Abstract
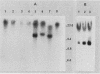
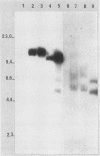
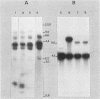
The effect of the chromosomal ends of Tetrahymena thermophila on the stability of linear transforming molecules in the filamentous fungus Podospora anserina was tested. A derivative of an integrative vector for this fungus has been constructed, so that after linearization, the ends of the plasmid are the telomeric sequences of T. thermophila. After transformation, this linear molecule was maintained as an extrachromosomal plasmid with no integrated copies in about 50% of the transformants. Under selective conditions, there was approximately one linear molecule per 5 to 10 nuclei, and these extrachromosomal molecules were rapidly lost under nonselective conditions. The circular plasmid carrying an inverted repeat of T. thermophila telomeres could be linearized and processed in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballance D. J., Turner G. Development of a high-frequency transforming vector for Aspergillus nidulans. Gene. 1985;36(3):321–331. doi: 10.1016/0378-1119(85)90187-8. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Bégueret J., Razanamparany V., Perrot M., Barreau C. Cloning gene ura5 for the orotidylic acid pyrophosphorylase of the filamentous fungus Podospora anserina: transformation of protoplasts. Gene. 1984 Dec;32(3):487–492. doi: 10.1016/0378-1119(84)90023-4. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Lambowitz A. M., Rambosek J. A., Kinsey J. A. Transformation of Neurospora crassa with recombinant plasmids containing the cloned glutamate dehydrogenase (am) gene: evidence for autonomous replication of the transforming plasmid. Mol Cell Biol. 1984 Oct;4(10):2041–2051. doi: 10.1128/mcb.4.10.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hsiao C. L., Carbon J. High-frequency transformation of yeast by plasmids containing the cloned yeast ARG4 gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3829–3833. doi: 10.1073/pnas.76.8.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Pedigree analysis of plasmid segregation in yeast. Cell. 1983 Oct;34(3):961–970. doi: 10.1016/0092-8674(83)90553-6. [DOI] [PubMed] [Google Scholar]
- Méchali M., Kearsey S. Lack of specific sequence requirement for DNA replication in Xenopus eggs compared with high sequence specificity in yeast. Cell. 1984 Aug;38(1):55–64. doi: 10.1016/0092-8674(84)90526-9. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paietta J. V., Marzluf G. A. Gene disruption by transformation in Neurospora crassa. Mol Cell Biol. 1985 Jul;5(7):1554–1559. doi: 10.1128/mcb.5.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razanamparany V., Bégueret J. Positive screening and transformation of ura5 mutants in the fungus Podospora anserina: characterization of the transformants. Curr Genet. 1986;10(11):811–817. doi: 10.1007/BF00418527. [DOI] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Szostak J. W. Replication and resolution of telomeres in yeast. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1187–1194. doi: 10.1101/sqb.1983.047.01.134. [DOI] [PubMed] [Google Scholar]
- Williamson D. H. The yeast ARS element, six years on: a progress report. Yeast. 1985 Sep;1(1):1–14. doi: 10.1002/yea.320010102. [DOI] [PubMed] [Google Scholar]