Abstract
In this work, we identified a high affinity and potency metallocene-containing triazole peptide conjugate that suppresses the interactions of HIV-1 envelope gp120 at both its CD4 and co-receptor binding sites. The ferrocene-peptide conjugate, HNG-156, was formed by an on-resin copper-catalysed [2 + 3] cycloaddition reaction. Surface plasmon resonance interaction analysis revealed that, compared to a previously reported phenyl-containing triazole conjugate HNG-105 (105), peptide 156 had a higher direct binding affinity for several subtypes of HIV-1 gp120 due mainly to the decreased dissociation rate of the conjugate-gp120 complex. The ferrocene triazole conjugate bound to gp120 of both clade A (92UG037-08) and clade B (YU-2 and SF162) virus subtypes with nanomolar KD in direct binding and inhibited the binding of gp120 to soluble CD4 and to antibodies that bind to HIV-1YU-2 gp120 at both the CD4 binding site and CD4-induced binding sites. HNG-156 showed a close-to nanomolar IC50 for inhibiting cell infection by HIV-1BaL whole virus. The dual receptor site antagonist activity and potency of HNG-156 make it a promising viral envelope inhibitor lead for developing anti-HIV-1 treatments.
Keywords: HIV-1 gp120, entry inhibitors, peptide triazoles, surface plasmon resonance, cell infection
INTRODUCTION
Acquired immunodeficiency syndrome (AIDS), the global epidemic caused by HIV-1 infection, has created an urgent need for new classes of antiviral agents (WHO/UNAIDS, December 2006). Entry inhibitors and membrane fusion inhibitors are gaining momentum for antiretroviral therapies, especially given their potential use in microbicide formulations as well as post-infection therapy (Veazey et al., 2005; Ketas et al., 2007). Currently, the development of effective HIV entry inhibitors is focused mainly on natural ligands (Munk et al., 2003; Gallo et al., 2006), monoclonal antibodies (Zhang et al., 2003; Cardoso et al., 2005; Zhang and Dimitrov, 2007), synthetic compounds obtained by high-throughput screening of compound libraries (Ferrer and Harrison, 1999; Lin et al., 2003; Zhao et al., 2005) and compounds derived by structure-guided rational design to interfere with the gp120 interaction with CD4 or co-receptor interaction (Vita et al., 1999; DeMarco et al., 2006). The recently introduced fusion inhibitor Enfuvirtide (Fuzeon, T-20) exemplifies the potential of entry inhibitors to provide an expanded range of treatment options for anti-HIV therapy (Moore and Doms, 2003; Tsibris and Kuritzkes, 2007).
Recently, we reported a peptide inhibitor generated from the combination of peptide and click chemistry (Gopi et al., 2006). Click-derived triazoles were constructed from both aryl and alkyl acetylenes on an internally incorporated azidoproline, present at position 6, within a variant of the previously reported peptide 12p1 (RINNIPWSEAMM) (Ferrer and Harrison, 1999; Biorn et al., 2004). Using this strategy, we identified a conjugated peptide HNG-105 (105), through reaction with phenylacetylene, that exhibited a sub-micromolar affinity to gp120 that was two orders of magnitude greater than the affinity of the initial 12p1 (Gopi et al., 2006). Peptide 105 showed broad spectrum inhibition of the molecular interactions of CD4 with gp120s derived from viruses of clades A, B, C, D and CRF07_BC (Cocklin et al., 2007). In addition, 105 inhibited infection of recombinant luciferase-containing viruses pseudotyped with envelopes from clades A, B and C (Cocklin et al., 2007). From this starting point, we examined the structure–activity relationships between structural variation of the substituted triazole and affinity for gp120 (Gopi et al., 2008). This has led us to a ferrocene-conjugated peptide derivative that has strikingly potent affinity and inhibition activity against HIV-1 gp120. We report here the characteristics of the gp120 antagonist activity of this inhibitor and in particular its ability to inhibit HIV-1 Env interactions with multiple ligands of the gp120 molecule.
MATERIALS AND METHODS
Materials
All Fmoc-protected amino acids, HBTU, HOBt, and Hyp(OMe)·HCl were purchased from Novabiochem. Rink amide resin was obtained from Applied Biosystems. Solvents and other chemicals were purchased from Aldrich or Fisher and used without further purification. Fmoc-cis-4-azidoproline was synthesized starting with commercially available Hyp(OMe)·HCl. HIV-1 strain BaL (catalogue no. 510) was obtained from the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID). This strain of HIV-1, which was prepared using primary human cells of monocytic origin, uses CCR5 as its co-receptor.
Peptide synthesis and click conjugation
Peptides were synthesized by manual solid phase synthesis using Fmoc chemistry on Rink amide resin at 0.1 mmol scale. The [3 + 2] cycloaddition reaction of azide and terminal alkynes was carried out by an on-resin method (Gopi et al., 2006). The peptides were cleaved from the resin by using a cocktail mixture of 95:2:2:1 trifluoroacetic acid/1,2-ethanedithiol/water/thioanisole. Crude peptides were purified by using a C4 preparative column by HPLC (Beckmann Coulter) with gradient between 95:5:0.1 and 5:95:0.1 water/acetonitrile/trifluoroacetic acid. Peptide purity and mass were confirmed by using a C18 analytical column (HPLC) and by MALDI-TOF, respectively.
Protein reagents
HIV-1YU2 gp120 was produced as described previously in Drosophila S2 cells (Biorn et al., 2004; Pancera et al., 2005). Cells were spun down and supernatant sterile filtered. Supernatant was purified over an F105-antibody column (NHS-activated Sepharose, Amersham; mAb F105 coupled according to manufacturer’s instructions). HIV-1YU2 was eluted from the column with glycine buffer, pH 2.4, dialysed against PBS and frozen at −80°C. sCD4 was expressed in CHO cells in a hollow fibre bioreactor. Supernatant from the hollow fibre bioreactor was purified with an SP-column and bound fractions were then run over a Q-column. Unbound material was concentrated and analysed by SDS-PAGE. The gp120 proteins from HIV-1SF162 and HIV-192UG037-08 were used in previous inhibitor binding studies (Cocklin et al., 2007); HIV-1SF162 gp120 was obtained through NIH AIDS Research and Reference Reagent Program from DAIDS and NIAID, while HIV-192UG037-08 gp120 was a gift from Dr James Arthos as reported in Cocklin et al. (2007). The following monoclonal antibodies were obtained through the NIH AIDS Research and Reference Reagent Program: 2G12 from Dr Hermann Katinger; F105 from Dr Marshall Posner and Dr Lisa Cavacini and b12 from Dr Dennis Burton and Carlos Barbas.
Optical biosensor binding assays
All surface plasmon resonance (SPR) experiments were performed on a Biacore 3000 optical biosensor (Biacore, Inc., Uppsala, Sweden). A CM5 sensor chip was derivatized by amine coupling by using N-ethyl-N-(3-dimethylaminopropyl)carbodiimide/N-hydroxy-succinimide (Ishino et al., 2006) with either HIV-1YU2 gp120, soluble CD4, mAb 17b Fab, or as a control surface mAb 2B6R (antibody to human IL-5 receptor α). For direct binding experiments, HIV-1YU2 gp120 was immobilized on the surface (~4000 RU); peptide analytes in PBS buffer were passed over the surface at a flow rate of 50 μL/min. with 5 min. association phase and 5 min dissociation phase. The gp120 proteins from HIV-1SF162 and HIV-192UG037-08 were immobilized on the sensor chip surface and direct binding analyses carried out, similarly as described above for HIV-1YU2 gp120. For competition experiments, ligands (sCD4, 17b mAb, b12, and F105) were immobilized on a surface with a density of approximately 2000 RU. The indicated analytes were passed over the surfaces at a flow rate of 50 μL/min with 2.5 min association phase and 2.5 min dissociation phase. Surfaces were regenerated by using 35 mM NaOH and 1.3 M NaCl for sCD4 and HIV-1YU2 gp120 surfaces, and 10 mM HCl for 17b surface.
Data analysis was performed using BIAEvaluation® 4.0 software (GE Healthcare, Uppsala, Sweden). The responses of a buffer injection and responses from the control surface to which the mAb 2B6R was immobilized, were subtracted to account for nonspecific binding. Experimental data were fitted to a simple 1:1 Langmuir binding model with a parameter included for mass transport. The average kinetic parameters (association [ka] and dissociation [kd] rates) generated from a minimum of four datasets were used to define equilibrium association (KA) and dissociation constants (KD).
Inhibition of HIV-1 infection using whole virus assay
P4-CCR5 MAGI cells (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID) were cultured in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% fetal bovine serum (FBS), sodium bicarbonate (0.05%), antibiotics (penicillin, streptomycin and kanamycin, 40 μg/mL each), and puromycin (1 μg/mL) (Charneau et al., 1994). P4-CCR5 cells were seeded at a density of 1.2 × 104 cells/well in a 96-well plate approximately 18 h prior to experiment. The cells were then incubated for 2 h with HIV-1BaL (2.4 ng/mL final concentration) in the presence of HNG-156 (156), or dextran sulphate as a positive control. After the 2 h incubation, cells were washed, cultured for an additional 46 h, and subsequently assayed for HIV-1 infection using the Galacto-Star®-Galactosidase Reporter Gene Assay System for Mammalian Cells as per manufacturer’s instructions (Applied Biosystems, Bedford, MA). Infectivity remaining is expressed relative to mock-treated, HIV-1-infected cells. Data were fit to a sigmoidal inhibition model using Prism GraphPad software to yield values for IC50, the concentration at which exposure to the compound resulted in a 50% decrease in infectivity relative to mock-treated, HIV-1-infected cells. The above assay design was similar to that used in prior studies (Krebs et al., 1999).
In vitro cytotoxicity
P4-CCR5 cells were seeded at a density of 4 × 104 cells/well in a 96 well plate approximately 18 h prior to experiment. Cells were then exposed to the indicated concentrations of 156 and dextran sulphate for 2 h. The cells were subsequently washed and assessed for viability using a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay of viability (previously described in Krebs et al. (1999). Concentrations were tested in triplicate in two independent assays.
RESULTS AND DISCUSSION
Identification of the high affinity HIV-1 gp120 synthetic peptide inhibitor HNG-156
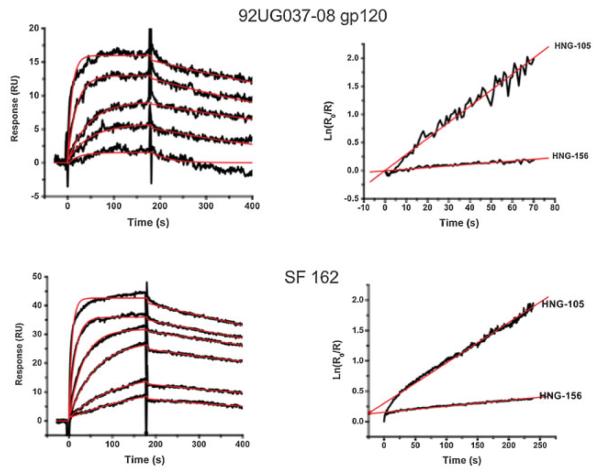
A Biacore® 3000 SPR optical biosensor was used to assess both the direct interactions of peptide conjugates with various subtypes of gp120 and their inhibitory effects on interactions of gp120 at its CD4 and co-receptor binding sites (Zhang et al., 1999; Dowd et al., 2002; Biorn et al., 2004; Cocklin et al., 2007). We evaluated synthetic conjugate variants of the recently reported 105, focusing on more bulky hydrophobic groups to replace the phenyl group substituent on the triazole (Gopi et al., 2008). The binding and dual inhibition activities of the peptide conjugate with the bulky ferrocene triazole (Figure 1), herein denoted 156, were improved strongly over those of 105. To test for conjugate binding, gp120 was covalently immobilized on a CM5 biosensor chip (5000 RU) using standard amine coupling. Interactions were measured by injecting various concentrations of 156 over the immobilized gp120 sensor chip in PBS buffer. 156 peptide was found to bind to a number of HIV-1 gp120s including those from several subtypes. The most striking improvement in affinity observed to date was for the gp120 derived from the clade A virus HIV-1 92UG037-08. Here, the KD was approximately two orders of magnitude lower (greater affinity) than that for 105. Representative sensorgrams depicting the direct binding of 156 to HIV-192UG037-08 gp120 binding are shown in Figure 2 (top left). Shown in Figure 2 (top right) is the comparison of the off-rate kinetic analysis for binding of both 156 and 105 to HIV-1 92UG037-08 gp120. Similar data are shown in Figure 2 (bottom) for HIV-1SF162 gp120. SPR-derived kinetic interaction parameters, determined for the set of HIV-1 gp120 variants tested, are given in Table 1. The SPR binding data reveal that the high binding affinity of 156 versus 105 is due largely to a slower off-rate. This was observed with several subtypes of HIV-1 gp120. Interestingly, a recent study correlating the kinetics of SIV gp120 interaction to monoclonal antibodies with neutralization efficiencies found that a slower dissociation rate correlated with enhanced neutralization capacity of a given mAb in viral assays (Steckbeck et al., 2005). As such, improving this feature both in this derivative, and future peptide conjugates and their truncates, could be advantageous for antagonist design.
Figure 1.
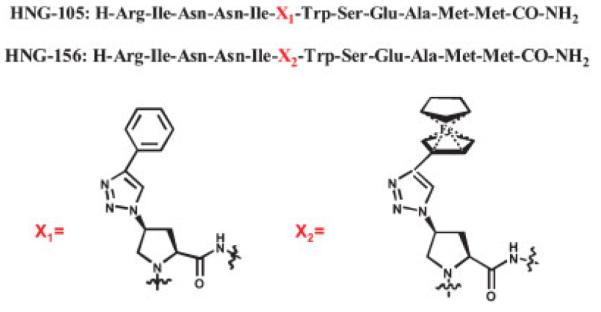
Sequences of conjugate peptides 105 and 156. X1 = (2S, 4S)-4-(4-phenyl-1H-1, 2, 3-triazol-1-yl) pyrrolidine-2-carboxylic acid and X2 = (2S, 4S)-4-(4-ferrocenyl-1H-1, 2, 3-triazol-1-yl) pyrrolidine-2-carboxylic acid. The general procedures for synthesis of triazole conjugates of 12p1 have been reported (Gopi et al., 2006, 2008). The specific synthesis protocol for 156 is given in the text.
Figure 2.
Direct binding of 156 to surface-immobilized HIV-192UG037-08 gp120 (top) and HIV-1SF162 gp120 (bottom). Left: Sensorgrams depicting the interaction of 156 with gp120 at 156 concentrations of 0.125, 0.25, 0.5, 1, 2, and 4 μM. Black lines indicate experimental data, whereas red lines indicate fitting to a 1:1 Langmuir binding model with a parameter included for mass transport. Right: comparison of the linearized dissociation rates of 105 and 156.
Table 1.
Kinetic parameters derived by SPR for the interactions of 156 to different gp120s determined by direct interaction SPR analysis, with comparison to those results obtained for 105
| Peptide | HIV-1 Env | ka(1/Ms) | kd (1/s) | KD (M × 10−9) |
|---|---|---|---|---|
| 12p1 | YU2 (B) | 1.36×104 | 0.072 | 5400 |
| 105 | YU2 (B) | 3.39±0.22x 105 | 7.79±3.2×10−3 | 22.9 |
| 156 | YU2(B) | 1.29±0.2×105 | 9.55±0.15×10−4 | 7. 4 |
| 105 | 92UG037-08 (A) | 1.63±0.8×104 | 3.0±2×10−2 | 1840 |
| 156 | 92UG037-08 (A) | 1.06±0.2x105 | 1.73±0.8×10−3 | 16.3 |
| 105 | SF162 (B) | 1.05±0.3x105 | 7.4±0.18×10−3 | 70.5 |
| 156 | SF162(B) | 1.22±0.3×105 | 1.1±0.4×10−3 | 9.0 |
These data are taken from Gopi et al. (2008). Designations in parentheses are clades of HIV-1 from which gp120 subtypes were derived. The peptide denoted “12p1” is the parent peptide (RINNIPWSEAMM) (Ferrer and Harrison 1999; Biorn et al., 2004), from which peptides HNG-105 and HNG-156 were derived using the click conjugation.
Suppression of binding at multiple interaction sites of HIV-1 gp120 by HNG-156
Competition SPR analysis was used to measure the inhibitory effects of 156 on the binding of gp120 to CD4, to the gp120 CD4-binding site (CD4bs) monoclonal antibodies (Yuan et al., 2006) F105 and IgG b12, and to the CD4-induced (CD4i) mAb 17b (Kwong et al., 1998), which shares its epitope with the co-receptor binding site of gp120. The analyte HIV-1YU2 gp120 (100 nM) was passed over the immobilized protein ligands sCD4, mAb 17b, mAb b12, mAb F105 and control mAb 2B6R in the absence or presence of increasing amounts of 156. In control experiments, 156 exhibited no direct binding to any of the immobilized protein ligands (data not shown). Figure 3 shows that increasing the concentration of 156 suppressed gp120 binding to all of the protein ligands tested that recognize receptor and co-receptor sites. In contrast, 156 did not show any effect on the binding of another broadly neutralizing antibody, 2G12 (Trkola et al., 1996), which binds to a discontinuous epitope involving glycosylation sites in the envelope protein (data not shown).
Figure 3.
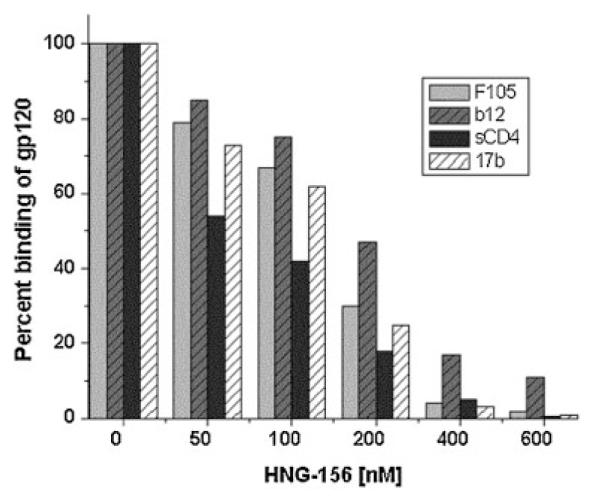
Inhibition by 156 of the binding of HIV-1YU2 gp120 to CD4bs and CD4i antibodies and to soluble CD4. mAb F105, IgG b12, sCD4 and mAb 17b were immobilized on the surface of biosensor CM5 chip. A constant concentration of HIV-1YU2 gp120 was passed over these immobilized ligands in the presence of increasing concentrations (0–600 nM) of 156. The per cent (%) binding of gp120 to immobilized antibodies is plotted against the concentration of the peptide. 156 inhibited the binding of HIV-1YU2 gp120 to F105, b12, CD4 and 17b at IC50 values of 131 (±30), 200 (±42), 94 (±38) and 137 (±39) nM, respectively.
Inhibition of HIV-1 cell infection by whole virus
In vitro experiments were conducted to measure the anti-HIV-1 activity of 156. This assay was carried out using the subtype B strain HIV-1BaL (R5 phenotype), as this was the strain used previously to measure antiviral activity for the parent peptide 12p1 and derivatives (McFadden et al., 2007; Gopi et al., 2008). We found (Figure 4) that 156 exhibited an IC50 96 nM. This potency was close to three orders of magnitude more than that (48 μM) measured previously for 12p1 (Gopi et al., 2008; McFadden et al., 2007) and 15-fold more than that (1.43 μM) measured for 105 (Gopi et al., 2008). Importantly, 156 had no effect on P4-CCR5 cell viability when assessed at concentrations as high as 0.1 mg/mL (59 μM). The strong potency in the HIV-1BaL infection assay argues for follow-up studies, currently under way, to evaluate the breadth of antiviral potency with both whole virus and pseudoviral cell infection assays.
Figure 4.
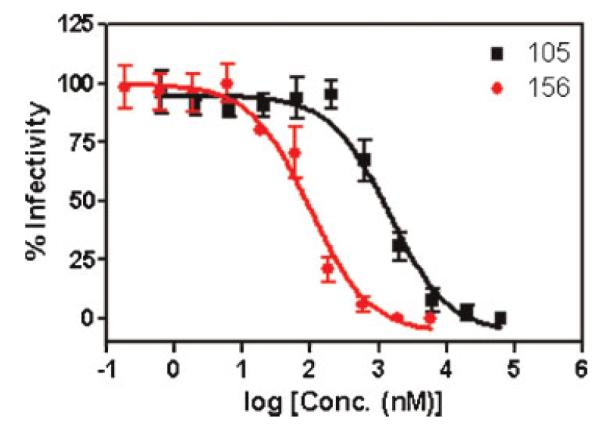
Analysis of activity of 156 in inhibiting infection of P4-CCR5 cells by HIV-1BaL whole virus. The data points for 156 and 105 were fit to a simple sigmoidal inhibition model using Prism GraphPad software to derive the best-fit lines (solid lines) and IC50 values. 156 IC50 = 96 ±0.1 nM; 105 IC50 = 1430 ±100 nM.
Implications of improved interaction kinetics for HIV-1 gp120 targeted drug discovery and affinity capture
Results with the new metallocene-conjugated peptide 156 argue for its potential usefulness as a starting point to develop entry inhibitor leads for AIDS treatments. This is suggested by its low nM affinity for HIV-1gp120 and substantial potency in inhibiting cell infection. Furthermore, the binding data reported here hint at the possibility that HNG-156 may have a broad HIV-1 subtype specificity, a property that is currently being evaluated. The presence of the triazole-indole side chain cluster at positions 6 and 7 suggests the potential to focus on this locus in developing smaller molecular weight derivatives. While the structural nature for the latter cannot be predicted at present, it could include the triazole grouping itself, which is chemically stable (Rostovtsev et al., 2002), or other structural elements that recapitulate the specific binding properties of the triazole grouping. The binding kinetics of 156, taken with the multi-clade specificity of this peptide, also suggest the potential to utilize this peptide conjugate as a molecular tool for developing broad-specificity affinity capture ligands for HIV-1 gp120. The slow dissociation rate of the gp120–156 noncovalent complex suggests that immobilized forms of 156 could retain gp120 strongly. We have recently found (unpublished results) that we can form the peptide HNG-105C, containing the 105 sequence with the C-terminal extension Gly-Gly-γOrn-Cys(SH)-CONH2. The HNG-105C derivative, when covalently attached via the Cys-SH to an SPR sensor chip surface, retained high binding affinity for gp120 analyte. This preliminary result opens up the possibility to use the homologous HNG-156C as a low-cost immobilized ligand in chromatographic and other affinity surfaces for separation and sensing of HIV-1 envelope protein and envelope complexes.
Acknowledgements
We thank Drs Ernesto Freire, Judith LaLonde, Wayne Hendrickson, Amos Smith III and Joseph Sodroski for many helpful discussions during the course of this study. Funding for this work was provided by NIH P0156550 (IC P.I.), NIH R21 AI 071965 (IC P.I.), NIH-CHAVI, NIH U01 AI067854-02 (IC Pilot Project, P.I.) and International Partnership for Microbicides.
Abbreviations used
- 12p1
12-residue peptide #1
- CD4
cluster of differentiation-4 (receptor on leukocytes)
- CHO
Chinese hamster ovary
- CM
carboxy-methyl
- CRF07_BC
circulating subtype B-C recombinant form of HIV-1
- Env
envelope protein
- Fab
fragment antigen binding
- Fmoc−
9-fluorenylmethyl-oxycarbonyl
- gp120
glycoprotein 120
- HBTU
2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HIV-1
human immunodeficiency virus type 1
- HNG-156
the peptide Hosahudya N Gopi −156
- HOBt
1-hydroxybenzotriazole
- HPLC
high performance liquid chromatography
- Hyp
trans-4R-hydroxy-l-proline
- IC50
50% inhibitory concentration
- IL-5
interleukin 5
- mAb
monoclonal antibody
- MALDI-TOF
matrix-assisted laser desorption/ionization-time of flight
- NHS
N-hydroxy-succinimde
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- PBS
phosphate buffer saline
- Q
quaternary amino
- RU
response units
- sCD4
soluble CD4
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- SIV
simian immunodeficiency virus
- SP
sulphopropyl
- SPR
surface plasmon resonance
REFERENCES
- Biorn AC, Cocklin S, Madani N, Si Z, Ivanovic T, Samanen J, Van Ryk DI, Pantophlet R, Burton DR, Freire E, Sodroski J, Chaiken IM. Mode of action for linear peptide inhibitors of HIV-1 gp120 interactions. Biochemistry. 2004;43:1928–1938. doi: 10.1021/bi035088i. [DOI] [PubMed] [Google Scholar]
- Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusio-n-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- Cocklin S, Gopi H, Querido B, Nimmagadda M, Kuriakose S, Cicala C, Ajith S, Baxter S, Arthos J, Martin-Garcia J, Chaiken IM. Broad-spectrum anti-human immunodeficiency virus (HIV) potential of a peptide HIV type 1 entry inhibitor. J. Virol. 2007;81:3645–3648. doi: 10.1128/JVI.01778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco SJ, Henze H, Lederer A, Moehle K, Mukherjee R, Romagnoli B, Robinson JA, Brianza F, Gombert FO, Lociuro S, Ludin C, Vrijbloed JW, Zumbrunn J, Obrecht JP, Obrecht D, Brondani V, Hamy F, Klimkait T. Discovery of novel, highly potent and selective beta-hairpin mimetic CXCR4 inhibitors with excellent anti-HIV activity and pharmacokinetic profiles. Bioorg. Med. Chem. 2006;14:8396–8404. doi: 10.1016/j.bmc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Dowd CS, Leavitt S, Babcock G, Godillot AP, Van Ryk D, Canziani GA, Sodroski J, Freire E, Chaiken IM. Beta-turn Phe in HIV-1 Env binding site of CD4 and CD4 mimetic miniprotein enhances Env binding affinity but is not required for activation of co-receptor/17b site. Biochemistry. 2002;41:7038–7046. doi: 10.1021/bi012168i. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Harrison SC. Peptide ligands to human immunodeficiency virus type 1 gp120 identified from phage display libraries. J. Virol. 1999;73:5795–5802. doi: 10.1128/jvi.73.7.5795-5802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo SA, Wang W, Rawat SS, Jung G, Waring AJ, Cole AM, Lu H, Yan X, Daly NL, Craik DJ, Jiang S, Lehrer RI, Blumenthal R. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 2006;281:18787–18792. doi: 10.1074/jbc.M602422200. [DOI] [PubMed] [Google Scholar]
- Gopi HN, Tirupula KC, Baxter S, Ajith S, Chaiken IM. Click chemistry on azidoproline: high-affinity dual antagonist for HIV-1. envelope glycoprotein gp120. ChemMedChem. 2006;1:54–57. doi: 10.1002/cmdc.200500037. [DOI] [PubMed] [Google Scholar]
- Gopi H, Umashankara M, Pirrone V, LaLonde J, Madani N, Tuzer F, Baxter S, Zentner I, Cockin S, Jawanda N, Miller S, Schon A, Klein J, Freire E, Krebs F, Smith A, Sodroski J, Chaiken I. Structural determinants for affinity enhancement of a dual antagonist peptide entry inhibitor of human immunodeficiency virus type-1 envelope gp120. J. Med. Chem. 2008 doi: 10.1021/jm070814r. http:dx.doi/org/10.1021/jm070814r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Pillalamarri U, Panarello D, Bhattacharya M, Urbina C, Horvat S, Sarkhel S, Jameson B, Chaiken I. Asymmetric usage of antagon-ist charged residues drives interleukin-5 receptor recruitment but is insufficient for receptor activation. Biochemistry. 2006;45:1106–1115. doi: 10.1021/bi0518038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketas TJ, Schader SM, Zurita J, Teo E, Polonis V, Lu M, Klasse PJ, Moore JP. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology. 2007;364:431–440. doi: 10.1016/j.virol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Krebs FC, Miller SR, Malamud D, Howett MK, Wigdahl B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antiviral Res. 1999;43:157–173. doi: 10.1016/s0166-3542(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PF, Blair W, Wang T, Spicer T, Guo Q, Zhou N, Gong YF, Wang HG, Rose R, Yamanaka G, Robinson B, Li CB, Fridell R, Deminie C, Demers G, Yang Z, Zadjura L, Meanwell N, Colonno R. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc. Natl. Acad. Sci. USA. 2003;100:11013–11018. doi: 10.1073/pnas.1832214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden K, Cocklin S, Gopi H, Baxter S, Ajith S, Mahmood N, Shattock R, Chaiken I. A recombinant allosteric lectin antagonist of HIV-1 envelope gp120 interactions. Proteins. 2007;67:617–629. doi: 10.1002/prot.21295. [DOI] [PubMed] [Google Scholar]
- Moore JP, Doms RW. The entry of entry inhibitors: a fusion of science and medicine. Proc. Natl. Acad. Sci. USA. 2003;100:10598–10602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk C, Wei G, Yang OO, Waring AJ, Wang W, Hong T, Lehrer RI, Landau NR, Cole AM. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retroviruses. 2003;19:875–881. doi: 10.1089/088922203322493049. [DOI] [PubMed] [Google Scholar]
- Pancera M, Lebowitz J, Schon A, Zhu P, Freire E, Kwong PD, Roux KH, Sodroski J, Wyatt R. Soluble mimetics of human immunodeficiency virus type 1 viral spikes produced by replacement of the native trimerization domain with a heterologous trimerization motif: characterization and ligand binding analysis. J. Virol. 2005;79:9954–9969. doi: 10.1128/JVI.79.15.9954-9969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Steckbeck JD, Orlov I, Chow A, Grieser H, Miller K, Bruno J, Robinson JE, Montelaro RC, Cole KS. Kinetic rates of antibody binding correlate with neutralization sensitivity of variant simian immunodeficiency virus strains. J. Virol. 2005;79:12311–12320. doi: 10.1128/JVI.79.19.12311-12320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibris AM, Kuritzkes DR. Chemokine antagonists as therapeutics: focus on HIV-1. Annu. Rev. Med. 2007;58:445–459. doi: 10.1146/annurev.med.58.080105.102908. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- Vita C, Drakopoulou E, Vizzavona J, Rochette S, Martin L, Menez A, Roumestand C, Yang YS, Ylisastigui L, Benjouad A, Gluckman JC. Rational engineering of a miniprotein that reproduces the core of the CD4 site interacting with HIV-1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA. 1999;96:13091–13096. doi: 10.1073/pnas.96.23.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/UNAIDS AIDS Epidemic Update. 2006 Dec; ISBN 9291735426 ( http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2006/)
- Yuan W, Bazick J, Sodroski J. Characterization of the multiple conformational States of free monomeric and trimeric human immunodeficiency virus envelope glycoproteins after fixation by cross-linker. J. Virol. 2006;80:6725–6737. doi: 10.1128/JVI.00118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MY, Dimitrov DS. Novel approaches for identification of broadly cross-reactive HIV-1 neutralizing human monoclonal antibodies and improvement of their potency. Curr. Pharm. Des. 2007;13:203–212. doi: 10.2174/138161207779313669. [DOI] [PubMed] [Google Scholar]
- Zhang W, Canziani G, Plugariu C, Wyatt R, Sodroski J, Sweet R, Kwong P, Hendrickson W, Chaiken I. Conformational changes of gp120 in epitopes near the CCR5 binding site are induced by CD4 and a CD4 miniprotein mimetic. Biochemistry. 1999;38:9405–9416. doi: 10.1021/bi990654o. [DOI] [PubMed] [Google Scholar]
- Zhang MY, Shu Y, Phogat S, Xiao X, Cham F, Bouma P, Choudhary A, Feng YR, Sanz I, Rybak S, Broder CC, Quinnan GV, Evans T, Dimitrov DS. Broadly cross-reactive HIV neutralizing human monoclonal antibody Fab selected by sequential antigen panning of a phage display library. J. Immunol. Meth. 2003;283:17–25. doi: 10.1016/j.jim.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Ma L, Jiang S, Lu H, Liu S, He Y, Strick N, Neamati N, Debnath AK. Identification of N-phenyl-N’-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4. Virology. 2005;339:213–225. doi: 10.1016/j.virol.2005.06.008. [DOI] [PubMed] [Google Scholar]