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Goncalves O., Meuth M. Structure of mutant alleles at the aprt locus of Chinese hamster ovary cells. J Mol Biol. 1983 Jul 5;167(3):575–594. doi: 10.1016/s0022-2836(83)80099-0. [DOI] [PubMed] [Google Scholar]
- Patel P. I., Framson P. E., Caskey C. T., Chinault A. C. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol Cell Biol. 1986 Feb;6(2):393–403. doi: 10.1128/mcb.6.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. I., Nussbaum R. L., gramson P. E., Ledbetter D. H., Caskey C. T., Chinault A. C. Organization of the HPRT gene and related sequences in the human genome. Somat Cell Mol Genet. 1984 Sep;10(5):483–493. doi: 10.1007/BF01534853. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Simons J. W., Arwert F., Natarajan A. T., van Zeeland A. A. Mutations induced by X-rays at the HPRT locus in cultured Chinese hamster cells are mostly large deletions. Mutat Res. 1985 Dec;144(4):281–286. doi: 10.1016/0165-7992(85)90065-x. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]