Abstract
Spermine oxidase (SMO), the most recently characterized polyamine metabolic enzyme, catalyzes the direct back-conversion of spermine to spermidine in an FAD-dependent reaction that also yields the byproducts hydrogen peroxide (H2O2) and 3-aminopropanal. These metabolites, particularly H2O2, have been implicated in cytotoxic cellular responses to specific antitumor polyamine analogs, as well as in the inflammation-associated generation of DNA damage. This chapter describes a rapid, sensitive, and inexpensive method for the chemiluminescent measurement of SMO (or alternatively, N1-acetyl polyamine oxidase, APAO) enzyme activity in cultured cell lysates, without the need for radioactive reagents or the use of high performance liquid chromatography (HPLC). Specifically, H2O2 production by SMO is coupled to chemiluminescence generated by the horseradish peroxidase-catalyzed oxidation of luminol. Detailed protocols for preparation of reagents, harvesting cell lysates, generation of a standard curve, assaying of samples, and calculation of SMO enzyme activity are presented.
Keywords: Spermine oxidase, Polyamine catabolism, Hydrogen peroxide, Chemiluminescence
1. Introduction
While the enzymatic biosynthesis of polyamines is irreversible, a group of tightly regulated, complementary enzymes facilitate the back-conversion of the higher polyamines spermine and spermidine to spermidine and putrescine, respectively (1). The first pathway involves acetylation of spermine or spermidine to N1-acetylspermine or N1-acetylspermidine by spermidine/spermine N1-acetyltransferase (SSAT) (2). The acetylated polyamine then undergoes oxidation to spermidine or putrescine by acetyl polyamine oxidase (APAO), with generation of hydrogen peroxide (H2O2) as a byproduct (3, 4). Alternatively, spermine oxidase (SMO), the most recently characterized polyamine catabolic enzyme (5, 6), catalyzes the direct back-conversion of spermine to spermidine, 3-aminopropanal, and H2O2, without an acetylated intermediate. This stoichiometric production of H2O2 by SMO facilitates quantification of SMO enzyme activity by the chemiluminescent method described in this chapter.
The production of H2O2 by SMO and APAO activity has been associated with downstream oxidative damage and has important implications in the etiology and treatment of human diseases (7–9). The cytotoxic effect of numerous polyamine analogs under investigation as chemotherapeutic agents has been ascribed to the generation of reactive oxygen species, resulting from massive upregulation of polyamine catabolism through the SSAT/APAO pathway (10–12). Recent studies, however, suggest that SMO is the more critical source of H2O2 with respect to analog-induced cell death (13, 14). Furthermore, oxidative damage following modest upregulation of SMO in epithelial tissues by inflammatory stimuli is hypothesized to contribute to inflammation-associated carcinogenesis (reviewed by (1)). The following protocol, based on a method originally reported by Fernandez et al. (15), has been altered to provide greater sensitivity, allows for real-time analysis of polyamine oxidase activity (SMO or APAO) (16, 17), and provides the potential for modification to high-throughput applications, such as the screening of potential antitumorigenic compounds for induction of oxidase activity in cancer cell lysates.
In preparation for the measurement of SMO activity, cells are harvested in glycine buffer and lysed by freezing at −80°C. The assay reaction mix contains cell lysate, horseradish peroxidase (HRP), luminol, pargyline (monoamine oxidase inhibitor), and aminoguanidine (diamine oxidase inhibitor), in a glycine buffer at pH 8.0. This reaction is incubated briefly at 37°C, followed by addition of spermine (substrate for SMO) and integration of chemiluminescence. The slightly basic pH (8.0) permits the HRP to catalyze the oxidation of luminol in a reaction coupled to the production of H2O2 by SMO. Upon oxidation of luminol, a light-emitting molecule (excited 3-aminophthalate ion) is produced (Fig. 1). The amount of chemiluminescence measured in a sample corresponds to the relative oxidation of spermine by SMO in the cell lysate.
Fig. 1.
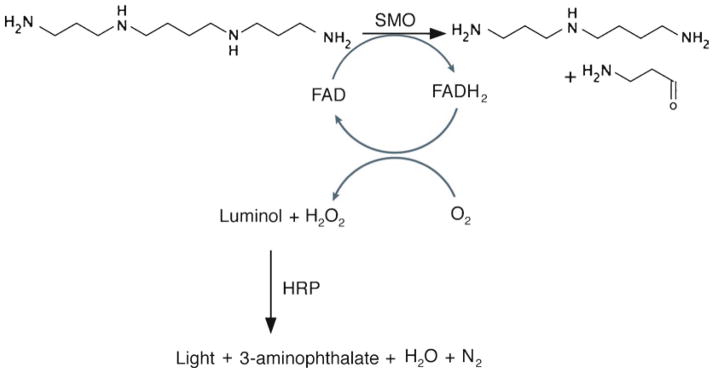
Schematic illustrating the coupled enzyme reactions that comprise the enzyme activity assay described in this chapter. First, spermine oxidase (SMO) catalyzes the FAD-dependent oxidation of spermine to spermidine, 3-aminopropanal, and H2O2. Second, in the presence of this H2O2, luminol is oxidized by horseradish peroxidase (HRP), resulting in production of chemiluminescence.
2. Materials
2.1. Reagents That May Be Prepared in Advance and Stored at −20°C
Aminoguanidine (Sigma #A7009; FW: 123.1 g/mol). Dissolve 123.1 mg/10 mL HPLC-grade water to yield 100 mM stock solution; aliquot in 1.5 mL tubes and storeat −20°C.
Luminol (3-aminophthalhydrazide; Fluka Biochemika #09253; FW: 177.16 g/mol). Dissolve 177.2 mg/10 mL dimethyl sulfoxide (DMSO) to yield 100 mM stock solution; aliquot in light-protected 1.5 mL tubes and store at −20°C.
Pargyline (N-methyl-N-(2-propynyl)benzylamine hydrochloride; Sigma #P8013). Dissolve in HPLC-grade water to yield 2.3 mg/mL stock solution; aliquot in 1.5 mL tubes and store at −20°C.
Spermine tetrachloride (see Note 1) (Sigma #S2846; FW: 348.19 g/mol). Dissolve 52.2 mg/10 mL HPLC-grade water to yield 15 mM stock solution; aliquot in 1.5 mL tubes and store at −20°C. On the day of the assay, dilute to a 1.5 mM working stock with HPLC-grade water.
2.2. Reagents That Must Be Prepared Fresh for Each Assay
Glycine buffer (J.T. Baker# 4059-02; FW: 75.07 g/mol). Dissolve 375.4 mg/10 mL HPLC-grade water to yield 0.5 M stock solution and adjust pH to 8.0 with NaOH. Make a further dilution to 0.083 M (1 part 0.5 M glycine, pH 8.0 plus 5 parts HPLC-grade water) for harvesting cells and enzyme assay blank (see Note 2).
HRP (Roche #10814407001, EIA grade, lyophilized). Dissolve in HPLC-grade water to yield a 0.4 mg/mL solution (see Note 3).
H2O2 (3% w/v solution) (see Note 4).
2.3. Common Laboratory Reagents and Disposables
Bradford protein assay reagent (Bio-Rad #500-0006).
Cell lifters (Fisher #08-773-1).
Dimethyl sulfoxide (DMSO; Sigma #D1435).
HPLC-grade water (J.T. Baker #4218-03).
Light-shielded (1.5, 15, 50 mL) and standard microcentrifuge tubes (1.5 mL).
Luminometer cuvettes (12 × 75 mm; BD Biosciences #556862).
3. Methods
3.1. Harvesting of Adherent Cells and Preparation of Cell Lysates
Remove culture media, rinse adherent cells with ice-cold 1× PBS, aspirate PBS.
Cover cells with 0.083 M glycine buffer, pH 8.0 (approximately 300 μL/well of 6-well dish or 700 μL/25 cm2 flask), and place in −80°C freezer to lyse cells (see Note 5).
On day of assay, thaw flasks or dishes on ice, scrape cells with disposable cell lifters, transfer lysates to 1.5 mL tubes, and vortex 10s.
Centrifuge cell lysates (10 min, >12,000 × g, 4°C) and keep lysates on ice at all times during assay.
3.2. Prepare Assay Master Mix and Determine Hydrogen Peroxide Standard Curve
Preheat water bath containing a rack suitable for holding luminometer cuvettes to 37°C.
Thaw spermine, aminoguanidine, and pargyline on ice and thaw luminol at room temperature.
In a light-shielded conical tube on ice, prepare the assay reaction mix according to the chart in Table 1 for the desired number of reactions (see Notes 3 and 6) and mix by vortexing.
-
In light-shielded 15 mL tubes, serially dilute H2O2 in HPLC-grade water:
Dilution A: add 100 μL H2O2 (3% w/v solution, 0.882 M) to 8.72 mL HPLC-grade water to yield a 10 mM solution. Mix gently by inversion (see Note 7).
Dilution B: add 100 μL Dilution A to 10 mL HPLC-grade water to yield a 100 μM solution, and mix gently by inversion.
Dilution C: add 100 μL Dilution B to 10 mL HPLC-grade water to yield a 1 μM solution, and mix gently by inversion.
-
Prepare a series of solutions containing increasing concentrations of H2O2 (0–100 pmol/100 μL) for production of a standard curve relating H2O2 concentration to RLU, in a total volume of 300 μL:
0 pmol: 1,000 μL HPLC-grade water.
20 pmol: 800 μL HPLC-grade water + 200 μL Dilution C from step 3.2.4.
40 pmol: 600 μL HPLC-grade water + 400 μL Dilution C.
60 pmol: 400 μL HPLC-grade water + 600 μL Dilution C.
80 pmol: 200 μL HPLC-grade water + 800 μL Dilution C.
100 pmol: 1,000 μL Dilution C.
Pipette 200 μL reaction mix into a luminometer cuvette and incubate for 2 min in 37°C water bath.
Remove cuvette from water bath, remove condensation with Kimwipe, pipette 100 μL of the “0 pmol” solution into the reaction mix, and immediately read the luminescence for a 20 s integration time (see Note 8).
Repeat steps 3.2.6 and 3.2.7 to generate a standard curve with triplicate measurements of reactions containing 0, 20, 40, 60, 80, and 100 pmol H2O2 (18 total reactions) (see Note 9).
Using Microsoft Excel or other preferred method, fit a trend line to the standard curve data and calculate the r2 value to validate the assay reaction mix (Fig. 2a) and for interpolation of raw sample RLU data.
Table 1.
SMO enzyme activity assay setup and workflow
| Reagent | Stock concentration | Final concentration | Per reaction (μL) | Per 100 reactions |
|---|---|---|---|---|
| Glycine, pH 8.0 | 0.5 M | 0.083 M | 50 | 5 mL |
| Aminoguanidine | 100 mM | 1 mM | 3 | 300 μL |
| Pargyline | 2.3 mg/mL | 0.019 mg/mL | 2.5 | 250 μL |
| Luminol | 100 mM | 0.017 mM | 0.05 | 5 μL |
| HPLC-grade water | 94.5 | 9.45 mL | ||
| Horseradish peroxidase | 0.4 mg/mL | 0.067 mg/mL | 50 | 5 mL |
|
| ||||
| Reaction mix | 200 | |||
| Cell lysate or 0.083 M glycine blank | 50 | |||
|
| ||||
| Vortex cuvette briefly and incubate for 2 min in 37 °C water bath | ||||
| Spermine | 1.5 mM | 0.25 mM | 50 | |
| Final reaction volume | 300 | |||
|
| ||||
| Inject substrate and integrate luminescence for 40 s | ||||
The necessary assay reagents are indicated above as well as calculations for preparing a master mix for 100 reactions (see Subheading 2 for preparation instructions). The general assay scheme is also summarized (see Subheading 3 for detailed protocol)
Fig. 2.
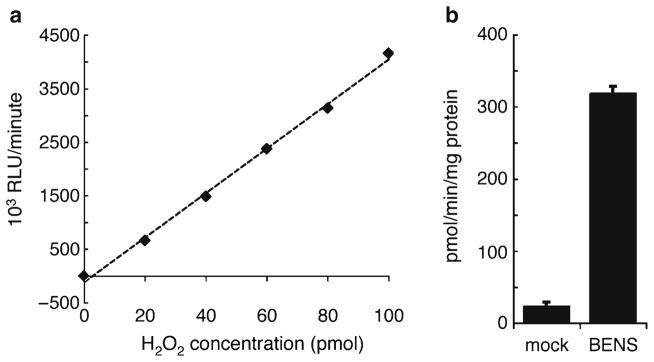
(a) Representative plot of H2O2 standard curve displaying H2O2 concentration (0–100 pmol in 300 μL reaction; x-axis) versus 103 RLU/min (y-axis). A best-fit line is also plotted (r 2 >0.99). (b) Representative data. A549 lung adenocarcinoma cells were treated for 24 h with or without 10 μM of the SMO-inducing polyamine analog, bis(ethyl)-norspermine (BENS). Cell lysate was harvested and SMO enzyme activity measured as described in this chapter.
3.3. Determination of SMO Enzyme Activity from Cell Lysates
If luminometer is equipped with an autoinjector, load and prime with 1.5 mM spermine substrate solution. Set the luminometer program to inject 50 μL/sample, followed by a 10 s delay and a 40 s integration of luminescence. Alternatively, the substrate can be manually pipetted into each cuvette as in step 3.2.3.
For blank, pipette 200 μL reaction mix into a luminometer cuvette, add 50 μL 0.083 M glycine buffer, pH 8.0, vortex briefly, and incubate 2 min in a 37°C water bath.
Remove cuvette from water bath, remove condensation with Kimwipe, and read luminescence as described in step 3.3.1.
Repeat steps 2 and 3 as necessary to measure blank and each cell lysate (50 μL supernatant from step 4) in triplicate. Alternatively, this protocol may be amended to assay enzyme kinetics of purified, recombinant SMO (see Note 10; Fig. 3).
Determine total protein concentration in each cell lysate supernatant using Bradford or other preferred method.
Calculate SMO activity as pmol H2O2 per min per mg protein, based on interpolation on the H2O2 standard curve (Fig. 2b).
Fig. 3.
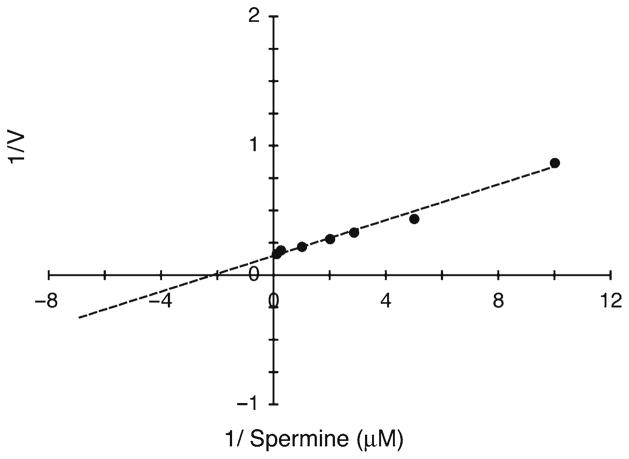
Data generated using alterations to the described protocol, as described in Note 10. Purified, recombinant spermine oxidase protein was used with the current protocol for the determination of kinetic properties. Plot depicts Lineweaver–Burk transformation (r 2 >0.99) of SMO/PAOh1, assayed in the presence of increasing concentrations of spermine (0–250 μM).
Footnotes
Alternatively, to measure the enzyme activity of APAO instead of SMO, use N1-acetylspermine (Fluka Biochemika #01467; FW: 353.76 g/mol) as the substrate.
0.083 M Glycine buffer may be stored at 4°C for several weeks exclusively for use in harvesting cells. However, for optimal results, the 0.5 M glycine to be used in the reaction mix should be made fresh on each assay date.
Prepare the HRP solution only after all other components have been added to the assay reaction mix, immediately prior to proceeding with the assay protocol. Working quickly, dissolve HRP in HPLC-grade water to yield a 0.4 mg/mL solution, add the appropriate amount to the assay reaction mix on ice, and proceed with assay.
While 30% w/v H2O2 solutions are available from chemical vendors (i.e., Sigma-Aldrich #216763), we have found 3% w/v solutions sold in supermarkets and drug stores to be preferable. These solutions are significantly less expensive, yield equivalent results, and can be replaced with a fresh bottle after several uses.
Alternatively, following trypsinization of adherent cells, or for suspension cells, cells can be centrifuged, washed in ice-cold 1× PBS, resuspended in 0.083 M glycine, and then lysed at −80°C. In this case, thaw tubes containing cell lysates on ice and proceed with the protocol at step 3.1.4. Note: due to the presence of divalent metal cations in tissue homogenates, this assay protocol is not suitable for the measurement of oxidase activity in tissue samples.
All samples should be assayed in triplicate; allow at least 18 reactions for the standard curve, 3 reactions for the blank, and three times the number of cell lysates to be assayed.
Whenever working with H2O2 solutions, it is critical to work quickly and minimize the amount of time that the tubes containing H2O2are open and exposed to air.
It is imperative to develop efficient and consistent timing and actions when adding the H2O2 to the reaction mix. For best results, we recommend placing the cuvette containing the 200 μL reaction mix into the luminometer, then pipetting the 100 μL H2O2 solution directly into the reaction mix (not down the side of the cuvette), and immediately starting the luminescence measurement. If this technique is followed, it is not necessary or advantageous to vortex the reaction mix + H2O2 mixture before reading.
After becoming comfortable with the mechanics of the assay technique, it is possible to process samples (for both the standard curve and experimental cell lysates) more quickly using a staggered strategy. After starting the first 2-min incubation, wait about 45 s and then start preparing the second sample.
For assay of purified SMO (or APAO) enzyme, the MAO and DAO inhibitors (pargyline and aminoguanidine) can be omitted and replaced with H2O. Purified enzyme is diluted to 2 ng/μL in 0.083 M glycine buffer, pH 8.0, then 50 μL is added to the reaction mix for each reaction, incubated 2 min at 37°C, substrate is added, and chemiluminescence is integrated over 20 s (16, 17).
References
- 1.Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J. 2009;421:323–338. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casero RA, Jr, Pegg AE. Spermidine/ spermine N1-acetyltransferase – the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 3.Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J. 2003;370:19–28. doi: 10.1042/BJ20021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu T, Yankovskaya V, McIntire WS. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, n1-acetylated polyamine oxidase. J Biol Chem. 2003;278:20514–20525. doi: 10.1074/jbc.M302149200. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Devereux W, Woster P, Stewart T, Hacker A, Casero R., Jr Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. [PubMed] [Google Scholar]
- 6.Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J. 2002;367:665–675. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA, Jr, Wilson KT. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA, Jr, Wilson KT. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin AC, Jadallah S, Toubaji A, Lecksell K, Hicks JL, Kowalski J, Bova GS, De Marzo AM, Netto GJ, Casero RA., Jr Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate. 2008;68:766–772. doi: 10.1002/pros.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha HC, Woster PM, Yager JD, Casero RA., Jr The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci U S A. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res. 2001;61:6437–6444. [PubMed] [Google Scholar]
- 12.Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, Woster PM, Casero RA. Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother Pharmacol. 2003;52:383–390. doi: 10.1007/s00280-003-0662-4. [DOI] [PubMed] [Google Scholar]
- 13.Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA., Jr Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem. 2005;280:39843–39851. doi: 10.1074/jbc.M508177200. [DOI] [PubMed] [Google Scholar]
- 14.Pledgie-Tracy A, Billam M, Hacker A, Sobolewski MD, Woster PM, Zhang Z, Casero RA, Davidson NE. The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother Pharmacol. 2010;65(6):1067–1081. doi: 10.1007/s00280-009-1112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez C, Sharrard RM, Talbot M, Reed BD, Monks N. Evaluation of the significance of polyamines and their oxidases in the aetiology of human cervical carcinoma. Br J Cancer. 1995;72:1194–1199. doi: 10.1038/bjc.1995.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster P, Casero R., Jr Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304:605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 17.Murray-Stewart T, Wang Y, Goodwin A, Hacker A, Meeker A, Casero RA., Jr Nuclear localization of human spermine oxidase isoforms – possible implications in drug response and disease etiology. FEBS J. 2008;275:2795–2806. doi: 10.1111/j.1742-4658.2008.06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]