Abstract
Increased fear memory generalization is associated with post-traumatic stress disorder, but the circuit mechanisms that regulate memory specificity remain unclear. Here, we define a neural circuit, composed of the medial prefrontal cortex, the N. reuniens, and the hippocampus, that controls fear memory generalization. Inactivation of prefrontal inputs into the N. reuniens or direct silencing of N. reuniens projections enhanced fear memory generalization, whereas constitutive activation of N. reuniens neurons decreased memory generalization. Direct optogenetic activation of phasic and tonic action-potential firing of N. reuniens neurons during memory acquisition enhanced or reduced memory generalization, respectively. We propose that the N. reuniens determines the specificity and generalization of memory attributes for a particular context by processing information from the medial prefrontal cortex en route to the hippocampus.
Memories allow animals to adapt to a constantly changing environment. Memories are never completely precise but always partially generalized, which enables an animal to quickly and appropriately respond to novel stimuli that resemble a previous experience. The level of memory specificity and the degree of generalization are normally balanced. Generalization of fear memories protects animals by alerting them to potential dangers when animals are exposed to situations that are similar to previously experienced harmful circumstances, but over-generalization of fear memories can lead to inappropriate anxiety. This is evident with post-traumatic stress disorder (PTSD), in which re-experiencing of a past trauma is triggered by cues existing in a normally safe environment (1). Similarly, overgeneralization of episodic memories is a consistent problem in patients with severe depression (2). Since its initial demonstration (3), memory generalization has been extensively characterized, and multiple theories have been developed to explain it. In addition to the hippocampus which is critical for maintaining the specificity of memories (4,5), we recently found that the medial prefrontal cortex (mPFC) is essential for memory generalization (6). Specifically, we observed that global impairment of synaptic transmission in the mPFC unexpectedly caused over-generalization of contextual fear memories. This observation is potentially interesting because functional abnormalities of the mPFC have been consistently observed in patients with PTSD and other psychiatric disorders (1).
Mapping synaptic projections from the mPFC
The mPFC mediates the cognitive control of many high-level brain functions (7,8). Consistent with such cognitive control, inactivation of synaptic transmission by expressing the light chain of tetanus toxin (TetTox) in the mPFC does not block fear memory, but leads to overgeneralization of such fear memory (6). However, it is unclear which synaptic projections from the mPFC to subcortical regions are critical for maintaining the proper balance between retention and generalization of fear memory details.
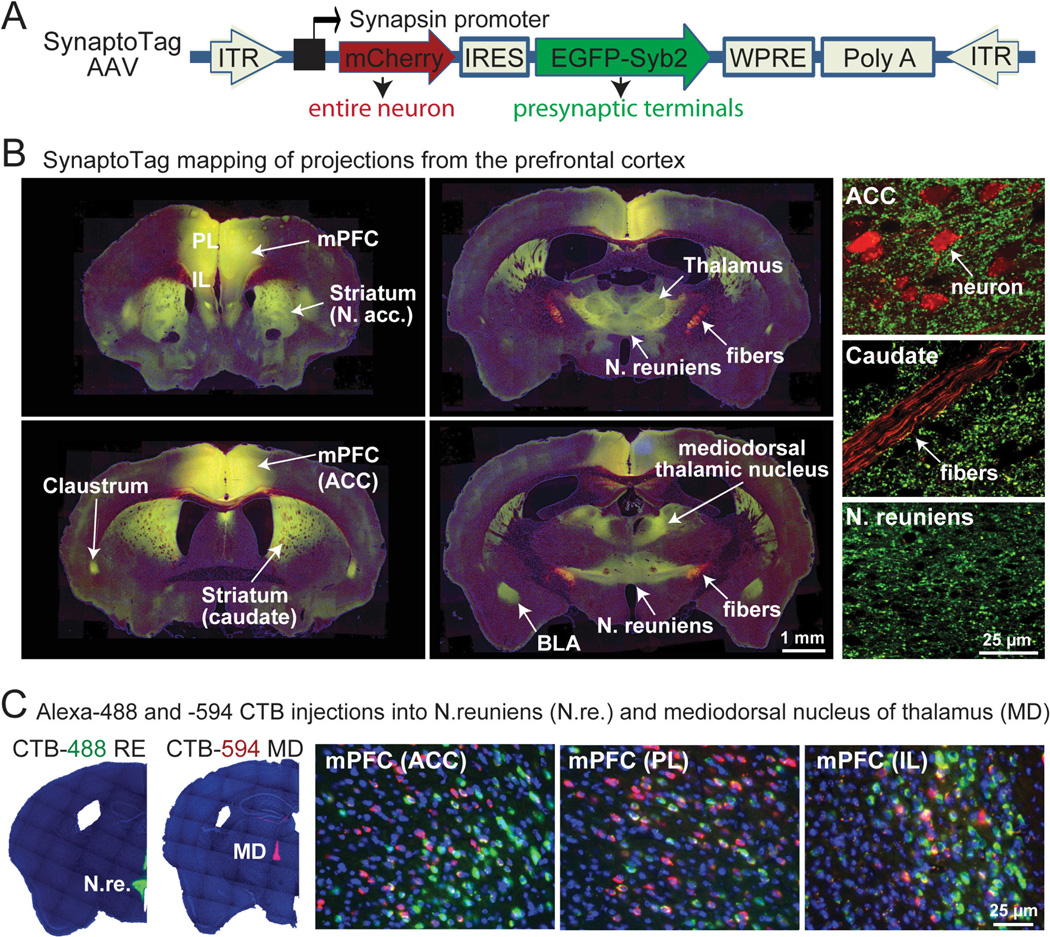
To quantitatively map the projections from the mPFC to subcortical regions, we developed a ‘SynaptoTag’ adeno-associated virus (AAV) which co-expresses red-fluorescent mCherry protein and enhanced green-fluorescent protein (EGFP) fused to the synaptic vesicle protein synaptobrevin-2 (Fig. 1A) (9). Neurons infected with SynaptoTag-AAV are filled with diffusible mCherry which is present throughout their cytoplasm, including axon fibers. These neurons selectively localize green-fluorescent synaptobrevin-2 to efferent synapses, allowing a quantitative assessment of the number of synapses formed in a target region by SynaptoTag-AAV infected neurons. This simple approach provides information about the distribution of both axonal fibers and synaptic terminals derived from a neuron.
Fig. 1. Distinct mPFC neurons project to different synaptic targets.
A, Design of SynaptoTag AAV used for tracing synaptic connections. The synapsin promoter in the AAV drives bicistronic expression of soluble mCherry and a presynaptic EGFP-synaptobrevin-2 fusion protein (EGFP-Syb2).
B, SynaptoTag AAV mapping of mPFC projections. Representative low-resolution (left and middle panels) and high-resolution images (right panels) illustrate synaptic targets for mPFC neurons. Red mCherry-labeling marks axonal fibers, while green EGFP-labeling marks synapses projecting from the mPFC (yellow = coincident red and green labeling; abbreviations: ACC, anterior cingulate cortex; BLA, basolateral nucleus of the amygdala; IL, infralimbic cortex; PL, prelimbic cortex; for complete sections, see Fig. S1).
C, Retrograde labeling of mPFC neurons after injection of Alexa Fluor-488 and -594 labeled cholera toxin-B (CTB-488 and CTB-594) into the N. reuniens (N.re.; green) and the mediodorsal thalamic nucleus (MD; red), respectively. Low-power micrographs (left panels) show injection areas, while high-power images (right panels) depict the three major mPFC regions (ACC, anterior cingulate cortex; PL, prelimbic cortex; IL, infralimbic cortex). Most traced neurons were dominated by the presence of one fluorophore (for additional mPFC projections, see Fig. S2).
We stereotactically injected SynaptoTag AAV into the mPFC of adult mice, and imaged the localization of synapses formed by mPFC neurons eight weeks later (Figs. 1B and S1). mCherry-positive axons of mPFC neurons formed a fiber bundle that extended caudoventrally through the corpus callosum, dorsal striatum, dorsal thalamus, hypothalamus, and midbrain structures. Axons continuously branched out of this bundle and formed synaptic connections with brain structures on its way. The intensity and density of the observed green synaptic puncta reflects the number of synaptic connections. Apart from a dense meshwork of synapses formed by mPFC neurons within the mPFC itself, mPFC neurons formed major synaptic projections in the mediodorsal striatum and N. accumbens, thalamus, claustrum, septohippocampal nucleus and basolateral amygdala (Fig. 1B). In the thalamus, most projections were targeted to the mediodorsal nucleus (MD) and the N. reuniens (N.re.). The mPFC also sent significant synaptic projections to the zona incerta, hypothalamic nuclei, midbrain, and periaqueductal gray.
The parallel connections of mPFC neurons to different subcortical nuclei raise the question whether the same mPFC neurons project to multiple targets. We therefore injected fluorescent Cholera Toxin-B (CTB), tagged with Alexa Fluor-488 or Alexa Fluor-594, into neighboring thalamic nuclei (the N. reuniens [N.re.] and the mediodorsal nucleus [MD]). We detected retrogradely labeled neurons in all three major sub-regions of the mPFC (the prelimbic [PL], infralimbic [IL], and anterior cingulate cortex [ACC]; Figs. 1C and S2). Most fluorescent mPFC neurons contained only one of the two fluorophores, indicating that these neurons preferentially project to only one of the two neighboring thalamic regions examined. We also injected the fluorescent CTB tracers into the mediodorsal striatum and either the mediodorsal thalamic nucleus or the N. reuniens, and observed a similar segregation of mPFC projection neurons (Fig. S2).
Which mPFC projections control fear memory?
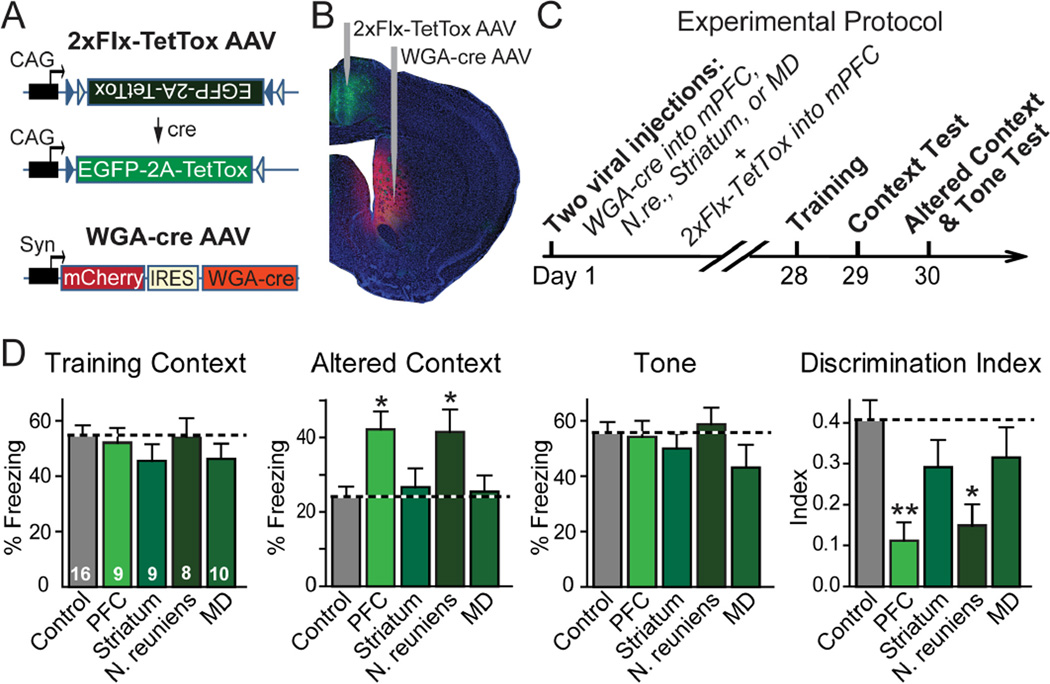
We have previously observed overgeneralization of contextual fear memory induced by global expression of TetTox in the mPFC (6). Because distinct subpopulations of mPFC neurons project to different brain regions, which of these projections participates in the circuit that controls fear memory generalization? To address this question, we used a trans-neuronally transported version of cre-recombinase that is fused to wheat-germ agglutinin (WGA-cre fusion protein; 10,11). We injected an AAV encoding a double-floxed, inverted EGFP and TetTox gene (2xFIx-TetTox) into the mPFC (Fig. 2A). This AAV only expressed EGFP and TetTox after inversion of the double-floxed expression cassette by cre-recombinase. At the same time, we injected a second AAV into one of the brain areas that are the targets of efferent synapses from the mPFC (Fig. 2B). The second AAV co-expressed red-fluorescent mCherry and WGA-cre fusion protein (Fig. 2A). We then tested whether expression of WGA-cre in target areas for the mPFC activated EGFP and TetTox expression in the mPFC. We found that WGA-cre AAV injections specifically induced EGFP expression in the mPFC (Figs. 2B and S3). Detection of WGA-cre mediated trans-neuronal transport was made possible by modifications in the AAV vectors, especially by using a shorter synapsin promoter (~0.5 kb) and the AAV-DJ serotype (6). Significantly fewer mPFC neurons were labeled with EGFP by the WGA-cre/2xFlx-TetTox approach than were traced with a CTB (Figs. 1C, 2B, S3, S4), suggesting that trans-synaptic WGA-cre transport is less efficient than retrograde labeling with an extracellular tracer. Quantifications showed that approximately a third of the mPFC neurons projecting to the striatum were captured by trans-neuronal transport of WGA-cre from the target area (Fig. S5). Despite its lower efficacy, we chose WGA-cre for our experiments instead of more efficacious rabies virus vectors (12) because rabies viruses in our hands induced rapid cytotoxicity, which may confound the interpretation of the behavioral results, whereas WGA-cre did not exhibit this problem.
Fig. 2. mPFC projection to the N. reuniens controls memory specificity.
A, Design of AAVs used for inactivating synaptic transmission in subsets of projection neurons with specific synaptic targets. Double-floxed inverted TetTox-AAV (2xFlx-TetTox AAV) encodes bicistronic expression of EGFP for visualizing infected neurons and of TetTox for blocking synaptic transmission. The coding region of the double-floxed inverted TetTox-AAV is not translated until cre-recombinase flips the inverted coding region into the correct orientation. WGA-cre AAV mediates bicistronic expression of mCherry and WGA-cre. When this AAV infects a neuron, WGA-cre is trans-neuronally transferred to connected neurons, whereas mCherry is only expressed in the infected neuron.
B, Coronal brain section of a mouse that was injected with 2xFlx-TetTox AAV in the mPFC, and with WGA-cre AAV in the dorsomedial striatum. The green EGFP fluorescence in the mPFC indicates that trans-synaptically transported WGA-cre activated expression of TetTox and EGFP in the mPFC. For high-magnification images, additional examples, and quantification of the trans-synaptic transport efficiency, see Figs. S3–S5.
C, Experimental protocol for analyzing the behavioral effects of selective TetTox expression in mPFC neurons that project to specific targets. 2xFIx-TetTox AAV was stereotactically injected into the mouse mPFC, and WGA-cre AAV was injected into the striatum, mediodorsal thalamic nucleus, N. reuniens, or mPFC (control = no WGA-cre AAV injection). Mice were tested four weeks later for contextual fear conditioning (context test), fear conditioning in an altered context to measure memory precision, and cued fear conditioning (tone test). For additional information, see Fig. S6.
D, Fear conditioning measured with the experimental strategy described in C in multiple independent experiments (numbers in bars = number of mice analyzed). The discrimination index was calculated as the difference between the percentage freezing in the training context and the altered context, divided by the sum of the two percentages. Data are means ± SEMs; statistical significance (* P<0.05; ** P<0.01) was assessed by (i) two-way mixed-model ANOVA with Bonferroni’s post-hoc test comparing the freezing levels, or (ii) one-way ANOVA followed by Turkey’s post-hoc test for the discrimination index.
We asked whether blocking specific projections from the mPFC to target areas alters fear memory generalization. We bilaterally injected the 2xFlx-TetTox AAV into the mPFC and the WGA-cre AAV into three target brain regions which receive synaptic inputs from the mPFC (the mediodorsal striatum, mediodorsal thalamic nucleus, and N. reuniens), as well as into the mPFC itself (as a positive control). We selected the mediodorsal striatum and the mediodorsal thalamic nucleus because they are major mPFC targets (Fig. 1B). We chose the N. reuniens because it forms an anatomical link between the mPFC and the hippocampus (13,14), both of which are essential for memory specificity (4–6), and because the N. reuniens has been shown to play a possible role in hippocampus-dependent learning and memory (15,16).
Four weeks after viral injections, we performed fear conditioning tests (Figs. 2C and S6). We trained the injected mice with three tone/footshock pairs in a conditioning chamber, and then measured “freezing” sequentially first in the training chamber to assess contextual fear memory; then in a similar but altered chamber to examine fear memory generalization; and finally in response to the conditioning tone in the altered chamber to measure cued fear memory (6). We quantified fear memory generalization as the discrimination index (the difference between freezing in the training and the altered context, divided by the sum of freezing in both conditions).
Global inactivation of the mPFC with TetTox did not impair cued or contextual fear conditioning, but induced overgeneralization of fear memories (Fig. 2D). Activation of TetTox specifically only mPFC neurons that projected to the striatum or the mediodorsal thamalic nucleus had no effect on any parameter during fear conditioning, including memory generalization. Activation of TetTox only in mPFC neurons that projected to the N. reuniens, however, caused overgeneralization of fear memories, similar to that observed with direct expression of TetTox in the mPFC (Fig. 2D). Given the incomplete efficiency of the retrograde transport of WGA-cre, our results do not exclude the possibility that the striatum and mediodorsal thalamic nucleus also play a more limited role in memory generalization, but suggest that this role is not inhibited by partial inactivation of the mPFC projection to these nuclei. In contrast, partial inactivation of the mPFC projection to the N. reuniens is sufficient to produce overgeneralization of fear memories.
N. reuniens bidirectionally controls fear memory generalization
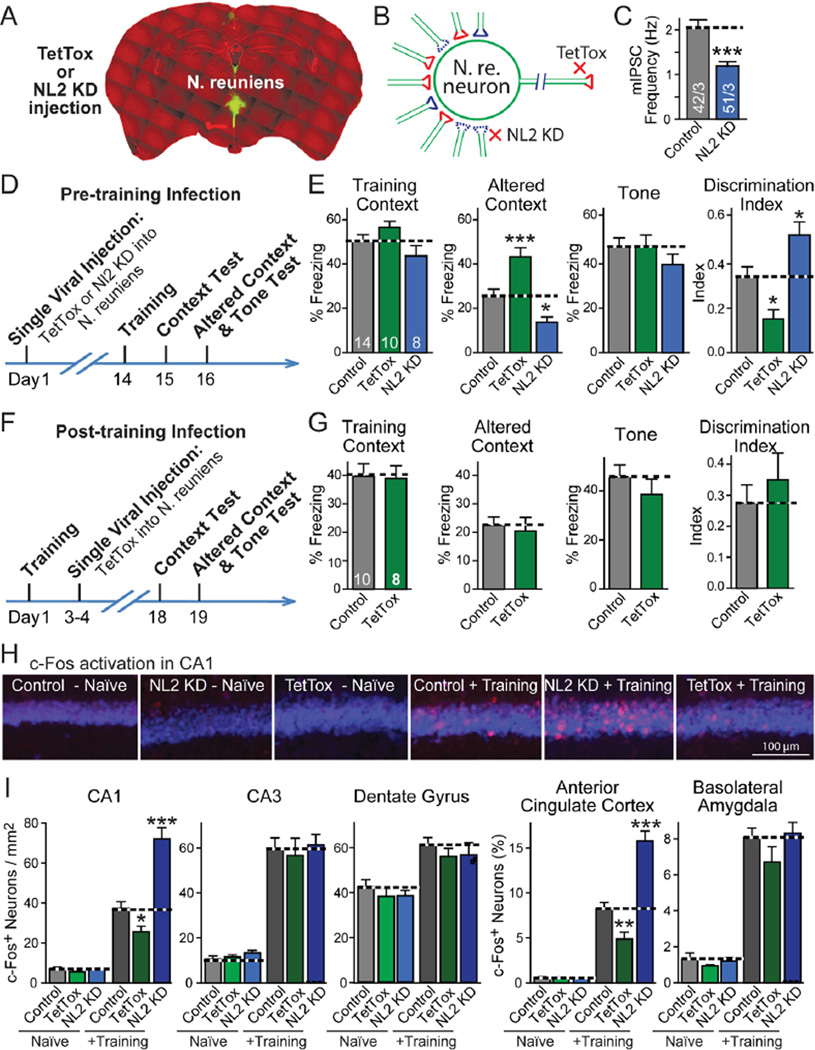
To explore the control of fear memory generalization by the N. reuniens, we injected into the N. reuniens recombinant lentiviruses encoding either EGFP alone (control), TetTox, or an shRNA that suppresses neuroligin-2 expression (NL2 KD; Figs. 3A and S7A; 17). Whereas TetTox suppressed propagation of synaptic signals from the N. reuniens, the neuroligin-2 knockdown decreased synaptic inhibition of N. reuniens neurons (N. reuniens lacks intrinsic inhibitory GABAergic neurons and receives inhibitory inputs from other brain regions), thereby increasing propagation of synaptic signals from the N. reuniens (Figs. 3 and S7).
Fig. 3. N. reuniens bidirectionally controls fear memory generalization.
A, Representative coronal brain section showing local expression of EGFP (green) after stereotactic injection into the N. reuniens of lentiviruses encoding EGFP and TetTox or the neuroligin-2 knockdown (NL2 KD).
B, Schema of the effects of TetTox expression or of the neuroligin-2 knockdown (NL2 KD) on the activity of neurons in the N. reuniens. The neuroligin-2 knockdown decreases inhibition of N. reuniens neurons, thereby activating these neurons, whereas TetTox blocks synaptic outputs from N. reuniens neurons.
C, Effect of neuroligin-2 knockdown on the frequency of spontaneous inhibitory miniature synaptic events (mIPSCs), recorded in acute N. reuniens slices from mice that were injected with neuroligin-2 knockdown lentivirus (number of neurons/mice analyzed is shown in the bars).
D, Experimental protocol for testing fear memory after TetTox expression or neuroligin-2 knockdown in the N. reuniens.
E, Bi-directional changes in fear memory generalization by neuronal silencing with TetTox or neuronal activation with the neuroligin-2 knockdown. Mice injected with lentivirus expressing only EGFP were used as control (mouse numbers are indicated in bars).
F and G, Same as C and D, except that mice were injected with control or TetTox virus after fear conditioning training.
H and I, Effect of fear conditioning training and of TetTox expression or neuroligin-2 knockdown in the N. reuniens on the activity levels of neurons in different target brain regions. Control, TetTox, or the neuroligin-2 knockdown (NL2 KD) lentiviruses were injected into the N. reuniens of adult mice. Mice were subjected to fear conditioning training (‘+training’) or received no training (‘naïve’) and sacrificed 90 min after training. Brain sections were stained for c-Fos (red) to measure neuronal activation and for NeuN to label all neuronal nuclei (blue). Panel H depicts representative images of the hippocampal CA1 region, and panel I quantification of c-Fos expression in the indicated brain regions (n= 12–18 brain sections from 4 mice in each group; for additional data, see Figs. S11 and S12).
Data shown are means ± SEMs. Statistical significance (* P<0.05; ** P<0.01; *** P<0.001) was assessed by 2-tailed Student’s t-test (C and G), two-way ANOVA followed by Bonferroni’s post-hoc test (E, comparing freezing levels, and I), or one-way ANOVA followed by Turkey’s post-hoc test (discrimination index in E).
Two weeks after viral injections, we measured fear conditioning (Figs. 3D and 3E). Similar to the effects induced by TetTox in the mPFC, expression of TetTox in the N. reuniens caused an over-generalization of contextual fear memory without significant effects on contextual or cued fear conditioning. This overgeneralization was specific for contextual memories as the generalization of cued memories was not affected (Fig. S8). In contrast to the effect of TetTox, suppression of neuroligin-2 expression reduced memory generalization (Fig. 3E).
Does the N. reuniens determine the precision of memory during memory acquisition and/or during memory retrieval? To address this question, we injected lentiviruses expressing TetTox into the N. reuniens after fear conditioning, and measured fear memories two weeks later (Figs. 3F and 3G). Expression of TetTox after training had no effect on memory generalization, establishing the specificity of the effects observed by TetTox on the generalization of fear memory during the acquisition stage.
The mPFC, N. reuniens, and hippocampus constitute a memory generalization circuit
The N. reuniens directly projects to the hippocampus and back to the mPFC (13,14), while the hippocampus in turn also projects to the mPFC (18), thus creating a closed loop with the projection from the mPFC to the N. reuniens (Fig. 1). In mapping experiments using SynaptoTag AAV injections into the N. reuniens and the hippocampus, we confirmed these conclusions (Figs. S9 and S10). A major question, however, is how much the activity of N. reuniens neurons actually influences neuronal excitation in the hippocampus, i.e. whether this is indeed a major signaling pathway during memory acquisition.
Previous studies indicate that memories with high specificity involve a high level of engagement of the hippocampus (19). Thus, we examined whether enhanced fear generalization upon TetTox expression or reduced fear generalization following neuroligin-2 suppression in the N. reuniens are associated with corresponding changes in the activation of hippocampal neurons, and whether such changes are specific to these neurons. We subjected control mice and mice with TetTox expression or neuroligin-2 knockdown in the N. reuniens to fear conditioning training, and analyzed c-Fos expression in multiple brain regions 90 min afterwards.
Consistent with previous reports (20,21), c-Fos positive neurons were increased after training in multiple brain regions, including the hippocampus, mPFC, amygdale, ventral tegmental area, and periaquaeductal gray in control mice (Figs. 3H, 3I, S11, and S12). Expression of TetTox or knockdown of neuroligin-2 in the N. reuniens had little effect on the basal c-Fos expression in any brain region. However, TetTox expression significantly and selectively decreased c-Fos activation in the CA1 region of the hippocampus and in the anterior cingulate cortex of the mPFC, whereas the neuroligin-2 knockdown significantly enhanced the effects of training on c-Fos expression in these two brain regions (Figs. 3I and S12).
Activity patterns of N. reuniens neurons control memory specificity
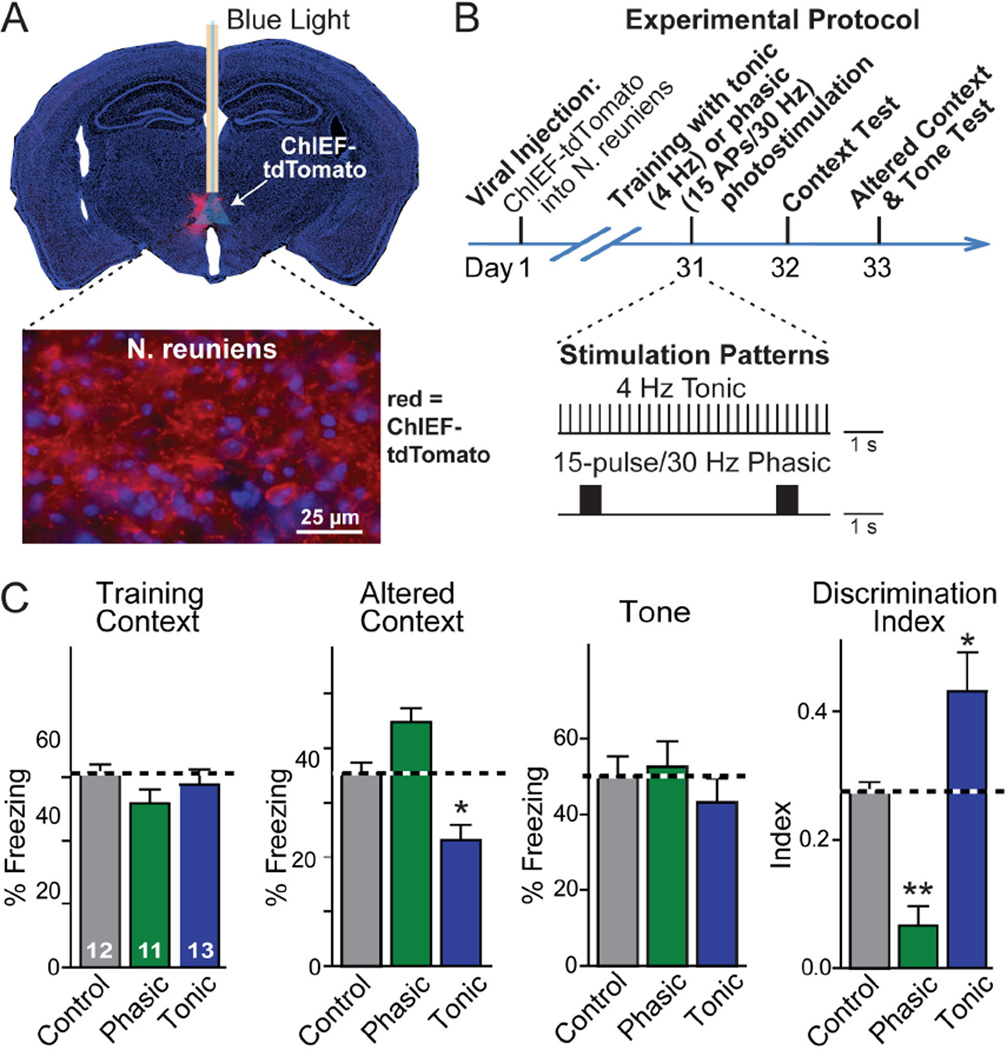
To directly test the role of the N. reuniens in balancing the precision of contextual fear memories, we stimulated firing of N. reuniens neurons in behaving mice during memory acquisition using optogenetics. We expressed the channelrodopsin-derivative ChIEF (22) in the N. reuniens, and stimulated N. reuniens neurons via an implanted optical fiber (Fig. 4A). Electrophysiological experiments have shown that ChIEF exhibits a fast kinetics and allows action-potential stimulation at frequencies of up to 50 Hz (22).
Fig. 4. Firing pattern of N. reuniens neurons dictates memory generalization.
A, Coronal brain section illustrating expression of ChIEF-tdTomato (red fluorescent channelrhodopsin) in the N. reuniens (top), and high-magnification micrograph showing ChIEF-tdTomato expressing N. reuniens neurons and their axonal fibers (bottom).
B, Experimental protocol for testing the effect of different optogenetic stimulation patterns of N. reuniens neurons on fear conditioning behavior, with the stimulation patterns illustrated below the time diagram. N. reuniens neurons were stimulated throughout the 6-min training period either by a 4 Hz tonic stimulation or a 30 Hz phasic stimulation, administered for 0.5 s every 5 seconds; stimulus light pulses were 15 ms.
C, Tonic and phasic optogenetic stimulation produced opposite effects on fear memory generalization. Control mice also expressed channelrhodopsin and contained an implanted optical fiber, but were not stimulated. Data shown are means ± SEMs; numbers in bars indicate number of mice analyzed. Statistical significance (* P<0.05; ** P<0.01) was assessed by two-way mixed-model ANOVA followed by Bonferroni’s post-hoc test comparing the freezing levels, or by one-way ANOVA followed by Turkey’s post-hoc test for the discrimination index.
We stimulated N. reuniens neurons during fear conditioning training using either tonic 4 Hz stimulus trains or phasic 15-pulse stimulus bursts (Fig. 4B; 23). Similar to the TetTox and neuroligin-2 knockdown manipulations in the N. reuniens, neither the phasic nor the tonic N. reuniens stimulation had detectable effects on contextual or cued fear conditioning (Fig. 4C). However, these stimulations induced opposite changes in fear memory generalization. Phasic N. reuniens stimulation during training caused increased freezing in the altered context, i.e. produced overgeneralization of fear memory. In contrast, tonic N. reuniens stimulation induced decreased freezing in the altered context, i.e. a reduction in fear generalization (Fig. 4C).
Because the two stimulation patterns used represent in principle the same manipulation – optogenetic stimulation of N. reuniens firing – but result in opposite effects, they control for each other, ruling out the possibility that the optogenetic manipulation simply impairs the functions of the N. reuniens instead of specifically stimulating it. Previous studies have shown that stimulation of the inputs from the N. reuniens to the hippocampus produces sub-threshold depolarization of CA1 pyramidal cells but above-threshold stimulation of inhibitory interneurons (24). The different behavioral phenotypes produced by the distinct stimulation patterns of N. reuniens neurons might arise from the relative impact of their stimulation on their downstream excitatory versus inhibitory neurons. Recent evidence indicates that the activity of the N. reuniens correlates with hippocampal oscillations, suggesting that the different behavioral effects may be related to changes in hippocampal oscillations (25).
Summary
Here, we establish that the mPFC controls memory specificity via signaling to the N. reuniens that in turn signals both to the hippocampus and back to the mPFC. The generalization of hippocampus-dependent memories is often discussed in the framework of complementary learning systems theory (26,27). In this theory, the hippocampus keeps separate representations of individual memory episodes (specific memories), while the cortex abstracts common features from multiple memories. Through systems consolidation in which memories are transferred from the hippocampus to the cortex, memories become generalized. Highly specific memories are proposed to be maintained through “pattern separation”, but can be generalized during retrieval through “pattern completion” (28,29). Complementary learning systems theory provides a plausible account of the time-dependent generalization of memories after memory acquisition and their generalization upon memory retrieval (30–32), but does not explain how memory generalization is controlled during acquisition. Taking advantage of the temporal precision of optogenetic stimulations, we found that the mPFC-N.reuniens-hippocampus circuit controls memory specificity and generalization during acquisition (Figs. 4).
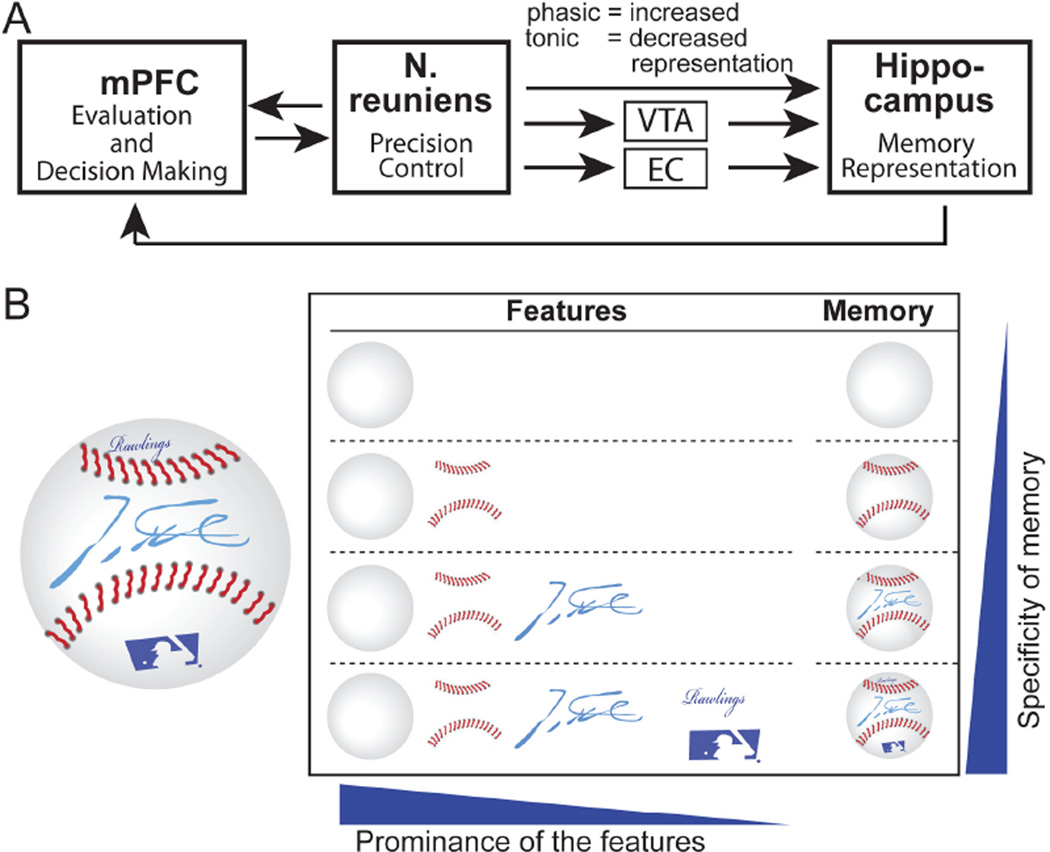
Since memories are not composed of simple unitary traces, but of flexible combinations of attributes or features of the remembered objects or situations (33, 34), generalization of memories may stem from overlap between the representations of the attributes/features of memories (35, 36). Different attributes of an object may not be remembered equally. For example, after seeing a baseball with a player’s autograph (Fig. 5B), readers may have memories of this baseball with different levels of specificity by memorizing distinct combinations of the features. When only the most prominent attributes are remembered, its memory representation is more likely to overlap with that of another memory and become generalized. But when more features are remembered, the overall representation is less likely to overlap with other memory representations, and hence becomes more specific. A plausible model that accounts for our findings is that the N. reuniens may exert a persistent regulation of the excitability of hippocampal neurons, thereby controlling memory generalization (24). Increased excitability may allow less prominent memory features to be incorporated into overall memories by facilitating the firing or synaptic plasticity of CA1 neurons (Fig. S13). Memories with more detailed attributes will then become more specific. This overall idea agrees with the general functions proposed for midline thalamic structures: instead of relaying specific sensory information, they are thought to adjust the activity level of cortical structures (including the hippocampus and mPFC; 37). Studies of hippocampal “place cells” indicate that these cells undergo significant “remapping” when encoding similar memories, especially in the CA3 region. Through “remapping”, subtle changes in the environment could produce profound alterations of a memory representation in the hippocampus, thereby increasing the distinction between similar memories (38). Thus, the mPFC-N.reuniens-hippocampus circuit may regulate memory generalization by actively controlling “remapping”. Notably, hippocampal “remapping” is modulated by motivational and emotional states (38, 39). Since the mPFC is centrally involved in motivational and emotional states of an animal (7, 8), the mPFC-N.reuniens pathway may convey the motivational and emotional value of the attributes of a memory to the hippocampus for memory encoding, which in turn may underly the regulation of memory generalization during acquisition.
Fig. 5. Model for the mechanism of N. reuniens’ control of memory generalization.
A, Schematic diagram of the synaptic interactions between the mPFC, N. reuniens, and hippocampus in controlling memory generalization.
B, Illustration of the modular composition of memory features. We posit that memories differentially incorporate a composite of specific attributes. The more prominent a feature is, the more likely it is included in memory, as illustrated here with a baseball containing additional features besides ‘ballness’. We propose that N. reuniens neurons control memory generalization by regulating the number of features that are incorporated into a memory. For a more detailed discussion, see Fig. S13.
Supplementary Material
Acknowledgments
We thank the following colleagues for providing plasmids and advice: Marc Fuccillo and Jaewon Ko (neuroligin-2 knockdown constructs), Jacqueline Burre (EGFP-synaptobrevin-2 construct), Claudio Acuna and Roger Tsien (ChIEF-tdTomato), Stephan Lammel (advice on optogenetics), and Karl Diesseroth (WGA-cre plasmid). This study was supported by grants from the Simons Foundation (177850), the NIMH (P50 MH086403), and the NINCDS (NS077906).
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
REFERENCES and NOTES
- 1.Mahan AL, Ressler KJ. Trends Neurosci. 2012 Jan;35:24. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotlib IH, Joormann J. Annu Rev Clin Psychol. 2010;6:285. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson JB, Rayner R. Am Psychol. 2000 Mar;55:313. doi: 10.1037//0003-066x.55.3.313. [DOI] [PubMed] [Google Scholar]
- 4.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. Behav Neurosci. 1998 Aug;112:863. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 5.Freeman FG, Kramarcy NR, Lee J. Physiol Behav. 1973 Aug;11:273. doi: 10.1016/0031-9384(73)90362-4. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, et al. Neuron. 2012 Mar 8;73:990. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalley JW, Cardinal RN, Robbins TW. Neurosci Biobehav Rev. 2004 Nov;28:771. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Wise SP. Trends Neurosci. 2008 Dec;31:599. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Experimental procedures are explained in the Materials & Methods section of the Supporting Materials.
- 10.Braz JM, Rico B, Basbaum AI. Proc Natl Acad Sci U S A. 2002 Nov 12;99:15148. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gradinaru V, et al. Cell. 2010 Apr 2;141:154. [Google Scholar]
- 12.Osakada F, et al. Neuron. 2011 Aug 25;71:617. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vertes RP. Neuroscience. 2006 Sep 29;142:1. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Brain Res Bull. 2007 Mar 30;71:601. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolleman-van der Weel MJ, Morris RG, Witter MP. Brain Struct Funct. 2009 Feb;213:329. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- 16.Loureiro M, et al. J Neurosci. 2012 Jul 18;32:9947. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lentiviruses were chosen for these experiments because they infect a small area of less than 0.8 mm diameter (Fig. 3A). TetTox inactivates all synaptic outputs from the N. reuniens. Neuroligin-2 is a synaptic adhesion molecule which is exclusively expressed in inhibitory synapses ( Varoqueaux F, Jamain S, Brose N. Eur J Cell Biol. 2004 Sep;83:449. doi: 10.1078/0171-9335-00410. ), and inactivation of neuroligin-2 depresses the function of a subset of inhibitory synapses ( Gibson JR, Huber KM, Sudhof TC. J Neurosci. 2009 Nov 4;29:13883. doi: 10.1523/JNEUROSCI.2457-09.2009. Poulopoulos A, et al. Neuron. 2009 Sep 10;63:628. doi: 10.1016/j.neuron.2009.08.023.
- 18.Swanson LW. Brain Res. 1981 Jul 27;217:150. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- 19.Wiltgen BJ, et al. Curr Biol. 2010 Aug 10;20:1336. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goshen I, et al. Cell. 2011 Oct 28;147:678. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Milanovic S, et al. Brain Res. 1998 Feb 16;784:37. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- 22.Lin JY, Lin MZ, Steinbach P, Tsien RY. Biophys J. 2009 Mar 4;96:1803. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Because only limited information about the natural firing partterns of N. reuniens neurons is available ( Morales GJ, Ramcharan EJ, Sundararaman N, Morgera SD, Vertes RP. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2480. doi: 10.1109/IEMBS.2007.4352831. 25), we first explored stimulation patterns to identify an optimal regimen. We found that tonic 4 Hz stimulus trains and phasic 15-pulse stimulus bursts (delivered at 30 Hz with 5 s intervals) produced significant but distinct behavioral effects, leading us to use these two stimulation paradigms for our experiments.
- 24.Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. J Neurosci. 1997 Jul 15;17:5640. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Yoshida T, Katz DB, Lisman JE. J Neurophysiol. 2012 Jun;107:3181. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumaran D, McClelland JL. Psychol Rev. 2012 Jul;119:573. doi: 10.1037/a0028681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClelland JL, McNaughton BL, O'Reilly RC. Psychol Rev. 1995 Jul;102:419. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 28.McHugh TJ, et al. Science. 2007 Jul 6;317:94. [Google Scholar]
- 29.Nakashiba T, et al. Cell. 2012 Mar 30;149:188. [Google Scholar]
- 30.Perkins CC, Weyant RG., Jr J Comp Physiol Psychol. 1958 Oct;51:596. doi: 10.1037/h0042550. [DOI] [PubMed] [Google Scholar]
- 31.Wiltgen BJ, Silva AJ. Learn Mem. 2007 Apr;14:313. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Riccio DC. Learn Motiv. 1996 Nov;27:400. doi: 10.1006/lmot.1996.0023. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H. Neuron. 2004 Sep 30;44:109. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. Neuron. 1999 Jun;23:209. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 35.Howard MW, Fotedar MS, Datey AV, Hasselmo ME. Psychol Rev. 2005 Jan;112:75. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM. Science. 2010 Dec 3;330:1408. doi: 10.1126/science.1194780. [DOI] [PubMed] [Google Scholar]
- 37.Groenewegen HJ, Berendse HW. Trends Neurosci. 1994 Feb;17:52. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 38.Colgin LL, Moser EI, Moser MB. Trends Neurosci. 2008 Sep;31:469. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. J Neurosci. 2004 Aug 4;24:7015. doi: 10.1523/JNEUROSCI.5492-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.