Abstract
Alzheimer’s disease (AD) is associated with impaired clearance of β-amyloid (Aβ) from the brain, a process normally facilitated by apolipoprotein E (apoE). ApoE expression is transcriptionally induced through the action of the nuclear receptors peroxisome proliferator–activated receptor gamma and liver X receptors in coordination with retinoid X receptors (RXRs). Oral administration of the RXR agonist bexarotene to a mouse model of AD resulted in enhanced clearance of soluble Aβ within hours in an apoE-dependent manner. Aβ plaque area was reduced more than 50% within just 72 hours. Furthermore, bexarotene stimulated the rapid reversal of cognitive, social, and olfactory deficits and improved neural circuit function. Thus, RXR activation stimulates physiological Aβ clearance mechanisms, resulting in the rapid reversal of a broad range of Aβ-induced deficits.
The most common form of Alzheimer’s disease (AD) occurs sporadically late in life and is typified by deposition of β-amyloid (Aβ) within the brain (1). Individuals with late-onset AD produce Aβ peptides at normal levels but have an impaired ability to clear them from the brain (2). Elevated levels of Aβ are associated with perturbations of synaptic function and neural network activity that probably underlie the cognitive deficits in AD (3). Moreover, Aβ accumulation leads to its deposition into plaques and is thought to drive a pathologic cascade, which ultimately leads to neuronal death.
The most influential genetic risk factor for sporadic AD is allelic variation in the apolipoprotein E (APOE) gene. Possession of an APOE4 allele markedly increases disease risk (4). ApoE acts normally to scaffold the formation of high-density lipoprotein (HDL) particles, which promote the proteolytic degradation of soluble forms of Aβ (5, 6).
The expression of apoE is transcriptionally regulated by the ligand-activated nuclear receptors, peroxisome proliferator–activated receptor gamma (PPARγ) and liver X receptors (LXRs) (7), which form obligate heterodimers with retinoid X receptors (RXRs). Transcriptional activity is regulated by ligation of either member of the pair (8). PPARγ:RXR and LXR:RXR act in a feed-forward manner to induce the expression of apoE, its lipid transporters ABCA1 and ABCG1, and the nuclear receptors themselves (7). Agonists of these receptors also act on macrophages and microglia to stimulate their conversion into “alternative” activation states (9) and promote phagocytosis (10). Chronic administration of LXR and PPARγ agonists reduces Aβ levels and improves cognitive function in mouse models of AD (10).
We reasoned that an RXR agonist would enhance normal Aβ clearance mechanisms by activating PPARγ:RXR and LXR:RXR, inducing apoE expression, facilitating Aβ clearance, and promoting microglial phagocytosis. Bexarotene (Targretin) is a highly selective, blood-brain barrier–permeant (fig. S3A), RXR agonist approved by the U.S. Food and Drug Administration (FDA) (11) with a favorable safety profile (12). Treatment of primary microglia or astrocytes with bexarotene stimulated the expression of apoE, ABCA1, and ABCG1 (fig. S1, A and B) and secretion of highly lipidated HDL particles (fig. S1, C and D). Bexarotene treatment of primary microglia and astrocytes facilitated degradation of soluble Aβ42 (fig. S2, A and B) in a PPARγ-, LXR (fig. S2, C and D)–, and apoE (fig. S2, E and F)–dependent manner. The levels of Aβ proteases, insulin-degrading enzyme and neprilysin, were unchanged with bexarotene treatment (fig. S1, E and F).
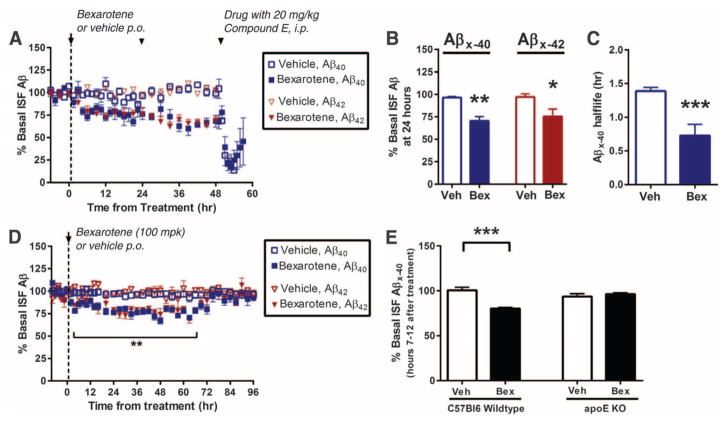
Brain interstitial fluid (ISF) Aβ levels were monitored by hippocampal in vivo microdialysis (13) of 2-month-old APPswe/PS1Δe9 (APP/PS1) mice. Bexarotene rapidly lowered ISF Aβ40 and Aβ42 levels within 6 hours of administration, with a 25% reduction by 24 hours (Fig. 1, A and B). One dose of bexarotene significantly decreased ISF Aβ40 and Aβ42 levels by 25% for more than 70 hours (Fig. 1D), with a return to baseline by 84 hours. The suppression of ISF Aβ was due to increased clearance, as the Aβ40 half-life was reduced from 1.4 to 0.7 hours (Fig. 1C). Bexarotene reduced murine Aβ levels in the C57Bl/6 mice to a similar extent as in APP/PS1 mice; however, it had no effect on Aβ levels in apoE-null mice (Fig. 1E), demonstrating that the enhanced clearance of soluble ISF Aβ required apoE.
Fig. 1.
ISF levels of Aβ decrease after bexarotene treatment. (A and B) ISF Aβx-40 and Aβx-42 levels were monitored by in vivo hippocampal microdialysis of 2-month-old APP/PS1 mice. Baseline Aβ levels were monitored for 6 hours, followed by daily orally administered bexarotene (100 mg kg−1 day−1) (Bex) or vehicle (Veh; water) for 3 days. Mice were coadministered compound E [20 mg kg−1 intraperitoneally (i.p.)] on day 3. (C) The elimination half-life of ISF Aβx-40 was measured. In 2-month-old APP/PS1 mice, baseline ISF Aβ levels were sampled after administration of a single oral dose of bexarotene (100 mg kg−1). (D) ISF Aβx-40 and Aβx-42 were sampled every 2 to 6 hours for 4 days after treatment. (E) Baseline ISF Aβ levels of nontransgenic (C57Bl/6) and apoE knockout (KO) mice (2 months) with and without bexarotene treatment. ISF Aβx-40 levels were measured between hours 7 and 12 after treatment; n = 5 mice per group (Student’s t test; mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001).
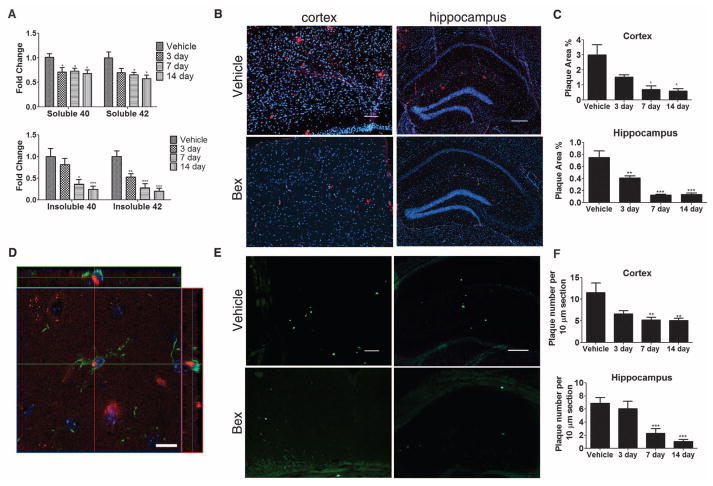
We observed the rapid removal of both diffuse and compact Aβ plaques in the cortex and hippocampus of APP/PS1 mice after acute treatment with bexarotene (Fig. 2). We orally administered bexarotene or vehicle daily to 6-month-old APP/PS1 mice for 3, 7, or 14 days. We observed the progressively enhanced expression of apoE, ABCA1, and ABCG1 and elevated HDL levels in both the hippocampus and cortex of bexarotene-treated mice (fig. S3, B and C). There was a sustained 30% reduction in soluble Aβ levels throughout the 14-day treatment period (Fig. 2A). Insoluble Aβ levels were reduced by 40% after 72 hours and progressively decreased over the subsequent 14 days (Fig. 2A). Total (Fig. 2, B and C) and thioflavin-S+ Aβ plaques (Fig. 2, E and F) were reduced by ~75% after 14 days of bexarotene treatment. Furthermore, we observed abundant Aβ-laden microglia after 3 days of bexarotene treatment, suggesting their involvement in the phagocytic removal of Aβ deposits (Fig. 2D).
Fig. 2.
Aβ levels and plaque burden are reduced by bexarotene treatment. APP/PS1 or nontransgenic (NonTg) mice (6 months) orally gavaged for 3, 7, and 14 days with bexarotene (100 mg kg−1 day−1) or vehicle (water). Soluble and insoluble Aβ40 and Aβ42 levels were measured by enzyme-linked immunosorbent assay. (A) Fold changes based on vehicle: 7.0445 ng/mg protein and 14.529 ng/mg protein of soluble Aβ40 and Aβ42, respectively, and 30.349 ng/mg protein and 36.8 ng/mg protein of insoluble Aβ40 and Aβ42, respectively. (B and E) Representative cortex and hippocampus sections of vehicle and 14-day bexarotene-treated mice stained with antibody against Aβ (6E10) (B) or thioflavin S (E) are shown and (C and F) plaque levels quantified; n ≥ 5 animals per group (Student’s t test; mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001). Scale bars (B and E): cortex, 100 μm; hippocampus, 200 μm. (D) Representative image of microglia in the cortex of a 6-month APP/PS1 mouse treated for 3 days with bexarotene (red: 6E10; green: Iba1; blue: DAPI). Scale bar: 10 μm.
To assess whether bexarotene could decrease Aβ burden in older animals with greater plaque deposition, we treated 11-month APP/PS1 mice with bexarotene for 7 days and found significantly reduced levels of soluble and insoluble Aβ40 and Aβ42 (fig. S4C), a 50% reduction in plaque number (fig. S4, D and E), and a concurrent increase in expression of apoE, the cholesterol transporters, and HDL levels (fig. S4, A and B). Thus, the efficacy of acute bexarotene treatment is evident in both early and later stages of pathogenesis in this mouse model.
We also tested the effect of chronic bexarotene treatment (3 months, daily) of APP/PS1 mice starting from 6 months of age. We found elevated levels of apoE, ABCA1, ABCG1, and HDL (fig. S5, A and B). Bexarotene reduced soluble Aβ levels by ~30%, consistent with its ability to enhance apoE-dependent Aβ proteolysis (fig. S6C). However, amyloid plaque burden was unchanged (fig. S5, D to G).
To evaluate the robustness of the effect of bexarotene, we treated an aggressive model of amyloidosis, the APPPS1-21 mouse (14), which possesses high levels of deposited Aβ at 7 to 8 months of age. APPPS1-21 mice treated for 20 days with bexarotene exhibited a reduced level of soluble and insoluble Aβ peptides (fig. S6C) and a 35% decrease in the number of thioflavin S+ plaques (fig. S6, D and E). Bexarotene treatment enhanced the expression of ABCA1, ABCG1, apoE, and its lipidated forms (fig. S6, A and B).
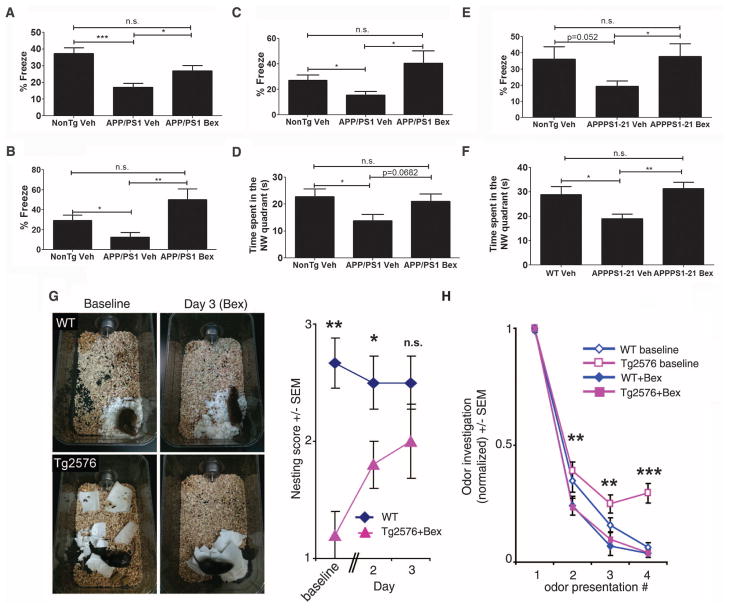
There is persuasive evidence that the cognitive and behavioral deficits characteristic of AD arise, in part, from impaired synaptic function due to soluble forms of Aβ. Bexarotene treatment rapidly restored cognition and memory, as assessed by contextual fear conditioning in APP/PS1 mice treated for 7 days at both early (6 months) and later (11 months) stages of plaque pathogenesis. Similarly, chronic treatment of 6-month-old APP/PS1 mice for 90 days (analyzed at 9 months of age) (Fig. 3, A to C) showed drug-induced behavioral improvements in the contextual fear conditioning task. Additionally, APP/PS1 mice treated for 90 days and APPPS1-21 mice treated for 20 days exhibited improved hippocampal function after bexarotene treatment, as assessed by Morris water maze performance (Fig. 3, D and F), as well as in the contextual fear conditioning assay (Fig. 3E).
Fig. 3.
Restoration of memory and cognition with bexarotene treatment. Contextual fear-learning assayed in (A) 6-month-old and (B) 11-month-old APP/PS1 mice treated for 7 days, or (C) in 9-month-old APP/PS1 mice treated for 90 days with vehicle or bexarotene. (E) APPPS1-21 mice 7 to 8 months of age were treated for 20 days and evaluated for performance. Percent time frozen was recorded in the 5-min test trial. (D and F) Spatial memory was assessed with the Morris water maze. Time spent in the northwest quadrant in the retention probe of (D) 9-month-old, 90-day-treated APP/PS1 mice and (F) 7- to 8-month-old, 20-day-treated APPPS1-21 mice with vehicle or bexarotene (Bex) (100 mg kg−1 day−1). [Nontransgenic littermates were controls (NonTg), n = 7 to 14 mice per group (Student’s t test; mean ± SEM, *P < 0.05, **P < 0.01)]. (G) Nest construction was quantified in 12- to 14-month NonTg and Tg2576 mice. Baseline data were obtained on day 0, after daily drug treatment and addition of paper towels in clean cages (two-tailed t test; *P < 0.05, **P < 0.01). (H) Odor habituation behavior in 12- to 14-month Tg2576 mice tested before (baseline) and after 9 days of bexarotene treatment; n = 5 mice per group (two-tailed t test; mean ± SEM, **P < 0.01, ***P < 0.001 for Tg2576 baseline versus Tg2576 Bex).
Nest construction is an affiliative, social behavior that becomes progressively impaired in Tg2576 mice (15). After just 72 hours of bexarotene treatment, nest construction behavior was restored in Tg2576 mice (Fig. 3G).
Finally, we explored whether bexarotene could rescue olfactory sensory impairments, (16), which are highly correlated with Aβ deposition in Tg2576 mice (17). Bexarotene treatment improved odor habituation behavior after 9 days of drug treatment in Tg2576 mice 12 to 14 months of age (Fig. 3H).
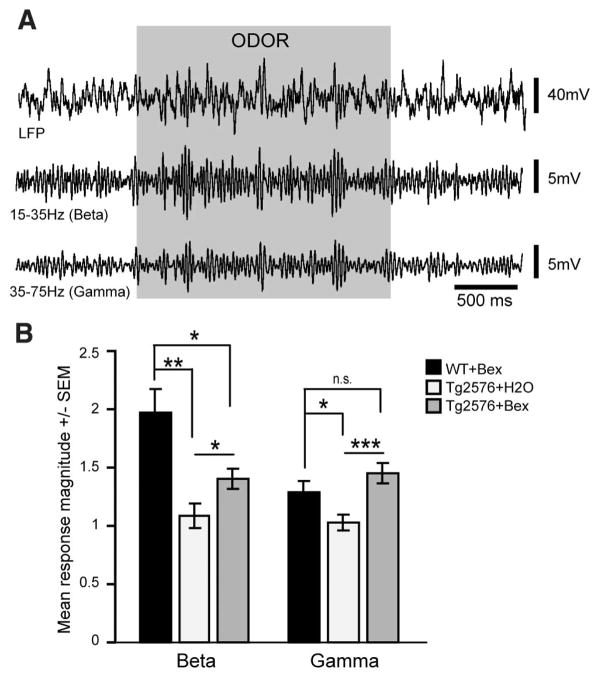
The improved behaviors observed in bexarotene–treated mice suggest global improvements of neural network function. Soluble Aβ interferes with synaptic function that subserves higher-order neural network information processing (3). Piriform cortex (PCX) circuit function is critical to odor-guided behaviors, and its disruption is implicated in impaired olfactory perception in both humans with AD and in Tg2576 mice (18). Therefore, we evaluated odor-evoked PCX local field potentials (LFPs) as a behaviorally relevant synaptic readout of neural circuit status. Odor-evoked high-frequency gamma-band oscillations (35 to 75 Hz) and beta-band oscillations (15 to 35 Hz), reflecting local circuit interactions and interregional network activity, respectively, are considered critical for normal olfactory function (18, 19). Tg2576 mice (12 to 14 months) treated with vehicle exhibited significantly less odor-evoked beta- and gamma-band LFP power compared to drug-treated nontransgenic mice (Fig. 4, A and B), which was restored by 3 days of bexarotene treatment. Odor habituation after bexarotene treatment was improved in these same mice (fig. S7, B and C), indicating a rapid drug-dependent normalization of local and regional circuit function in the primary olfactory pathway.
Fig. 4.
Rescue of cortical network activity with bexarotene. LFP recordings of Tg2576 or nontransgenic (NonTg) mice (12 to 14 months) gavaged with bexarotene (Bex) (100 mg kg−1 day−1) or vehicle (H2O) for 3 days after implantation of electrodes into PCX. PCX LFPs in response to the odor ethyl valerate in an awake nontransgenic, bexarotene-treated mouse. (A) Fifteen-to 35-Hz beta- and 35- to 75-Hz gamma-band power traces (second-order band pass). (B) PCX odor-evoked response magnitudes (2 s odor/2 s pre-odor) (n = 5 mice per group, four odor presentations per mouse; *P < 0.05, **P < 0.01, ***P < 0.001, mean ± SEM, two-tailed t tests of mean odor-evoked magnitudes within LFP bins). ns, not significant.
RXR activation stimulates the normal physiological processes through which Aβ is cleared from the brain. The dependence of soluble Aβ clearance on apoE validates the mechanistic linkage between the principal genetic risk factor for AD and the cognitive impairment that characterizes the disease (5, 6). Bexarotene acts rapidly to facilitate the apoE-dependent clearance of soluble forms of Aβ, accounting for the extremely rapid change in ISF Aβ metabolism. Bexarotene-mediated behavioral improvements were correlated with a reduction in soluble Aβ peptide levels of ~30%. These observations are consistent with previous observations that learning and memory can be improved through reducing brain-soluble Aβ levels, either upon the administration of β- or γ-secretase inhibitors (20, 21) or provision of antibodies against Aβ (22). However, the behavioral improvements were poorly correlated with the microglial-mediated removal of insoluble, deposited forms of Aβ. The dual actions of the nuclear receptors resulting in the enhanced expression and lipidation of apoE and modulation of the microglial-mediated immune response are consistent with recent genetic association analyses implicating them in the etiology of AD (23–25). The ability of bexarotene to rapidly reverse a broad range of deficits suggests that RXR agonists may be of therapeutic utility in the treatment of AD and its antecedent phases.
Supplementary Material
Acknowledgments
We thank Dr. Mangelsdorf for discussions and M. Pendergast, G. Casadesus, and I. Nagle for technical assistance. This work was supported by the Blanchette Hooker Rockefeller Foundation, Thome Foundation, Roby and Taft Funds for Alzheimer’s Research, Painstone Foundation, American Health Assistance Foundation, Cure Alzheimer’s Fund, Coins for Alzheimer’s Research Trust, and the National Institute on Aging (NIA) (grant AG030482-03S1 to G.E.L.); National Institute on Deafness and Other Communication Disorders (grant DC003906, RO1-AG037693 to D.A.W); NIA (grants K01 AG029524 and P50-AG005681), Shmerler family, and the Charles F. and Joanne Knight Alzheimer’s Disease Research Center at Washington University (to J.R.C.); and Marian S. Ware Alzheimer Program (to K.R.B.). All raw data are archived on \\gel-server1 for authorized users. P.E.C. and G.E.L. hold U.S. Provisional Patent Application no. 61/224,709 regarding bexarotene as a potential therapeutic for Alzheimer’s disease and are founding scientists of ReXceptor, Inc., which has licensing options from Case Western Reserve University on the use of bexarotene in the treatment of Alzheimer’s disease.
Footnotes
References and Notes
- 1.Querfurth HW, LaFerla FM. N Engl J Med. 2010;362:329. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Mawuenyega KG, et al. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palop JJ, Mucke L. Nat Neurosci. 2010;13:812. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roses AD, Saunders AM. Curr Opin Biotechnol. 1994;5:663. doi: 10.1016/0958-1669(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 5.Donkin JJ, et al. J Biol Chem. 2010;285:34144. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Q, et al. Neuron. 2008;58:681. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla A, et al. Mol Cell. 2001;7:161. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre P, Benomar Y, Staels B. Trends Endocrinol Metab. 2010;21:676. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Odegaard JI, Chawla A. Annu Rev Pathol. 2011;6:275. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandrekar-Colucci S, Landreth GE. Expert Opin Ther Targets. 2011;15:1085. doi: 10.1517/14728222.2011.594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. 1999 www.accessdata.fda.gov/drugsatfda_docs/nda/99/21055_Targretin.cfm.
- 12.Farol LT, Hymes KB. Expert Rev Anticancer Ther. 2004;4:180. doi: 10.1586/14737140.4.2.180. [DOI] [PubMed] [Google Scholar]
- 13.Cirrito JR, et al. J Neurosci. 2003;23:8844. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radde R, et al. EMBO Rep. 2006;7:940. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wesson DW, Wilson DA. Behav Brain Res. 2011;216:408. doi: 10.1016/j.bbr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy C. Physiol Behav. 1999;66:177. doi: 10.1016/s0031-9384(98)00262-5. [DOI] [PubMed] [Google Scholar]
- 17.Wesson DW, Levy E, Nixon RA, Wilson DA. J Neurosci. 2010;30:505. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesson DW, et al. J Neurosci. 2011;31:15962. doi: 10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Proc Natl Acad Sci USA. 2000;97:1867. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukumoto H, et al. J Neurosci. 2010;30:11157. doi: 10.1523/JNEUROSCI.2884-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comery TA, et al. J Neurosci. 2005;25:8898. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodart JC, et al. Nat Neurosci. 2002;5:452. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 23.Jones L, et al. PLoS ONE. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollingworth P, et al. Nat Genet. 2011;43:429. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naj AC, et al. Nat Genet. 2011;43:436. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.