Abstract
Background
Asthma is defined as a chronic inflammatory disease of the airways; however, the underlying physiologic and immunologic processes are not fully understood.
Objective
The aim of this study was to determine whether TH9 cells develop in vivo in a model of chronic airway hyperreactivity (AHR) and what factors control this development.
Method
We have developed a novel chronic allergen exposure model using the clinically relevant antigen Aspergillus fumigatus to determine the time kinetics of TH9 development in vivo.
Results
TH9 cells were detectable in the lungs after chronic allergen exposure. The number of TH9 cells directly correlated with the severity of AHR, and anti–IL-9 treatment decreased airway inflammation. Moreover, we have identified programmed cell death ligand (PD-L) 2 as a negative regulator of TH9 cell differentiation. Lack of PD-L2 was associated with significantly increased TGF-β and IL-1α levels in the lungs, enhanced pulmonary TH9 differentiation, and higher morbidity in the sensitized mice.
Conclusion
Our findings suggest that PD-L2 plays a pivotal role in the regulation of TH9 cell development in chronic AHR, providing novel strategies for modulating adaptive immunity during chronic allergic responses.
Keywords: Asthma, chronic airway hyperreactivity, Aspergillus fumigatus, TH9, costimulation, programmed cell death ligand 2, TGF-β
Allergic asthma is a complex inflammatory disorder caused by TH2-driven inflammatory responses in the presence of environmental factors, such as allergens. Allergic asthma is characterized by airway hyperreactivity (AHR), airway inflammation, intermittent reversible airway obstruction, excessive mucus production, and increased serum IgE and TH2 cytokine levels.1–3 The mechanisms of recognition of allergens by the immune system have been investigated extensively. Although it was initially thought that only TH2 cells and their cytokines, namely IL-4, IL-5, and IL-13, might orchestrate the allergic immune response to inhaled innocuous allergen, recent studies suggest that the immune response in asthmatic patients is now highly heterogeneous. 4 Several studies suggest that other cytokines, such as IL-9, might play an important role in chronic allergic immune responses. 5–8 IL-9 is a T cell–derived cytokine belonging to the IL-2 cytokine family, which was initially described as a T-cell and mast cell growth factor in mice.9 It has been shown to stimulate the proliferation of activated T cells and enhance the production of IgE from B cells and promotes the proliferation and differentiation of mast cells and hematopoietic progenitors. This multifunctional cytokine is secreted by many cell types, including activated T lymphocytes, eosinophils, mast cells, and neutrophils.10–13 Although described as one of several TH2 cytokines, other TH subsets also appear to have the potential for IL-9 production, such as TH17 and regulatory T (Treg) cells.14,15 Recent comparative analyses of different subsets of TH cells revealed that a population of IL-9–producing TH cells distinct from TH2, TH1, TH17, and Treg cell populations, termed TH9 cells, develop in the presence of IL-4 and TGF-β in patients with various chronic inflammatory disorders. 5,16–18 In patients with chronic asthma, a close association between the IL9 gene and allergic inflammation has been demonstrated,5,6,19 and selective overexpression of the IL9 gene within the lungs of transgenic mice results in increased airway inflammation with eosinophils and lymphocytes as the predominant infiltrating cells.7,8,20 Furthermore, IL-9 has been found to have direct and indirect effects on airway remodeling occurring during chronic asthma.7,21–24 Although all these data suggest a central role for the IL-9 pathway in the pathogenesis of chronic allergic asthma, the molecular regulation for TH9 differentiation in vivo remains unknown.
We recently reported that programmed cell death ligand (PD-L) 2, a member of the B7 family, has an important role in the regulation of acute AHR in mice.25 Here we have developed a novel protocol to expose mice to intranasal doses of Aspergillus fumigatus lysate for several weeks to induce chronic AHR. We observed that in the first 4 weeks of exposure, pulmonary TH2 cells were induced; however, by week 6, a significant population of TH9 cells started to accumulate in the lungs. Furthermore, using PD-L2–deficient mice, we probed the role of the PD-L2 pathways in the control of the TH9 response and in the development of chronic AHR. Our data suggest that blockade of the PD-L2 pathway significantly increased TGF-β and IL-1α levels in the lungs of sensitized mice, inducing an enhanced development of TH9 cells, which was directly correlated with the severity of lung inflammation, mucus production, and AHR. Thus PD-L2 plays a pivotal role in the regulation of TH9 cells in patients with chronic AHR, which provides novel strategies for modulating adaptive immunity during inflammatory/allergic responses.
METHODS
Mice
Female BALB/c ByJ mice (6 to 8 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, Me). PD-L2−/− mice were obtained from Dr Arlene Sharpe (Harvard Medical School, Boston, Mass) and backcrossed to BALB/cByJ mice, as previously described.26 All mice were maintained in a pathogen-free mouse colony at the Keck School of Medicine, University of Southern California, under protocols approved by the Institutional Animal Care and Use Committee.
Induction of chronic AHR and measurement of airway responsiveness
Mice were sensitized intranasally for 46 days with A fumigatus lysate (50 µg on weeks 1 and 2 and 20 µg on weeks 3–8 in 50 µL of saline solution; Cosmo Bio, San Diego, Calif) or PBS to induce chronic AHR. In some experiments mice were treated intraperitoneally with 500 µg of mouse anti-mouse IL-9 blocking antibody (clone MM9C1) produced by means of autovaccination, as previously described,27 or IgG2a isotype control antibody (BioXcell, West Lebanon, NH). On day 48 of the regimen, mice were anesthetized by using a 300-µL intraperitoneal injection of ketamine (10 mg/mL) and xylazine (1 mg/mL) and tracheotomized. Measurements of airway resistance and compliance were conducted with the FinePointe RC System (Buxco Research Systems, Wilmington, NC), in which mice were mechanically ventilated by using a modified version of a previously described method.28 Mice were sequentially challenged with aerosolized PBS (baseline), followed by increasing doses of methacholine ranging from 1.25 to 20 mg/mL. Maximum resistance and average compliance values were recorded during a 3-minute period after each challenge. We continuously computed lung resistance (RL) and dynamic compliance (Cdyn) by fitting flow, volume, and pressure to an equation of motion.
Collection of BAL fluid and lung histology
After measurement of AHR and death, the trachea was cannulated, the lungs were washed twice with 1 mL of PBS plus 2% FCS, and fluids were pooled, as previously described.29 The relative number of leukocyte populations was differentiated on slide preparations of BAL fluid stained with the DIFF stain kit (IMEB, San Marcos, Calif). After BAL was performed, transcardial perfusion of the lungs was performed with cold PBS, and subsequently, the lungs were fixed and harvested for histology with 4% paraformaldehyde buffered in PBS. After fixation, the lungs were embedded in paraffin, cut into 4-µm sections, and stained with hematoxylin and eosin and periodic acid–Schiff. Histologic pictures were acquired with a DFC290 Leica camera and analyzed with the Leica Application suite (Leica Microsystems, Bannockburn, Ill).
ELISA and lung lysates
Cytokines were analyzed in cell-culture supernatants by means of ELISA with Ready Set Go kits (eBioscience, San Diego, Calif), according to the manufacturer’s instructions. Briefly, lungs were collected and homogenized in 500 µL of Triton X-100 lysis buffer (0.5% Triton X-100, 150 mmol/L NaCl, 15 mmol/L Tris, 1 mmol/L CaCl2, and 1 mmol/L MgCl2) by using a homogenizer. The homogenates were then centrifuged for 20 minutes at 10,000g, and the supernatant was collected. Cytokine levels were then measured in the supernatants by means of ELISA. Data were normalized based on the background in the lungs of untreated mice.
Flow cytometric analysis
Lungs and draining lymph nodes (LNs) were collected and digested with collagenase D and DNase I, as previously described.29 CD45+ cells were then purified by using magnetic cell sorting (Miltenyi Biotec, Auburn, Calif) with mouse anti-CD45 microbeads (Miltenyi Biotec). The positively selected fractions were then cultured overnight in round-bottom 96-well plates (5 × 105 cells per well) in RPMI (Cellgro, Manassas, Va) supplemented with 10% FCS (OMEGA Scientific, Tarzana, Calif) without any antigen. For cytokine staining, cells were incubated in vitro for 6 hours with 0.15 µL per well Golgistop (BD Biosciences, San Jose, Calif), followed by surface staining on ice of 2 × 106 cells by using an antibody combination that included eFluor-450–CD44 (clone IM7, eBioscience) and allophycocyanin-Cy7–CD4 (clone L3T4, eBioscience) or peridinin-chlorophyll-protein complex–Cy5.5–DO11.10 (clone KJ1-26, eBioscience). Cells were then permeabilized with the BD Cytofix/Cytoperm kit (BD Biosciences), and intracellular cytokines were stained by using antibodies that included allophycocyanin–IL-9 (clone RM9A4; BioLegend, San Diego, Calif), phycoerythrin-Cy7–IL-4 (clone BVD6-24G2, eBioscience), phycoerythrin–IL-13 (clone ebio13A, eBioscience), phycoerythrin–IL-10 (clone JES5-16E3, BD PharMingen), and peridininchlorophyll-protein complex–Cy5.5–IL-17a (clone ebio17b7, eBioscience), according to the manufacturer’s instructions (BD PharMingen). Analytic flow cytometry was carried out with a FACSCanto II 8-color flow cytometer (BD Biosciences). The data were analyzed with FlowJo version 8.6 software (TreeStar, Ashland, Ore).
In vitro generation of TH9 cells
CD4+DO11.10+ T cells were purified from splenocytes of DO11.10+ mice by means of magnetic cell sorting (Miltenyi Biotec) with a mouse CD4+ isolation kit (Miltenyi Biotec). Purified CD4+DO11.10+ T cells were cultured for 3 days in round-bottom 96-well plates (1 × 105 cells per well) with ovalbumin (OVA)–loaded (OVA peptide 323–339) bone marrow–derived dendritic cells (DCs; ratio, 1:32) from wild-type (WT) or PD-L2−/− mice in the presence of 10 µg/mL anti–IFN-γ antibodies (clone XMG1.2; BioXcell, West Lebanon, NH), 2 ng/mL rTGF-β1 (eBioscience) and 10 ng/mL rIL-4 (eBioscience) with or without 10 µg/mL anti–PD-1 blocking antibodies (clone J43, eBioscience), anti–PD-L2 blocking antibodies (clone mAb 3.2, a gift of Dr. Gordon Freeman, Harvard Medical School25), or IgG1 isotype control antibodies (IgG1, MOPC-21, BioXCell).
Statistical analysis
Differences between groups were analyzed by using the 2-tailed, unpaired Student t test and considered significant when the P value was less than .05. Survival data were analyzed by using a Mantel Cox test with Prism version 4 software (GraphPad Software, La Jolla, Calif).
RESULTS
Specific development of pulmonary TH9 cells during chronic AHR
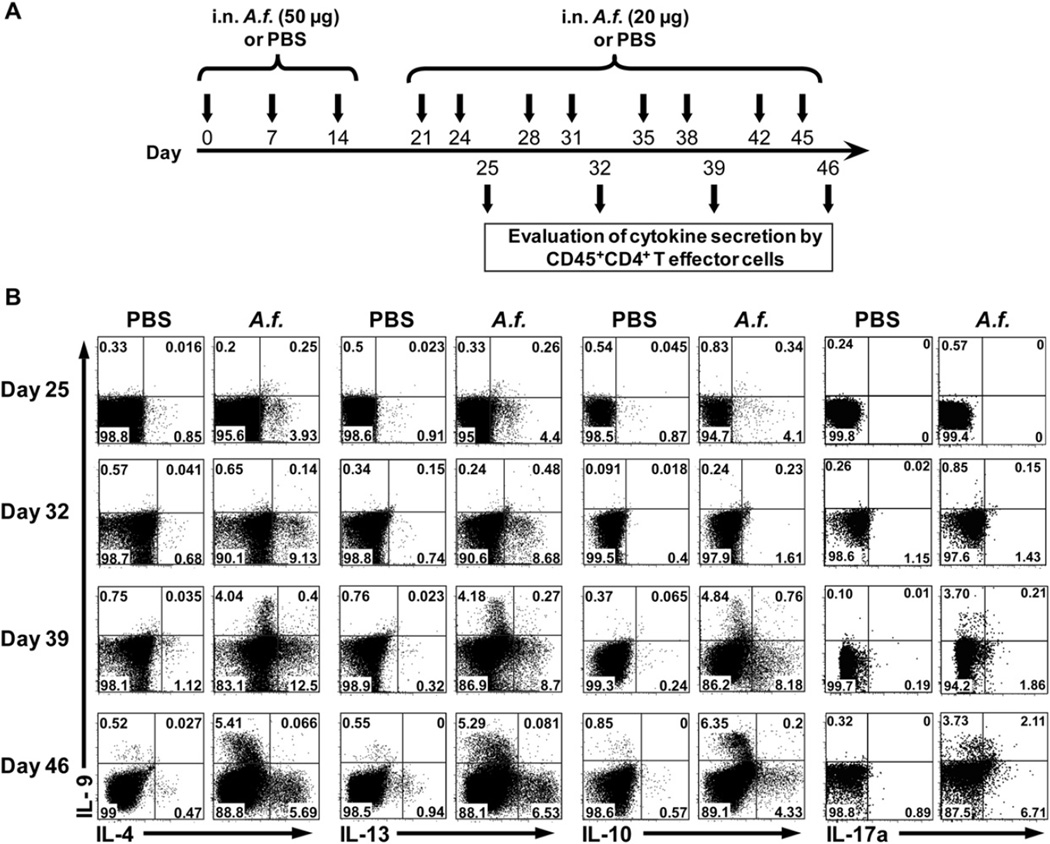
Several groups have reported that IL-9 production is enhanced in the lungs of human patients with chronic asthma.5,6 However, even though TH9 cells have recently been identified in vitro,16,18 it remains unclear whether the principal cellular sources of IL-9 during chronic allergic inflammation are TH9 cells. Therefore we have developed a novel protocol to induce chronic AHR in mice through intranasal exposure to doses of A fumigatus lysates for more than 6 weeks (Fig 1, A). We used this model of chronic AHR to assess the development of IL-9 and other key cytokines by effector T (Teff) cells. On days 25, 32, 39, and 46 of the exposure protocol, CD45+ cells were purified from the lungs and LNs and kept in culture overnight without any stimulation. The production of IL-9, IL-4, IL-10, IL-13, and IL-17a was then assessed by using an intracellular cytokine assay gated on the CD3+CD44+CD4+ Teff cells (see Fig E1, A, in this article’s Online Repository at www.jacionline.org). On days 25 and 32, we observed the generation of TH2 cells producing IL-4, IL-13, and IL-10 cytokines but not IL-9–producing CD4+ T cells both in the lungs and LNs of A fumigatus–sensitized mice (Fig 1, B, and see Fig E1, B). However, by days 39 and 46, although IL-4–, IL-10–, IL-13–, and IL-17a–producing CD4+ T cells were still detected both in the lungs and LNs of A fumigatus–sensitized mice, a significant population of IL-9–producing CD4+ T cells started to appear in the lungs (Fig 1, B) but not in the LNs (see Fig E1, B). These IL-9–producing CD4+ T cells did not coexpress IL-4, IL-13, and IL-10; however, they coexpress IL-17a. These results suggest that although CD4+ T cells producing TH2 cytokines are present in both the lungs and the LNs of mice during the acute and chronic phases of AHR, the development of TH9 cells is restricted to the lungs.
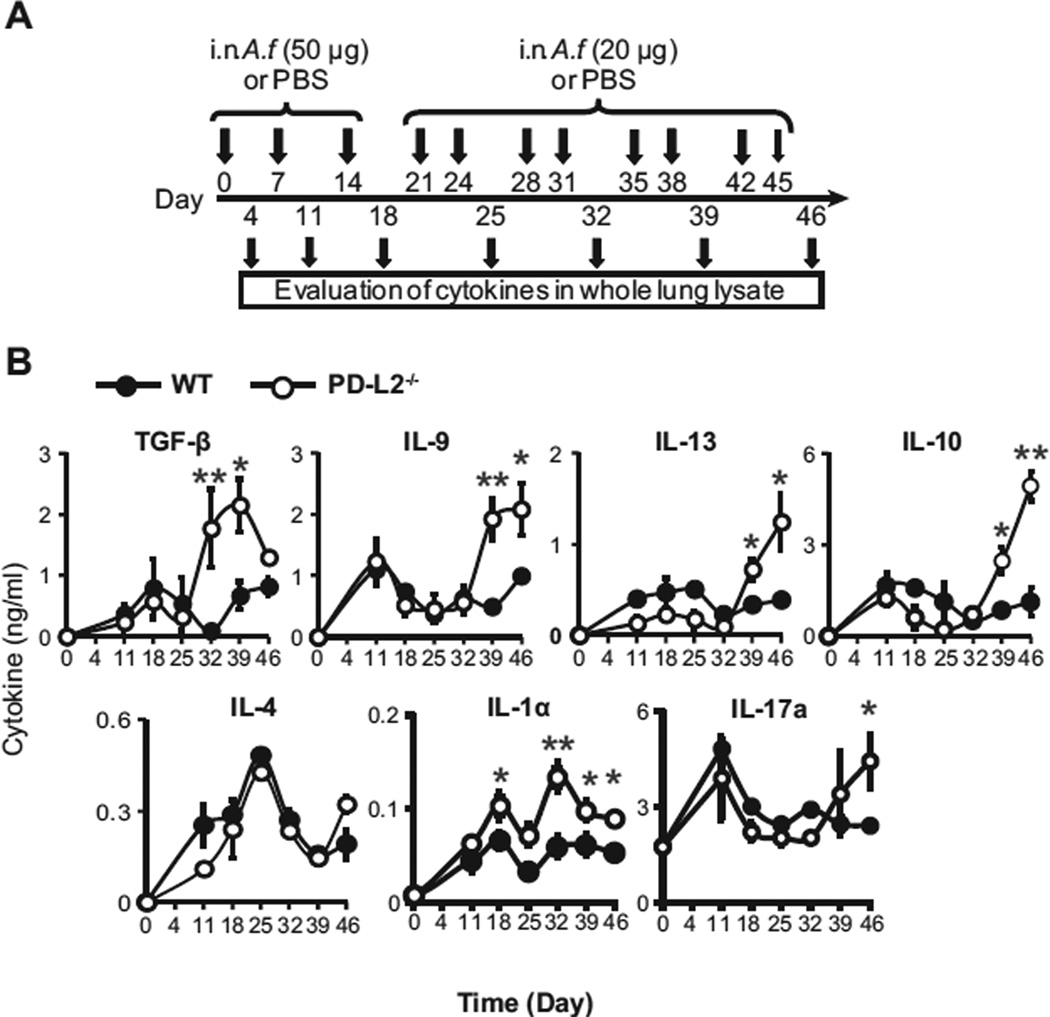
FIG 1.
Development of pulmonary TH9 cells after chronic exposure to Aspergillus fumigatus (A.f.). A, Protocol of immunization to induce chronic AHR by A fumigatus. Briefly, a group of naive BALB/c mice were immunized intranasally (i.n.) for 46 days with A fumigatus or PBS. B, On days 25, 32, 39, and 46, the percentages of cytokines (IL-9, IL-4, IL-13, IL-10, and IL-17a) secreting Teff cells in the lungs were then assessed by means of intracellular staining gated on the CD45+CD3+CD44+CD4+ population. Data are representative of 4 independent experiments (n = 5), with P values of less than .001 for IL-9 on days 39 and 46 between PBS- and A fumigatus–treated mice.
PD-L2 regulates TH9 development in vitro
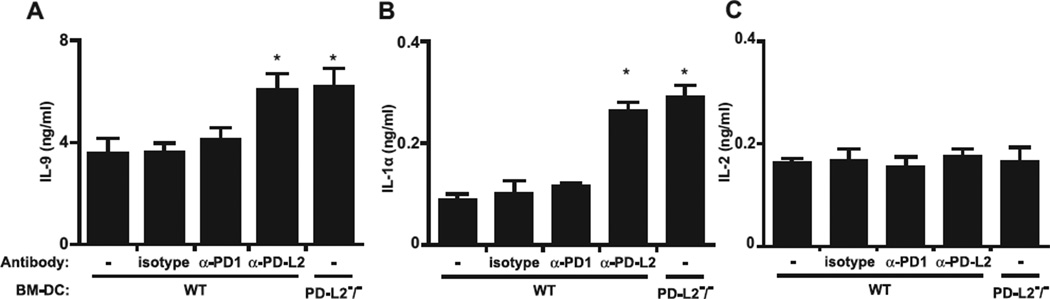
The current hypothesis is that TH9 cells develop from TH2 cells, and costimulatory molecules have been shown to influence T-cell polarization.30,31 In particular, PD-L2 has been shown to enhance the production of TH2 cytokines and therefore would be predicted to affect TH9 cell development.32–34 Therefore purified CD4+DO11.10+ cells were cultured under TH9 cell development conditions (TGF-β, IL-4, and anti–IFN-γ) and cocultured with OVA-loaded bone marrow–derived DCs from WT or PD-L2−/− mice because the effects of OVA have been well studied in these cells. Alternatively, anti–PD-L2, anti–PD-1 blocking, or isotype control antibodies were added to the abovementioned tissue culture. After 3 days of culture, IL-9, IL-2, and IL-1α production was determined by using ELISA performed on the supernatants. As expected, DO11.10 T cells produced high levels of IL-9 cytokine when cultured in the presence of OVA-loaded bone marrow–derived DCs from WT mice (Fig 2, A). Although the production of IL-9 and IL-1α by DO11.10 T cells was significantly increased in the absence of PD-L2 or after blockade of PD-L2 with anti–PDL2 blocking antibodies, it was not affected by anti–PD-1 blocking or isotype control antibodies (Fig 2, A and B). Furthermore, IL-2 levels did not change after using PD-1 or PD-L2 blocking antibodies (Fig 2, C). We also measured the gene expression levels of the TH9 lineage transcription factors PU-1 and interferon regulatory factor 4 (IRF4) and the TH2 lineage transcription factors signal transducer and activator of transcription 6 (STAT6) and GATA-320,35,36 and found that levels of IRF4 and PU-1, but not STAT6 and GATA-3, were increased in cocultures of T cells and bone marrow–derived DCs (see Fig E2 in this article’s Online Repository at www.jacionline.org). Taken together, our data suggest that PD-L2 interactions negatively regulate TH9 but not TH2 development in vitro.
FIG 2.
PD-L2 regulates TH9 cell development in vitro. Purified CD4+DO11.10+ T cells were put in culture (1×105 cells per well) with OVA-loaded bone marrow–derived DCs (BM-DC) from WT and PD-L2−/− mice in the presence of IL-4 (10 ng/mL), TGF-β (1 ng/mL), and anti–IFN-γ antibodies (10µg/mL) with or without anti–PD-1 blocking (α-PD1), anti–PD-L2 blocking (α-PD-L2), or isotype control antibodies (10 µg/mL). After 3 days of culture, production of IL-9, IL-1α, and IL-2 was measured by means of ELISA in the supernatant. Data are means ± SEMs of 3 experiments. *P < .05,WT isotype–treated versus WT anti–PD-L2–treated or PD-L2−/− mice.
PD-L2 regulates development of TH9 cells in vivo
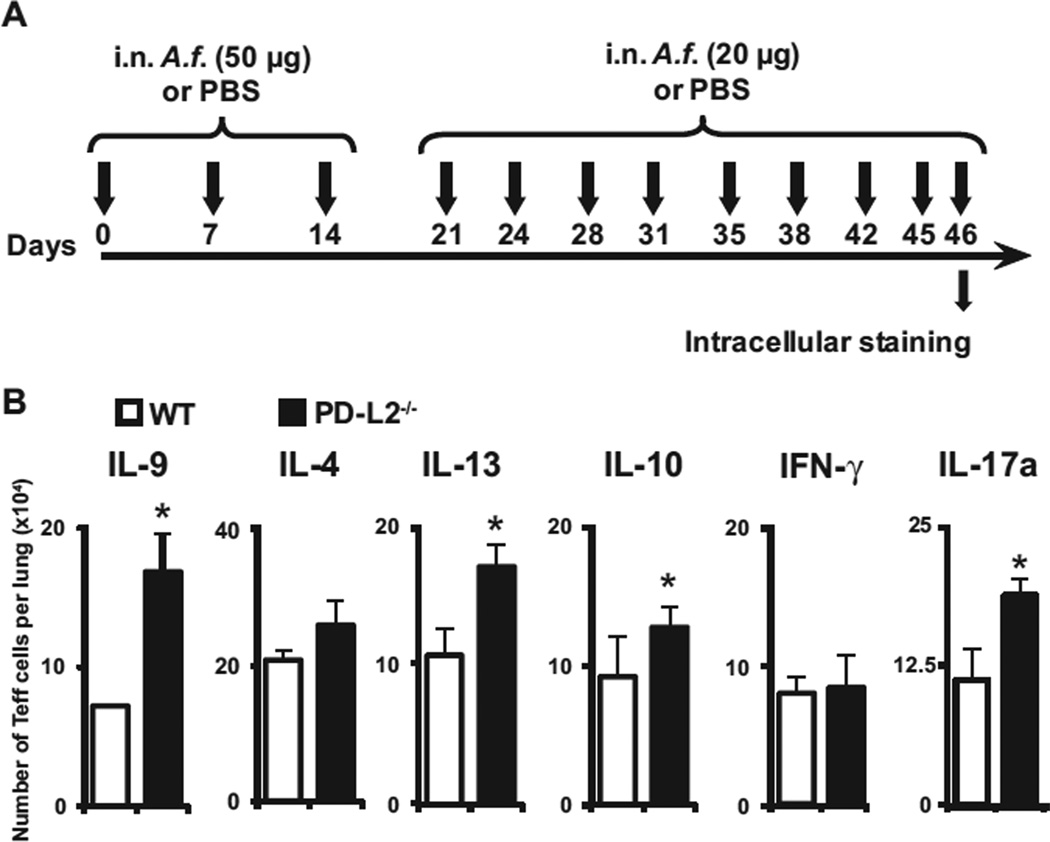
To confirm whether the aforementioned results in vitro hold true in vivo, we assessed the development of T-cell subsets in the lungs of mice lacking PD-L2−/− using our chronic exposure protocol (Fig 3, A). The absolute numbers of IL-9–, IL-4–, IL-10–, IL-13–, IL-17a–, and IFN-γ–producing Teff cells per lung were assessed 24 hours after the last intranasal immunization by using the intracellular cytokine assay. On day 46, IL-9–, IL-4–, IL-10–, IL-13–, IL-17a–, and IFN-γ–producing Teff cells were quantified in the lungs of WT and PD-L2−/− mice. Although the number of Teff cells producing IL-4 or IFN-γ in mice lacking PD-L2 was similar to that in WT mice, the number of Teff cells producing IL-9, IL-10, IL-17a, and IL-13 significantly increased (Fig 3, B). These data, in agreement with our in vitro findings, suggest that PD-L2 signaling significantly regulates the development TH9 effector cells in the lungs of mice chronically exposed to an allergen.
FIG 3.
PD-L2 regulates TH9 cell differentiation in vivo. A, Protocol of immunization to induce chronic AHR by A fumigatus (A.f.). B, Purified lung CD45+ cells were cultured 24 hours after the last intranasal (i.n.) immunization overnight without any stimulation and analyzed for the percentage of cytokine-secreting Teff cells by means of intracellular staining gated on the CD45+CD3+CD44+CD4+ cells. Statistical analyses were performed by using the Student t test: *P <s .05. Values are representative of 3 independent experiments (n = 5).
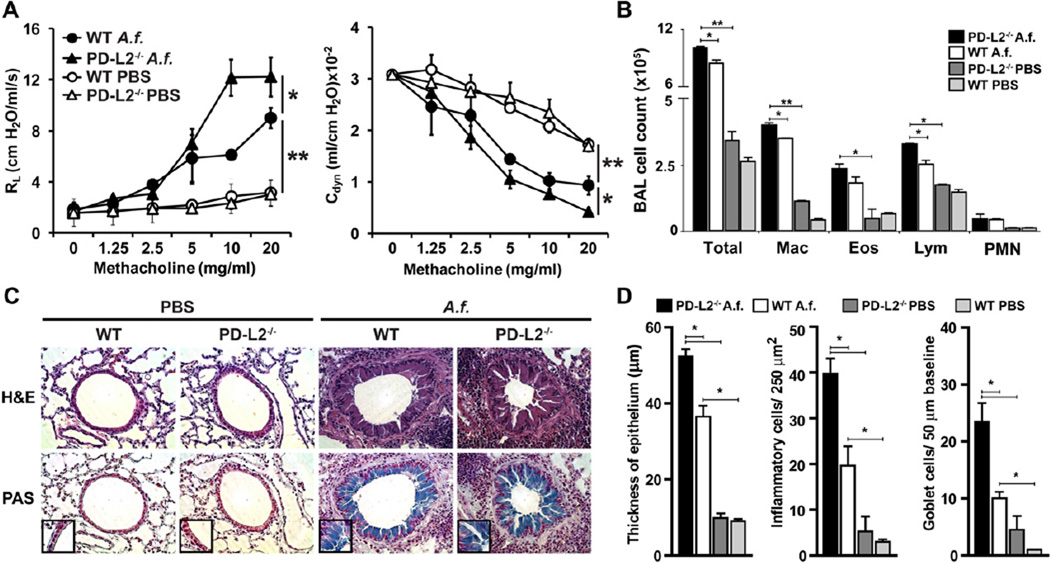
PD-L2 modulates the severity of chronic AHR
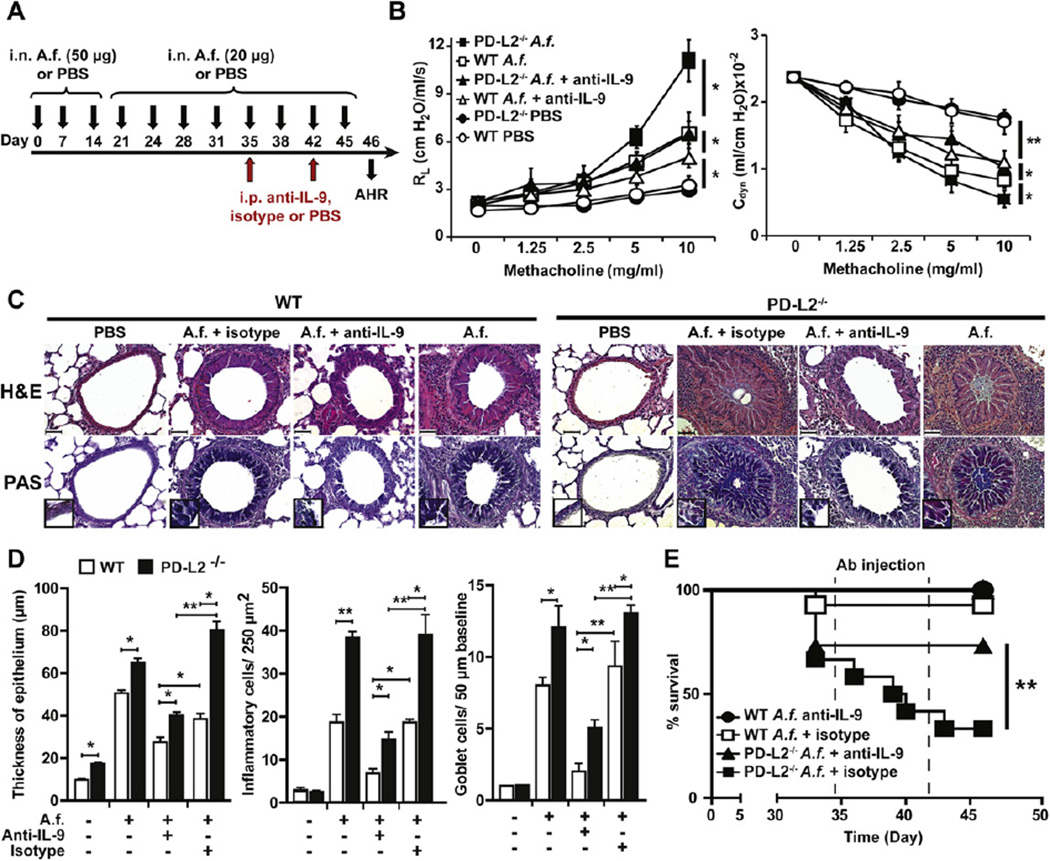
Recently, we reported that PD-L2 has an important role in the regulation of acute AHR in mice.25 To determine whether PD-L2 also plays a role in the development of allergen-induced chronic AHR, we compared the development of AHR in PD-L2−/− and WTBALB/c mice chronically exposed to A fumigatus, as outlined in Fig 1, A. AHR was measured 24 hours after the last allergen challenge by means of direct measurement of RL and Cdyn in anesthetized, tracheostomized, intubated, and mechanically ventilated mice (Fig 4, A). As expected, after chronic exposure to A fumigatus, BALB/c control mice experienced severe AHR characterized by increased RL and reduced Cdyn. However, in agreement with the increased TH9 cell development in the lungs of PD-L2−/− mice compared with that seen in control BALB/c mice, the severity of AHR was increased in PD-L2−/− mice (Fig 4, A). This increased AHR in PD-L2−/− mice was associated with a significant increase in the number of macrophages and lymphocytes in BAL fluid compared with those seen in the WT BALB/c group (Fig 4, B). We also examined the lung histology of mice shown in Fig 4, A, using hematoxylin and eosin to determine cellular infiltration and after periodic acid–Schiff staining to determine mucus production. Lung tissue of WT mice showed infiltration of inflammatory cells around the airways in the lumen (Fig 4, C, upper panel) and mucus production (Fig 4, C, lower panel) after chronic A fumigatus exposure. To assess the level of inflammation in the lungs, we quantified the thickness of airway epithelium and quantified the number of inflammatory and goblet cells (Fig 4, D). The degree of inflammation, cellular infiltration, and mucus production was significantly higher in PD-L2−/− mice compared with that seen in A fumigatus–sensitized WT mice. Altogether, these data demonstrate that PD-L2 modulates the severity of AHR and lung inflammation after chronic allergen exposure.
FIG 4.
Increased airway inflammation and AHR in PD-L2−/− mice. A, Groups of WT and PD-L2−/− BALB/c mice were immunized intranasally with A fumigatus (A.f.) or PBS, as described Fig 1, A. The mice were then assessed for AHR 24 hours after the last intranasal immunization by measuring RL and Cdyn. Data are means ± SEMs and representative of 3 separate experiments (n = 5). *P < .05 and **P < .03, Student t test. B, Bronchoalveolar lavage (BAL) fluid from the mice in Fig 4, A, was analyzed 24 hours after AHR measurement. Results are shown as the total number of cells in BAL fluid. *P < .05 and **P < .01, Student t test. Eos, Eosinophils; Lym, lymphocytes; Mac, monocyte/macrophage; PMN, neutrophils; Total, total cell number. C, Lung tissue from A fumigatus–sensitized PD-L2−/− or WT mice were stained with hematoxylin and eosin (H&E; upper panel) and analyzed for cell infiltration. Lung tissue from the same mice were stained with periodic acid–Schiff (PAS; lower panel) and analyzed for the presence of mucus. Arrows indicate the production of mucus in the lumen. Original magnification ×40 (inset, magnification ×100). D, Quantifications of lung histopathology shown as thickness of airway epithelium (left panel), number of inflammatory cells per 250 µm2 of lung tissue (middle panel), and number of goblet cells per 50 µm of epithelium at baseline (right panel). Data are shown as means ± SEMs (n = 5). *P < .05.
PD-L2 affects pulmonary TGF-β secretion during chronic allergen exposure
Several studies have identified that TGF-β is critical for the development of TH9 cells.16–18 To evaluate whether PD-L2 contributes to the regulation of TGF-β in patients with chronic asthma, we determined the amount of this cytokine in whole-lung lysates of PD-L2−/− and WT BALB/c mice by means of ELISA at 6 time points during the A fumigatus exposure protocol (Fig 5, A). During the course of chronic allergen exposure, 2 peaks of TGF-β were observed in the lungs of WT mice: one during the early stages of A fumigatus exposure (day 18) and a more intense peak during the later phases of A fumigatus exposure (day 39). In the lungs of PD-L2−/− mice, although the first peak of TGF-β was similar to that observed with WT mice, the second peak of TGF-β started slightly earlier and was significantly increased (Fig 5, B). In parallel, we also determined the pulmonary levels of IL-9, IL-13, IL-10, IL-4, IL-1α, and IL-17a. In the lungs of both WT and PD-L2−/− mice, although only 1 peak of IL-4 was observed, 2 peaks of secretion of IL-9, IL-13, IL-10, IL-4, IL-1α, and IL-17a were observed (Fig 5, B). Furthermore, as observed for TGF-β, the intensity of the first peaks of IL-9, IL-10, IL-13, and IL-17 were similar between WT and PD-L2−/− mice, whereas the intensity of the second peaks were enhanced in the lungs of PD-L2−/− mice. In regard to IL-1α, we observed significantly higher levels in both peaks when PD-L2−/− mice were compared with WT mice. Thus our results suggest that in addition to the direct role of PD-L2 interactions in polarization of TH9 cells, lack of PD-L2 increases TGF-β levels and can therefore enhance pulmonary TH9 differentiation.
FIG 5.
Variation of TGF-β production in lungs of PD-L2−/− mice. A, Protocol for A fumigatus (A.f.) immunization and preparation of lung lysate samples from WT and PD-L2−/− BALB/c mice. i.n., Intranasal. B, Concentrations of IL-9 and TGF-β, IL-13, IL-10, IL-4, IL-1α, and IL-17a were then assessed by means of ELISA in the whole-lung lysates, as described in the Methods section. Statistical analyses were performed with the Student t test. *P < .05 and **P < .01. Values are representative of 3 separate experiments (n = 5).
IL-9 regulates the severity of chronic AHR
Because we discovered that PD-L2−/− mice produce more IL-9 and have more severe AHR than WT mice when chronically exposed to A fumigatus, we investigated the contribution of IL-9 to the development and severity of chronic AHR by using anti–IL-9 blocking antibodies. A cohort of WT and PD-L2−/− mice were exposed to A fumigatus for 46 days and then treated with 500 µg of anti–IL-9 blocking antibodies or isotype control according to the protocol (Fig 6, A). Treatment with anti–IL-9 blocking antibody dramatically reduced the severity of AHR in the WT group compared with that seen in the isotype control group (RL: P = .02) and to a greater extent in PD-L2−/− mice (RL: P = .01; Fig 6, B). In agreement with lung function results, neutralization of IL-9 also reduced lung inflammation, mucus production, and cell recruitment, as demonstrated by the increased thickness of epithelial cells and increased number of inflammatory and goblet cells on lung histology (Fig 6, C and D). Finally, in PD-L2−/− mice there were high rates of mortality during the immunization process in the last 3 weeks of immunization; the percentage of survival among the group of PD-L2−/− mice treated with A fumigatus dramatically decreased compared with that in the relative group of WT mice (day 46: 30% vs 95%; Fig 6, E). Treatment with the anti–IL-9 blocking antibodies significantly increased the survival among PD-L2−/− mice immunized with A fumigatus (day 46: 30% vs 70%) to the extent that there were no further deaths in the anti–IL-9–treated PD-L2−/− mice. Altogether, these results clearly demonstrated that IL-9 regulates the severity of chronic AHR by acting on both lung inflammation and mucus production. Moreover, our data suggest that enhanced severity in PD-L2−/− mice is due to the differential capacity to develop pulmonary TH9 cells.
FIG 6.
IL-9 regulates the severity of chronic AHR. A, Protocol of immunization of WT and PD-L2−/− mice with A fumigatus (A.f.) and antibodies. i.n., Intranasal. B, A group of WT and PD-L2−/− BALB/c mice were immunized intranasally according to our protocol in Fig 6, A. Twenty-four hours after the last immunization (day 46), mice were assessed for the development of AHR by measuring RL and Cdyn. Data are means ± SEMs and representative of 3 experiments (n = 5). *P < .05 and **P < .01, Student t test. C, Lung tissue from A fumigatus (A.f.)–sensitized PD-L2−/− or WT mice were stained with hematoxylin and eosin (H&E; upper panel) and periodic acid–Schiff (PAS; lower panel) and analyzed for cell infiltration and mucus production, respectively. Arrows indicate the production of mucus in the lumen. Original magnification ×40 (inset, original magnification ×100). D, Quantifications of lung histopathology shown as thickness of airway epithelium (left panel), number of inflammatory cells per 250 µm2 of lung tissue (middle panel), and number of goblet cells per 50 µm of epithelium at baseline (right panel). Data are shown as means ± SEMs (n = 5). *P < .05. E, Survival curves of PD-L2−/− and WT mice immunized as described in Fig 6, A. **P < .01, as determined by using a Mantel Cox test. Values are representative of 3 independent experiments starting with 10 mice per group. Ab, Antibody.
DISCUSSION
Here we demonstrate that TH9 cells develop solely in the lungs in a murine model of A fumigatus–induced chronic AHR and that the costimulatory molecule PD-L2 can directly influence T-cell polarization toward the TH9 subset as PD-L2−/− or PD-L2 blocking antibodies enhance IL-9 production by CD4+ T cells in vitro. In addition to this direct role, PD-L2 influences the release of TGF-β and IL-1α in vivo. Modulation of TGF-β is important because it is a key cytokine for the induction of TH9 cells.16–18 Furthermore, using a chronic allergen exposure model, we demonstrated that TH9 cells are detectable in the lungs of sensitized mice and that PD-L2−/− mice have increased numbers of TH9 cells. As a result of the increase in TH9 cell numbers, the IL-9 concentration in the lungs is much higher in PD-L2−/− than in WT mice, resulting in more severe lung inflammation and AHR. Furthermore, neutralizing IL-9 in vivo resulted in a significant reduction in the severity of disease in WT mice and a greater reduction in PD-L2−/− mice.
IL-9 was initially identified more than 20 years ago as a T-cell growth factor and was originally considered a TH2-type cytokine.9,37 However, recent molecular and cellular evidence suggests that T cells that produce IL-9 are a distinct subset of CD4+ T cells and thus can be referred to as TH9 cells.5,16–18 Importantly, these cells do not coexpress IL-4 or IFN-γ cytokines on activation in vitro but can produce high amounts of IL-10 or IL-17. Furthermore, TH9 cells require the expression of PU-1 and IRF4 transcription factors20,35,36 but not the "master regulator" transcription factors associated with TH1 (T-bet), TH2 (GATA-3), TH17 (retinoic acid–related orphan receptor γt), or Treg (forkhead box protein 3) subsets of CD4+ T cells.18 In our experiments PU-1 and IRF4, but not STAT6 and GATA-3, were differentially expressed when PD-L2 was not expressed on antigen-presenting cells and compared with WT bone marrow–derived DCs. Studies in vitro have indicated that polarization to the TH9 subset requires the presence of TGF-β and IL-4 and the absence of IFN-γ.16–18,36,38 TGF-β bioactivity is synergized in the presence of IL-1α or IL-2.17,39 Consistent with previous findings, 40,41 here we show that IL-1α, but not IL-2, can enhance TGF-β–induced IL-9 production from CD4+ T cells in our model. IL-9 can also be produced by TH17 cells, which can proliferate in the absence of TGB-β and the presence of IL-6 in mice.14,15,42 Although these cells were present in the lungs and LNs in our model, TH9 cells were the most abundant source of IL-9 compared with IL-17+IL-9+ cells.
The exact role of costimulatory molecules in the polarization of CD4+ T cells to TH9 cells has yet to be investigated. Nevertheless, our results indicate that PD-L2 expression on antigen-presenting cells acts as a negative regulator of this process. In the absence of PD-L2 (either genetic or through the use of blocking antibodies), the production of IL-9 is significantly increased when CD4+ T cells are activated in optimal TH9 conditions (TGF-β, IL-4, and anti–IFN-γ). PD-L2 is recognized by PD-1 expressed on activated T cells, and although several groups have previously shown that signaling through PD-Ls influences T-cell polarization, the molecular mechanisms underlying this process have yet to be determined. 25,43 Several reports have demonstrated that the lack of PD-L2 expression results in increased AHR and lung inflammation and that PD-L2 expression in the lung protects against the initiation and progression of airway inflammation.25,44–46 PD-L2 is predominantly expressed by antigen-presenting cells, such as DCs and macrophages,31,47–49 but a recent report suggested that PD-L2 can also be expressed by the polarized T-cell subsets TH2, TH17, and Treg cells.50,51 Therefore PD-L2 can affect various cell types involved in asthma pathogenesis. Although the effect of IL-9 can affect IL-13 and IL-10 production by T cells, the effect of PD-L2 on various cells can also contribute to the reduction in IL-13, IL-10, and TGF-β levels. For instance, Matsumoto et al44 demonstrated that PD-L2 is highly expressed on pulmonary DCs and macrophages of sensitized mice, and administration of blocking antibodies against PD-L2 during allergen challenge enhances the AHR and production of TH2 cytokines.45,46 Furthermore, administration of PD-L2–Fc in a mouse model of allergic asthma resulted in increased serum IgE levels and increased eosinophilic and lymphocytic infiltration.52 Finally, engagement of PD-1 to PD-L2 in T cells, especially TH2 cells, downmodulated cytokine responses and cell proliferation.50,51
Despite the evidence for the role of IL-9 in allergic asthma, there have been no animal models demonstrating the in vivo differentiation of TH9 cells in response to chronic antigen exposure. 5,7,21–24 Therefore using chronic respiratory exposure to A fumigatus in the absence of systemic immunization represents a novel model and an important advance to the study of IL-9 in allergic asthma pathogenesis and IL-9–targeted therapies. TH9 cells were only detectable after prolonged exposure (>6 weeks), correlating with the increase in the pulmonary TGF-β concentration. Other models of chronic AHR with OVA or house dust mite also confirmed our observation that a higher level of TGF-β is present in the airways of WT mice after chronic antigen challenges.53 In agreement with the essential role of TGF-β for the development of TH9 cells, it was very recently demonstrated that blocking TGF-β and the TGF-β family member activin A prevents the development of pulmonary TH9 cells in an acute model sensitized with house dust mite.54 Here we found that PD-L2−/− mice had a significantly increased number of pulmonary TH9 cells, which correlated with the increased pulmonary TGF-β concentration. Our data suggest that pulmonary CD4+ T cells will initially polarize to TH2 cells and consequently convert to TH9 in the presence of TGF-β. Several studies suggest that the main sources of TGF-β in the lungs are epithelial cells and macrophages.53,55–58 The presence of IL-9 positively promotes TH2 differentiation and lung inflammation because it was previously demonstrated in a transgenic murine model.8,59
Chronic lung exposure to A fumigatus results in many disease features associated with human allergic asthma.60,61 This includes AHR, lung inflammation, increased mucus secretion, and pulmonary cellular infiltration. Along with promoting TH2-mediated processes, IL-9 contributes to the pathogenesis of asthma by playing important roles in the (1) expansion and differentiation of T cells,13 (2) activation of mast cells and eosinophils, 62,63 and (3) enhancement of the production of IgE from B cells and increase in mucus production from epithelial cells.64 Taken together, this can contribute to the morbidity seen among patients with severe asthma. In agreement with the increased numbers of TH9 cells, we observed that the pulmonary IL-9 concentration and disease severity were augmented in PD-L2−/− mice compared with WT mice. The severity was such that there was a substantial mortality rate in PD-L2−/− mice during the chronic exposure protocol. Interestingly, treatment with neutralizing IL-9 antibodies was sufficient to prevent death and significantly reduce many aspects of disease pathology in PD-L2−/− mice. Neutralization of IL-9 in vivo was also sufficient to reduce AHR, lung inflammation, and mucus secretion in WT BALB/c animals. However, neutralization of IL-9 did not completely abrogate airway inflammation and AHR in comparison with that seen in PBS control animals, and this might be due to the presence of IL-17a, which can mediate aspects of lung inflammation and function.65 Furthermore, our data indicated that TH9 cells do not produce IL-10, confirming the prior reports that production of IL-9 and IL-10 by TH9 cells is independently regulated.35,66
In conclusion, we have demonstrated that after chronic allergen exposure, TH9 cells develop in the lungs but not in the LNs. The restricted development of TH9 cells to the lung might be due to the absence of TGF-β in the LN milieu, whereby the conversion of CD4+ T cells to TH9 cells occurs after migration from the LNs to the lung, where TGF-β is produced by macrophages and epithelial cells. This is the first in vivo demonstration of TH9 polarization in a chronic allergen model, and we provide evidence that TH9 cells are pathogenically relevant for the development of allergic asthma. Furthermore, we have demonstrated that the costimulatory molecule PD-L2 directly and indirectly affects TH9 cell differentiation and normally acts as a negative regulator of this differentiation process. These new insights into TH9 cell development and function will have important implications for the design and development of therapies for chronic allergic asthma.
Supplementary Material
Key messages.
TH9 cells develop only in the lung after chronic exposure to A fumigatus, and blocking IL-9 decreases AHR and lung inflammation.
PD-L2 acts as a negative regulator of this process, and PD-L2−/− mice have enhanced disease severity, resulting in death.
Acknowledgments
We thank Dr Brigitta Stockinger (National Institute for Medical Research, London, United Kingdom) and William De Paolo (University of Southern California) for critical comments on the manuscript. We thank Dr Arlene Sharpe and Dr Gordon Freeman for the generous gift of the PD-L2−/− mice and anti–PD-L2 antibody, respectively.
Supported by the National Institutes of Health Public Health Service Grant R01 AI066020 (to O.A.), the American Lung Association Fellowship (to V.L.), and the Fonds National de la Recherche Scientifique Médicale de Belgique (to J.V.S.).
Abbreviations used
- AHR
Airway hyperreactivity
- Cdyn
Dynamic compliance
- DC
Dendritic cell
- IRF4
Interferon regulatory factor 4
- LN
Lymph node
- OVA
Ovalbumin
- PD-L
Programmed cell death ligand
- RL
Lung resistance
- STAT6
Signal transducer and activator of transcription 6
- Teff
Effector T
- Treg
Regulatory T
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: H. Maazi, B. Khoo, S. Geryak, J. Lam, and O. Akbari have received grants from the National Institutes of Health. J. Van Snick has received grants from Fonds National de la Recherche Scientifique Medicale de Belgique. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erpenbeck VJ, Hohlfeld JM, Discher M, Krentel H, Hagenberg A, Braun A, et al. Increased expression of interleukin-9 messenger RNA after segmental allergen challenge in allergic asthmatics. Chest. 2003;123(suppl):370S. [PubMed] [Google Scholar]
- 6.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000;105:108–115. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- 7.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J Clin Invest. 2002;109:29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 10.Hultner L, Moeller J. Mast cell growth-enhancing activity (MEA) stimulates interleukin 6 production in a mouse bone marrow-derived mast cell line and a malignant subline. Exp Hematol. 1990;18:873–877. [PubMed] [Google Scholar]
- 11.Renauld JC, Vink A, Louahed J, Van Snick J. Interleukin-9 is a major antiapoptotic factor for thymic lymphomas. Blood. 1995;85:1300–1305. [PubMed] [Google Scholar]
- 12.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A. 1988;85:6934–6938. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Snick J, Goethals A, Renauld JC, Van Roost E, Uyttenhove C, Rubira MR, et al. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40) J Exp Med. 1989;169:363–368. doi: 10.1084/jem.169.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 18.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ’reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 19.Bhathena PR, Comhair SA, Holroyd KJ, Erzurum SC. Interleukin-9 receptor expression in asthmatic airways In vivo. Lung. 2000;178:149–160. doi: 10.1007/s004080000018. [DOI] [PubMed] [Google Scholar]
- 20.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Longphre M, Li D, Gallup M, Drori E, Ordonez CL, Redman T, et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest. 1999;104:1375–1382. doi: 10.1172/JCI6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louahed J, Toda M, Jen J, Hamid Q, Renauld JC, Levitt RC, et al. Interleukin-9 upregulates mucus expression in the airways. Am J Respir Cell Mol Biol. 2000;22:649–656. doi: 10.1165/ajrcmb.22.6.3927. [DOI] [PubMed] [Google Scholar]
- 23.Reader JR, Hyde DM, Schelegle ES, Aldrich MC, Stoddard AM, McLane MP, et al. Interleukin-9 induces mucous cell metaplasia independent of inflammation. Am J Respir Cell Mol Biol. 2003;28:664–672. doi: 10.1165/rcmb.2002-0207OC. [DOI] [PubMed] [Google Scholar]
- 24.Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol. 2003;28:286–295. doi: 10.1165/rcmb.4887. [DOI] [PubMed] [Google Scholar]
- 25.Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc Natl Acad Sci U S A. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lombardi V, Stock P, Singh AK, Kerzerho J, Yang W, Sullivan BA, et al. A CD1d-dependent antagonist inhibits the activation of invariant NKT cells and prevents development of allergen-induced airway hyperreactivity. J Immunol. 2010;184:2107–2115. doi: 10.4049/jimmunol.0901208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 30.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 31.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radhakrishnan S, Iijima K, Kobayashi T, Rodriguez M, Kita H, Pease LR. Blockade of allergic airway inflammation following systemic treatment with a B7-dendritic cell (PD-L2) cross-linking human antibody. J Immunol. 2004;173:1360–1365. doi: 10.4049/jimmunol.173.2.1360. [DOI] [PubMed] [Google Scholar]
- 33.Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy. 2011;66:155–162. doi: 10.1111/j.1398-9995.2010.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lombardi V, Singh AK, Akbari O. The role of costimulatory molecules in allergic disease and asthma. Int Arch Allergy Immunol. 2010;151:179–189. doi: 10.1159/000242355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, Goenka S, et al. STAT6-dependent regulation of Th9 development. J Immunol. 2012;188:968–975. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 38.Wong MT, Ye JJ, Alonso MN, Landrigan A, Cheung RK, Engleman E, et al. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol Cell Biol. 2010;88:624–631. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt E, Beuscher HU, Huels C, Monteyne P, van Brandwijk R, van Snick J, et al. IL-1 serves as a secondary signal for IL-9 expression. J Immunol. 1991;147:3848–3854. [PubMed] [Google Scholar]
- 40.Wilhelm C, Turner JE, Van Snick J, Stockinger B. The many lives of IL-9: a question of survival? Nat Immunol. 2012;13:637–641. doi: 10.1038/ni.2303. [DOI] [PubMed] [Google Scholar]
- 41.Uyttenhove C, Brombacher F, Van Snick J. TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur J Immunol. 2010;40:2230–2235. doi: 10.1002/eji.200940281. [DOI] [PubMed] [Google Scholar]
- 42.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deppong C, Juehne TI, Hurchla M, Friend LD, Shah DD, Rose CM, et al. Cutting edge: B and T lymphocyte attenuator and programmed death receptor-1 inhibitory receptors are required for termination of acute allergic airway inflammation. J Immunol. 2006;176:3909–3913. doi: 10.4049/jimmunol.176.7.3909. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- 45.Radhakrishnan S, Iijima K, Kobayashi T, Kita H, Pease LR. Dendritic cells activated by cross-linking B7-DC (PD-L2) block inflammatory airway disease. J Allergy Clin Immunol. 2005;116:668–674. doi: 10.1016/j.jaci.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 46.Radhakrishnan S, Nguyen LT, Ciric B, Flies D, Van Keulen VP, Tamada K, et al. Immunotherapeutic potential of B7-DC (PD-L2) cross-linking antibody in conferring antitumor immunity. Cancer Res. 2004;64:4965–4972. doi: 10.1158/0008-5472.CAN-03-3025. [DOI] [PubMed] [Google Scholar]
- 47.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–3320. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 49.Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8alpha(+)beta(−) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 2012;5:432–443. doi: 10.1038/mi.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messal N, Serriari NE, Pastor S, Nunes JA, Olive D. PD-L2 is expressed on activated human T cells and regulates their function. Mol Immunol. 2011;48:2214–2219. doi: 10.1016/j.molimm.2011.06.436. [DOI] [PubMed] [Google Scholar]
- 51.Lesterhuis WJ, Steer H, Lake RA. PD-L2 is predominantly expressed by Th2 cells. Mol Immunol. 2011;49:1–3. doi: 10.1016/j.molimm.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Oflazoglu E, Swart DA, Anders-Bartholo P, Jessup HK, Norment AM, Lawrence WA, et al. Paradoxical role of programmed death-1 ligand 2 in Th2 immune responses in vitro and in a mouse asthma model in vivo. Eur J Immunol. 2004;34:3326–3336. doi: 10.1002/eji.200425197. [DOI] [PubMed] [Google Scholar]
- 53.Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012;129 doi: 10.1016/j.jaci.2011.12.965. 1000-10.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 56.Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, et al. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 2003;17:2139–2141. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- 57.Gandhi R, Anderson DE, Weiner HL. Cutting Edge: Immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-beta-dependent manner. J Immunol. 2007;178:4017–4021. doi: 10.4049/jimmunol.178.7.4017. [DOI] [PubMed] [Google Scholar]
- 58.Burton OT, Zaccone P, Phillips JM, De La Pena H, Fehervari Z, Azuma M, et al. Roles for TGF-beta and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in nonobese diabetic mice. J Immunol. 2010;185:2754–2762. doi: 10.4049/jimmunol.1001365. [DOI] [PubMed] [Google Scholar]
- 59.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int Immunol. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- 60.Fairs A, Agbetile J, Hargadon B, Bourne M, Monteiro WR, Brightling CE, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182:1362–1368. doi: 10.1164/rccm.201001-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pepys J, Riddell RW, Citron KM, Clayton YM, Short EI. Clinical and immunologic significance of Aspergillus fumigatus in the sputum. Am Rev Respir Dis. 1959;80:167–180. doi: 10.1164/arrd.1959.80.2.167. [DOI] [PubMed] [Google Scholar]
- 62.Hultner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rude E, et al. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9) Eur J Immunol. 1990;20:1413–1416. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 63.Gounni AS, Gregory B, Nutku E, Aris F, Latifa K, Minshall E, et al. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood. 2000;96:2163–2171. [PubMed] [Google Scholar]
- 64.Singhera GK, MacRedmond R, Dorscheid DR. Interleukin-9 and-13 inhibit spontaneous and corticosteroid induced apoptosis of normal airway epithelial cells. Exp Lung Res. 2008;34:579–598. doi: 10.1080/01902140802369372. [DOI] [PubMed] [Google Scholar]
- 65.Mizutani N, Goshima H, Nabe T, Yoshino S. Complement C3a-induced IL-17 plays a critical role in an IgE-mediated late-phase asthmatic response and airway hyperresponsiveness via neutrophilic inflammation in mice. J Immunol. 2012;188:5694–5705. doi: 10.4049/jimmunol.1103176. [DOI] [PubMed] [Google Scholar]
- 66.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol. 2010;185:6795–6801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.