Abstract
OBJECTIVE
To prospectively determine the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy (RAVEIL).
PATIENTS AND METHODS
Patients with T1-3N0 penile cancer were enrolled into a prospective phase I trial at a tertiary care institution from March 2010 to January 2012. All patients underwent an initial RAVEIL approach.
Verification of adequacy of dissection was performed by an independent surgeon via a separate open incision at the conclusion of the RAVEIL procedure.
Out of 10 patients, if more than two superficial inguinal fields with ≥ 2 nodes or more than four with ≥ 1 node remained within the superficial dissection field, the study would not proceed to phase II.
RESULTS
Of 10 enrolled patients two had inguinal metastases and all positive nodes were detected by RAVEIL. The remaining eight patients had no metastases, with a mean of nine (range 5–21) left and nine (range 6–17) right nodes removed. One inguinal field RAVEIL was converted to an open dissection.
The verifying surgeon confirmed that 18 of 19 inguinal fields (94.7% in nine patients) had an adequate dissection. Two benign nodes were found just beneath Scarpa’s fascia above the inguinal dissection field.
Limitations of the study include an inability to determine decisively what specific wound complications were related to RAVEIL because of the protocol-specified creation of a small inguinal incision for verification of adequate dissection.
CONCLUSIONS
RAVEIL allowed adequate staging of disease in the inguinal region among patients with penile cancer at risk for inguinal metastases.
Keywords: penile cancer, lymph nodes, robotic surgery, lymphadenectomy, squamous cell carcinoma, prospective study
INTRODUCTION
Inguinal lymphadenectomy (ILND) is the current standard of care for patients with penile squamous cell carcinoma and resectable inguinal lymph node metastasis. Because the presence and extent of inguinal metastasis dictate survival in patients with metastatic penile cancer [1–5], determining these features at the earliest possible time point maximizes the possibility of a surgical cure. Recent data suggest a survival advantage for patients who undergo prophylactic ILND and have positive nodes compared with those treated with surveillance and delayed dissection for positive nodes [6]. Furthermore, recent guidelines suggest strategies to select high risk patients most likely to benefit from ILND [7,8].
While prophylactic ILND seems to be preferable, it has disadvantages as well. The incidence of postoperative complications following ILND has been reported to approach 50% or even up to 75% [9–12]. Patients with comorbidities or locally advanced disease are at particular risk for postoperative morbidity [13]. Wound dehiscence and skin necrosis following ILND have also been reported [14–17]. More recently, complication rates among men with no palpable adenopathy have been reported to be decreasing in comparison with historical experience, but overall complication rates still range from 10% to 46% [18].
Alternative lymph node staging procedures have been proposed to reduce morbidity and complication rates. Dynamic sentinel lymph node biopsy with recent modifications has decreased false-negative findings to approximately 7%, and there is little morbidity associated with the procedure [19,20].
Another potential technique that could limit morbidity while sampling all the nearby superficial lymph nodes is endoscopic inguinal lymphadenectomy. The initial endoscopic inguinal lymphadenectomy technique for penile cancer was described by Bishoff and associates [21] and further developed by both Tobias-Machado and associates (who called their method video-endoscopic inguinal lymphadenectomy [VEIL]) and Sotelo et al. [22–24]. The first published study on the VEIL technique described a single patient with penile cancer who underwent a successful bilateral ILND [22]. A subsequent study of 10 patients who underwent bilateral ILND using standard lymphadenectomy on one side and the VEIL technique on the contralateral side was reported by the same centre [23]. Sotelo and colleagues reported a similar procedure in eight patients, with no intraoperative or wound-related complications [24]. A more recent development has been the incorporation of robotic assistance as an enabling tool for performing endoscopic/laparoscopic ILND; two case reports of robotic assisted lymph node dissection have been published on the subject [25,26].
We sought to prospectively evaluate the oncological adequacy of robotic assisted VEIL (RAVEIL) as a staging tool in patients with penile cancer at risk for inguinal metastasis. We assessed and confirmed that all the superficial first-echelon lymph nodes in the inguinal region would be successfully removed using RAVEIL.
PATIENTS AND METHODS
SETTING AND PATIENTS
This prospective study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center. The RAVEIL procedures were performed between March 2010 and January 2012 at this single tertiary care institution. Patients with high risk penile cancer, including those classified as T1 with vascular invasion or poor differentiation (i.e. T1b) or as ≥T2, were eligible for the study. Patients with prior chemotherapy for penile cancer, prior radiation therapy to the inguinal region or inguinal adenopathy clinically suggestive of metastasis were excluded. All patients gave written consent for participation. Two surgeons performed all of the procedures together: one (SFM) is a surgeon skilled in both laparoscopic and robotic surgery and the other (CAP) has extensive experience with penile cancer and robotic surgery. In each case, one surgeon dissected the left side and the other dissected the right side, alternating sides between patients.
SURGICAL TECHNIQUE
Patients were prepared and draped for the standard ILND surgical procedure; they were placed in the supine position with leg abduction using Allen stirrups. All procedures were performed with robotic assistance (da Vinci Surgical System, Intuitive Surgical Corp., Sunnyvale, CA, USA) using a four-port technique (three robotic, one assistant). The space was initially developed by making a 1.5-cm incision 2–3 cm inferior to the apex of the femoral triangle (Fig. 1). The subcutaneous fat was separated and Scarpa’s fascia was incised. Using a mosquito clamp the plane below Scarpa’s fascia was developed enough to introduce a balloon dilator (PDB1000, Covidien, Princeton, NJ, USA). This was introduced carefully to maintain the correct plane and inserted until the entire balloon was below Scarpa’s. The balloon was then inflated to 800 mL, deflated, and repositioned and reinflated as necessary until the entire required field was developed. A self-retaining 12-mm balloon port (Applied Medical, Rancho Santa Margarita, CA, USA) was then placed and the inguinal space was insufflated to 15 mmHg with carbon dioxide. A 10-mm, 0° camera was used, and two 8-mm robotic cannulae were placed under direct visualization in a triangulated fashion about 2–3 cm away from the borders of the triangle (Fig. 1). An additional 12-mm trocar was placed for the assistant between two robotic port sites.
FIG. 1.
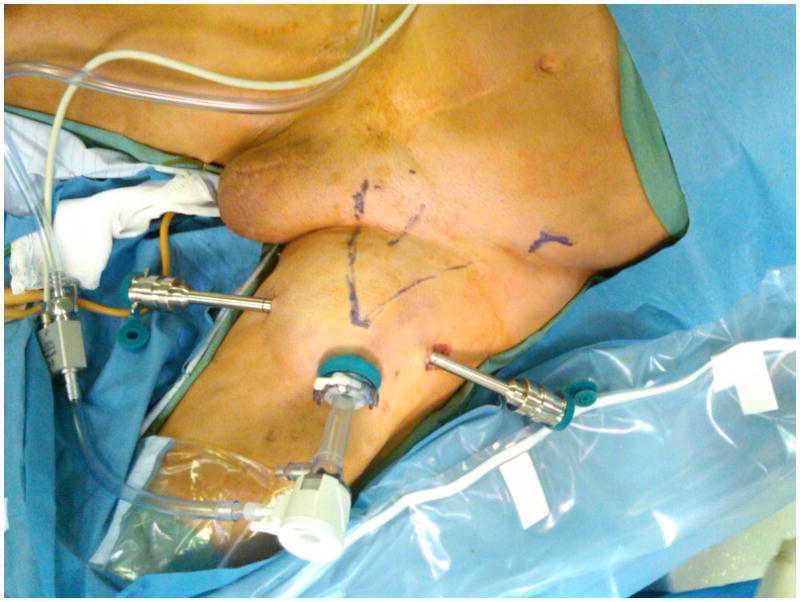
Robotic port placement in the left groin of a patient with penile cancer after penectomy. The ports are placed 2–3 cm away from the femoral triangle. Monopolar scissors were used in the dominant hand and bipolar Maryland forceps in the non-dominant hand. A 12-mm assistant port (not shown) is placed between the cameral port and one of the robotic ports.
Next, the patient-side cart was positioned from the opposite side of the inguinal field at about a 45° angle (Fig. 2). When moving to the other side, we initially tried to perform docking without repositioning the surgical cart, but that configuration caused the arms to frequently clash into one another. The patient-side cart was thus brought over the hip of the contralateral leg for the second side of the dissection without any difficulty (Fig. 2).
FIG. 2.
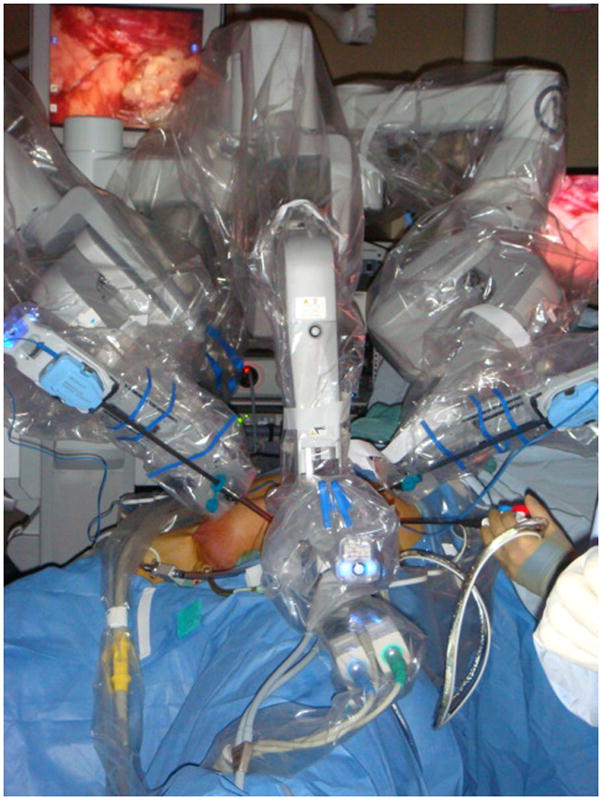
Docking alignment for the RAVEIL procedure. The machine is docked at about a 45° angle from the opposite side of the leg being operated on.
The surgical technique for the RAVEIL superficial nodal dissection utilized the same anatomical landmarks as a standard open ILND. The borders of dissection were as follows: superior, 2–3 cm above the inguinal ligament (exposing the ipsilateral spermatic cord); lateral, the midpoint of the sartorius muscle; and medial, the adductor longus muscle extending to the apex of the femoral triangle. It was important that we dissected lymph node tissue superficial to the inguinal field (i.e. below Scarpa’s fascia) as specified above. We preserved the saphenous vein when possible while removing nodal tissue above the fascia lata of the thigh. We removed the superficial nodal packets over the femoral vessels but did not skeletonize the vessels within the femoral sheath. Dissection was performed using monopolar scissors in the dominant hand and bipolar Maryland forceps in the non-dominant hand. Lymphatic and vascular structures were controlled prior to transection using either bipolar cautery or extensive use of surgical clips. All lymphatic tissue was removed and sent to the pathology laboratory for frozen section evaluation in accordance with the protocol.
Once the ipsilateral dissection was completed, we made an inguinal incision long enough to allow proper inspection of the surgical field (3–4 cm) by a urological oncologist not associated with the case. The verifying surgeon’s role was to inspect the surgical field and, with additional dissection if necessary, ensure that no additional superficial inguinal lymph nodes (e.g. above the fascia lata of the thigh) remained within the operative field. If additional tissue was removed, it was sent to the pathology laboratory and assessed to define whether it was nodal in origin and whether it contained metastasis.
If all removed ipsilateral nodes were negative for cancer, we then placed a closed suction drain and closed the wound (Fig. 3). Patients with metastasis detected in the ipsilateral lymph nodes underwent an open completion inguinal dissection with skeletonization of the vessels and an ipsilateral deep pelvic lymph node dissection. Tissue removed during the open portion of the procedure was sent to the pathology laboratory as a separate specimen. Pathological data pertaining to the total number of lymph nodes removed using RAVEIL and the presence of lymph nodes in the additional tissue collected during the verification procedure were documented.
FIG. 3.
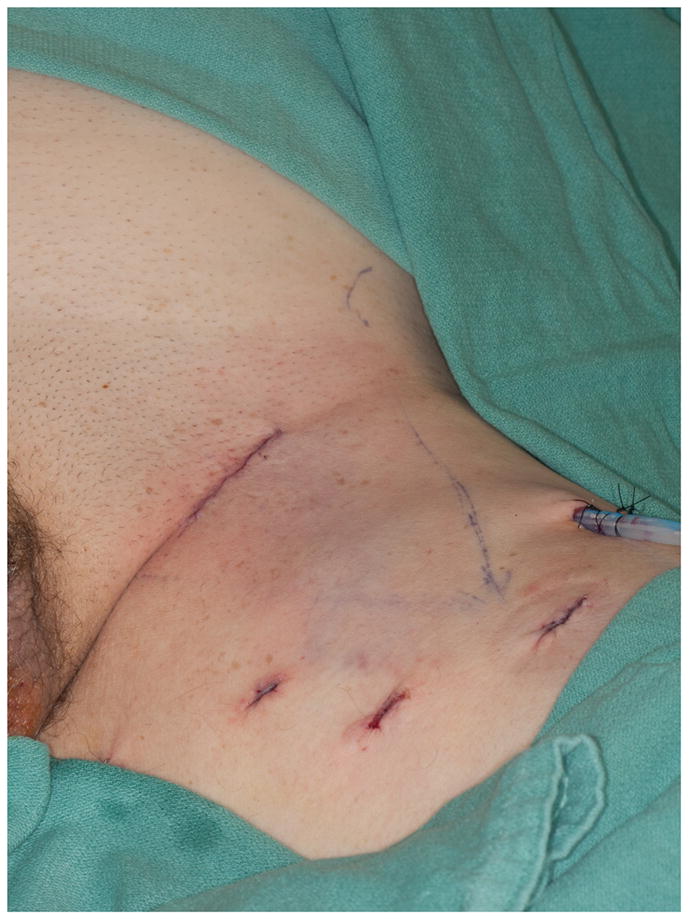
Final appearance of a successfully performed RAVEIL on the left side with an inguinal incision used for verification.
STATISTICAL CONSIDERATIONS
The statistical criteria and stopping rules for this phase I study were prospectively established between the study statistician (XH) and clinical investigators. It was defined that for 10 patients undergoing bilateral RAVEIL procedures, if more than two superficial inguinal fields with ≥ 2 lymph nodes remained or more than four with ≥ 1 node remained within the superficial dissection field, as determined by the verifying surgeon, we would not proceed to a phase II study. If, however, the RAVEIL procedure results met these criteria we would then proceed with a larger phase II study to formally assess the incidence of complications.
RESULTS
Ten patients were enrolled, and all 10 underwent a bilateral RAVEIL procedure. Patient demographics are listed in Table 1. Three patients had T1b disease, four had T2 disease and three had T3 disease. The median patient age was 62 years (range 28–84), and median body mass index was 31.1 kg/m2 (range 21.5–40.7), with four of the 10 patients considered morbidly obese (i.e. body mass index > 35 kg/m2). The median estimated blood loss was 100 mL (range 10–200). Although we did not specifically track procedure time during this portion of the study, each side of the dissection took between 90 and 120 min to complete.
TABLE 1.
Clinical and pathological characteristics and lymph node and inguinal field assessment
| Patient number | Age (years) | Body mass index (kg/m2) | Tumour classification | Clinical node classification | No. positive superficial inguinal nodes/total removed | No. positive deep nodes/total removed | No. positive pelvic nodes/total removed | Verifying surgeon’s assessment | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |||||
| Lymph node metastases | ||||||||||||
| 1 | 70 | 36.2 | T3 | N1* | 2/10 | 1/9 | 0/2 | † | 0/15 | 0/18 | Complete | Complete |
| 3 | 28 | 40.7 | T3 | N0 | 2/8 | 5/15 | 1/2 | † | 0/11 | 0/19 | Complete | Complete† |
| No lymph node metastases | ||||||||||||
| 2 | 67 | 38.4 | T1b | N0 | 0/21 | 0/17 | – | – | – | – | Complete | Complete |
| 4 | 84 | 26.9 | T3 | N0 | 0/6 | 0/8 | – | – | – | – | Complete | Complete |
| 5 | 68 | 26.4 | T2 | N0 | 0/6 | 0/7‡ | – | – | – | – | Complete | NA‡ |
| 6 | 60 | 28.9 | T1b | N0 | 0/5 | 0/6 | – | – | – | – | Incomplete§ | Complete |
| 7 | 59 | 35.7 | T2 | N0 | 0/6 | 0/7 | – | – | – | – | Complete | Complete |
| 8 | 60 | 33.4 | T2 | N0 | 0/15 | 0/14 | – | – | – | – | Complete | Complete |
| 9 | 54 | 21.5 | T2 | N0 | 0/11 | 0/9 | – | – | – | – | Complete | Complete |
| 10 | 64 | 25.8 | T1b | N0 | 0/5 | 0/6 | – | – | – | – | Complete | Complete |
Based on imaging, impalpable.
Fibrofatty tissue removed, no lymph nodes found.
Initial RAVEIL approach, converted to open.
Two additional lymph nodes found, benign.
NA, not assessed.
Among the 10 patients, two had inguinal lymph node metastases and subsequently underwent open completion ILND in the same setting (Table 1). For patient 1, the completed RAVEIL superficial dissection removed all three metastatic nodes. The deep inguinal and pelvic lymph nodes removed with completion dissection were negative for metastasis. For patient 2, seven of eight disease-positive nodes were removed with the RAVEIL technique. The only remaining disease-positive node was from the deep dissection removed during open portion.
The other eight patients underwent an attempted bilateral RAVEIL procedure and had no lymph node metastasis (pN0). However, in one patient, subsequent to dilatation of the working space on the right, a plane beneath the sartorius muscle was entered. Because of the subsequent loss of landmarks and an inability to progress endoscopically, we converted to an open technique and completed the procedure. No structural injuries were related to this event, and the left-side dissection was completed using the RAVEIL technique without difficulty.
Among the eight patients with pN0 disease, the mean numbers of nodes removed were nine (range 5–21) on the left side and nine (range 6–17) on the right side. The number of lymph nodes removed was consistent in each patient despite the fact that the two study surgeons alternated sides for the RAVEIL dissections. Fifteen independent sides among the eight pN0 patients were evaluated by a verifying surgeon. In one of these inguinal fields, two residual lymph nodes were dissected from below Scarpa’s fascia along the superficial aspect of the inguinal field near the spermatic cord. No metastases were detected in these additional nodes. Among all patients undergoing RAVEIL, 18 of 19 fields (94.7%) were adequately dissected.
There were no intraoperative vascular or neurological injuries. Among the eight patients who underwent a RAVEIL procedure alone, two (patients 2 and 6 in Table 1) were re-admitted to the hospital within 60 days for treatment of cellulitis, with one patient requiring incision and drainage of an abscess. Two additional patients (patients 4 and 9 in Table 1) were treated as outpatients, one for an area of wound breakdown (over the verification incision site) and the other for an area of skin necrosis (overlying the dissected field).
DISCUSSION
Minimally invasive ILND is a potential strategy for decreasing some of the adverse effects associated with more traditional open strategies, such as wound necrosis and dehiscence, whilst at the same time maintaining oncological adequacy [27,28]. We showed in this study that the RAVEIL technique adequately removes the superficial inguinal nodes when performed by surgeons with experience in the treatment of penile cancer and minimally invasive surgery.
Daseler et al. [29] performed anatomical inguinal dissections in 450 lower extremities of cadavers and found that the number of nodes in the superficial field ranged from 4 to 25, with an average of 8.25 per extremity. This is consistent with our average of 9 nodes removed per extremity using the RAVEIL technique. In a review of the initial experience from two South American centres, Sotelo et al. [24] noted that the average number of lymph nodes removed from the series of patients studied by Tobias-Machado and colleagues was 10.8 (range 7–16) and it was 9 (range 4–15) in their own series. Thus, it is likely that a carefully performed minimally invasive procedure accomplishes the oncological goal of removing all of the first-echelon lymph nodes.
There is a potential risk, however, for leaving behind lymph nodes within the field, particularly if some lymph node(s) are erroneously elevated at the start of blunt dissection of the potential space and therefore may remain undetected superficial to the inguinal field. In our study, the initial blunt dissection was also the cause of the one conversion to open surgery. Thus, we would caution that all surgeons engage in a similar initial quality-control study upon adoption of the RAVEIL technique because important subtle differences exist between endoscopic and open approaches for ILND, most notably for the initial blunt dissection of the correct plane of dissection.
The RAVEIL procedure detected all cases of superficial metastatic disease in our patients. The enhanced visualization and dexterity provided by RAVEIL would readily allow a deep dissection to be performed robotically. In the future, we plan to complete a deep inguinal dissection using RAVEIL for patients with microscopic metastases in superficial nodes (Fig. 4). Similarly, if a pelvic dissection is indicated, it also could be performed with robotic assistance at the same time.
FIG. 4.
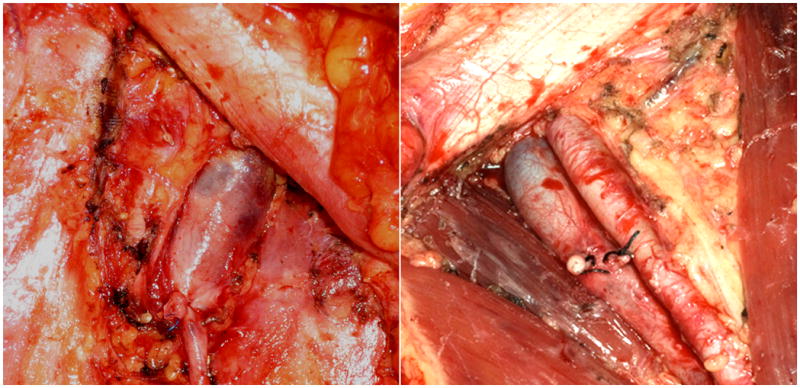
Photographs from a non-protocol open dissection showing differences between a superficial and a deep dissection. Left panel: Superficial dissection. The fascia lata covers the adductor and sartorius muscles. The lymph nodes are removed above the femoral vessels without opening the femoral sheath to expose the artery. Right panel: Deep dissection. Completed superficial and deep dissection removing the fascia lata from the muscles, including the femoral sheath fascia, completely exposing the vessels with removal of the saphenous vein.
The long-term results of endoscopic inguinal lymphadenectomy in the setting of limited metastatic disease are sparse, and conclusions about oncological efficacy await further follow-up and larger series. Tobias-Machado and associates [28] reported four inguinal fields with microscopic metastases, with no recurrences at 30 months. In other reported series, the follow-up period has been short. Thus, information on the long-term results of endoscopic ILND for limited metastatic disease is sparse, and conclusions about its efficacy await further follow-up and larger numbers of patients.
Whether RAVEIL will be shown to reduce the incidence of overall or specific complications in a prospective manner is uncertain at this time. In retrospect, we determined that in two of our cases with skin complications the dissection of nodes along the roof of the inguinal canal traversed Scarpa’s fascia, devascularizing the overlying skin. Both of these patients were relatively thin with relatively little subcutaneous fat, which may have been an influential factor in these dermal complications.
The results of two previous series suggested that endoscopic ILND could result in less morbidity than the standard open procedure. Tobias-Machado et al. [28] saw a 50% absolute reduction in the incidence of complications. Delman et al. [27] reported a series of 45 video-endoscopic ILNDs in 32 patients with a variety of malignancies. The overall complication rate was 25%, which was more favourable than their historical experience of 43%.
CONCLUSIONS
We have shown in this phase I pilot study that RAVEIL can adequately stage disease in the superficial inguinal region among patients with penile cancer at risk for inguinal metastases. We are encouraged to proceed with the next phase to formally determine the incidence and types of complications as well as longer-term oncological efficacy of RAVEIL in this high risk, clinically node-negative population. In the meantime, open ILND continues to be the standard of care for patients presenting with penile cancer and clinically evident metastases.
What’s known on the subject? and What does the study add?
Several lymph node staging strategies have been proposed as a response to the high morbidity seen after standard inguinal lymphadenectomy for penile cancer. A video-endoscopic (laparoscopic and robotic) approach has been proposed as a less morbid procedure in several retrospective studies. To date, none has evaluated the oncological adequacy with regard to whether all relevant nodes have been removed.
To the authors’ knowledge this is the first prospective study of a robotic or laparoscopic inguinal lymphadenectomy that evaluates the oncological adequacy of this approach for penile cancer. The study shows that robotic inguinal lymphadenectomy allowed adequate staging of disease in the inguinal region by removing all relevant lymph nodes as assessed by an independent evaluating urological oncologist.
Acknowledgments
This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672. Zachary Bohannan reviewed the paper for grammatical and editorial content.
Abbreviations
- ILND
inguinal lymphadenectomy
- VEIL
video-endoscopic inguinal lymphadenectomy
- RAVEIL
robotic assisted VEIL
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Horenblas S, van Tinteren H, Delemarre JF, Moonen LM, Lustig V, van Waardenburg EW. Squamous cell carcinoma of the penis. Iii. Treatment of regional lymph nodes. J Urol. 1993;149:492–7. doi: 10.1016/s0022-5347(17)36126-8. [DOI] [PubMed] [Google Scholar]
- 2.Marconnet L, Rigaud J, Bouchot O. Long-term followup of penile carcinoma with high risk for lymph node invasion treated with inguinal lymphadenectomy. J Urol. 2010;183:2227–32. doi: 10.1016/j.juro.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Ornellas AA, Nobrega BL, Wei Kin Chin E, Wisnescky A, da Silva PC, de Santos Schwindt AB. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354–9. doi: 10.1016/j.juro.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93:133–8. doi: 10.1002/jso.20414. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas V, Morse MJ, Herr HW, Sogani PC, Whitmore WF., Jr Penile cancer: relation of extent of nodal metastasis to survival. J Urol. 1987;137:880–2. doi: 10.1016/s0022-5347(17)44281-9. [DOI] [PubMed] [Google Scholar]
- 6.Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. 2005;173:816–9. doi: 10.1097/01.ju.0000154565.37397.4d. [DOI] [PubMed] [Google Scholar]
- 7.Pompeo ACL, Heyns CF, Abrams P, editors. International Consultation on Penile Cancer. Montreal: Societe Internationale d’Urologie; 2009. Penile cancer. [Google Scholar]
- 8.Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol. 2010;57:1002–12. doi: 10.1016/j.eururo.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Baas PC, Schraffordt Koops H, Hoekstra HJ, van Bruggen JJ, van der Weele LT, Oldhoff J. Groin dissection in the treatment of lower-extremity melanoma. Short-term and long-term morbidity. Arch Surg. 1992;127:281–6. doi: 10.1001/archsurg.1992.01420030043008. [DOI] [PubMed] [Google Scholar]
- 10.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur J Surg Oncol. 2006;32:785–9. doi: 10.1016/j.ejso.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Ingvar C, Erichsen C, Jonsson PE. Morbidity following prophylactic and therapeutic lymph node dissection for melanoma – a comparison. Tumori. 1984;70:529–33. doi: 10.1177/030089168407000610. [DOI] [PubMed] [Google Scholar]
- 12.Tonouchi H, Ohmori Y, Kobayashi M, et al. Operative morbidity associated with groin dissections. Surg Today. 2004;34:413–8. doi: 10.1007/s00595-003-2738-5. [DOI] [PubMed] [Google Scholar]
- 13.Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the MD Anderson Cancer Center experience. J Urol. 2002;167:1638–42. [PubMed] [Google Scholar]
- 14.Abraham V, Ravi R, Shrivastava BR. Primary reconstruction to avoid wound breakdown following groin block dissection. Br J Plast Surg. 1992;45:211–3. doi: 10.1016/0007-1226(92)90079-d. [DOI] [PubMed] [Google Scholar]
- 15.Chester DL, Waters R. Adverse alteration of wound flora with topical negative-pressure therapy: a case report. Br J Plast Surg. 2002;55:510–1. doi: 10.1054/bjps.2002.3890. [DOI] [PubMed] [Google Scholar]
- 16.Han LY, Schimp V, Oh JC, Ramirez PT. A gelatin matrix–thrombin tissue sealant (floseal) application in the management of groin breakdown after inguinal lymphadenectomy for vulvar cancer. Int J Gynecol Cancer. 2004;14:621–4. doi: 10.1111/j.1048-891X.2004.14411.x. [DOI] [PubMed] [Google Scholar]
- 17.Rouzier R, Haddad B, Dubernard G, Dubois P, Paniel BJ. Inguinofemoral dissection for carcinoma of the vulva: effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg. 2003;196:442–50. doi: 10.1016/S1072-7515(02)01895-1. [DOI] [PubMed] [Google Scholar]
- 18.Spiess PE, Hernandez MS, Pettaway CA. Contemporary inguinal lymph node dissection: minimizing complications. World J Urol. 2009;27:205–12. doi: 10.1007/s00345-008-0324-6. [DOI] [PubMed] [Google Scholar]
- 19.Kroon BK, Horenblas S, Estourgie SH, Lont AP, Valdes Olmos RA, Nieweg OE. How to avoid false-negative dynamic sentinel node procedures in penile carcinoma. J Urol. 2004;171:2191–4. doi: 10.1097/01.ju.0000124485.34430.15. [DOI] [PubMed] [Google Scholar]
- 20.Leijte JA, Hughes B, Graafland NM, et al. Two-center evaluation of dynamic sentinel node biopsy for squamous cell carcinoma of the penis. J Clin Oncol. 2009;27:3325–9. doi: 10.1200/JCO.2008.20.6870. [DOI] [PubMed] [Google Scholar]
- 21.Bishoff JT, Basler JW, Teichman JM, Thompson IM. Endoscopic subcutaneous modified inguinal lymph node dissection (ESMIL) for squamous cell carcinoma of the penis. J Urol. 2003;169:78, Abstract 301. [Google Scholar]
- 22.Tobias-Machado M, Tavares A, Molina WR, Jr, et al. Video endoscopic inguinal lymphadenectomy (VEIL): initial case report and comparison with open radical procedure. Arch Esp Urol. 2006;59:849–52. doi: 10.4321/s0004-06142006000800020. [DOI] [PubMed] [Google Scholar]
- 23.Tobias-Machado M, Tavares A, Ornellas AA, Molina WR, Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol. 2007;177:953–8. doi: 10.1016/j.juro.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 24.Sotelo R, Sanchez-Salas R, Carmona O, et al. Endoscopic lymphadenectomy for penile carcinoma. J Endourol. 2007;21:364–7. doi: 10.1089/end.2007.9971. [DOI] [PubMed] [Google Scholar]
- 25.Dogra PN, Saini AK, Singh P. Robotic-assisted inguinal lymph node dissection: a preliminary report. Indian J Urol. 2011;27:424–7. doi: 10.4103/0970-1591.85458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Josephson DY, Jacobsohn KM, Link BA, Wilson TG. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology. 2009;73:167–71. doi: 10.1016/j.urology.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 27.Delman KA, Kooby DA, Rizzo M, Ogan K, Master V. Initial experience with videoscopic inguinal lymphadenectomy. Ann Surg Oncol. 2011;18:977–82. doi: 10.1245/s10434-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 28.Tobias-Machado M, Tavares A, Silva MN, et al. Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J Endourol. 2008;22:1687–91. doi: 10.1089/end.2007.0386. [DOI] [PubMed] [Google Scholar]
- 29.Daseler EH, Anson BJ, Reimann AF. Radical excision of the inguinal and iliac lymph glands; a study based upon 450 anatomical dissections and upon supportive clinical observations. Surg Gynec Obstet. 1948;87:679–94. [PubMed] [Google Scholar]


