Abstract
Background
The transformation of noninfective epimastigotes into infective metacyclic trypomastigotes (metacyclogenesis) is a fundamental step in the life cycle of Trypanosoma cruzi, comprising several morphological and biochemical changes. GP82 and GP90 are glycoproteins expressed at the surface of metacyclic trypomastigote, with opposite roles in mammalian cell invasion. GP82 is an adhesin that promotes cell invasion, while GP90 acts as a negative regulator of parasite internalization. Our understanding of the synthesis and intracellular trafficking of GP82 and GP90 during metacyclogenesis is still limited. Therefore, we decided to determine whether GP82 and GP90 are expressed only in fully differentiated metacyclic forms or they start to be expressed in intermediate forms undergoing differentiation.
Methods
Parasite populations enriched in intermediate forms undergoing differentiation were analyzed by quantitative real-time PCR, Western blot, flow cytometry and immunofluorescence to assess GP82 and GP90 expression.
Results
We found that GP82 and GP90 mRNAs and proteins are expressed in intermediate forms and reach higher levels in fully differentiated metacyclic forms. Surprisingly, GP82 and GP90 presented distinct cellular localizations in intermediate forms compared to metacyclic trypomastigotes. In intermediate forms, GP82 is localized in organelles at the posterior region and colocalizes with cruzipain, while GP90 is localized at the flagellar pocket region.
Conclusions
This study discloses new aspects of protein expression and trafficking during T. cruzi differentiation by showing that the machinery involved in GP82 and GP90 gene expression starts to operate early in the differentiation process and that different secretion pathways are responsible for delivering these glycoproteins toward the cell surface.
Keywords: Trypanosoma cruzi, Metacyclogenesis, Surface proteins, Gene expression, Intracellular trafficking
Background
The metacyclogenesis, a process that involves the transformation of noninfective epimastigotes into infective metacyclic trypomastigotes, is a fundamental step in the life cycle of the protozoan Trypanosoma cruzi, the etiological agent of Chagas disease. It occurs at the hindgut of the insect vector, where epimastigotes attach to the superficial cuticle layer of the gut epithelium prior to differentiation into metacyclic trypomastigotes [1-3]. Metacyclic forms detached from the hindgut wall are released in the insect’s feces during its blood meal and enter mammalian host cells through skin lesions. Another mode of transmission is the oral infection (reviewed in [4]), where whole triatomine insects or their feces containing metacyclic forms are potential sources of food contamination.
It is not clear how the differentiation process is triggered, but nutritional stress and adhesion to substrate are important requirements, with the involvement of free fatty acids, cyclic AMP and adenylate cyclase [5-8]. Several changes occur during metacyclogenesis, including nuclear structure modifications [9], chromatin remodeling and differential mRNA stability [10,11], which result in differential protein expression [12], changes in cell morphology [13], proliferation and infectivity. It has been demonstrated that the expression of some stage-specific genes precedes morphological changes during metacyclogenesis [11,14,15]. Additionally, changes in membrane lipids [16] and carbohydrate composition [17] were observed to precede morphological transformation.
The trans-sialidase family members, GP82 and GP90, are developmentally regulated proteins expressed in metacyclic trypomastigotes [18-21], in which their mRNA half-life is longer than in epimastigotes [22]. These molecules play distinct roles in parasite internalization: the cell invasion-promoting GP82 induces a transient increase in host cell intracellular Ca2+ concentration [23,24] and actin cytoskeleton disruption [25], leading to the recruitment of lysosomes to the site of entry [26], an event required for the biogenesis of parasitophorous vacuole and host cell invasion [27]. GP90 binds to mammalian cells without triggering Ca2+ signals and functions as a down regulator in cell invasion so that its expression is inversely correlated with the parasite’s capacity to invade mammalian cells [24,28].
Despite GP82 and GP90 have been known for many years, our understanding of their synthesis and intracellular trafficking during the metacyclogenesis is still limited. A previous attempt to clarify this issue gave contradictory results that were unable to be clarified due to technical limitations imposed by the in vivo system [29]. Therefore, here we decided to use reproducible axenic culture conditions [30] to study the metacyclogenesis and determine whether GP82 and GP90 are expressed only in fully differentiated metacyclic forms or they start to be expressed in intermediate forms undergoing differentiation. Isolated intermediate forms were analyzed by a combination of techniques, revealing that GP82 and GP90 mRNAs and proteins are already expressed. Unexpectedly, GP82 and GP90 presented distinct cellular localizations in intermediate forms, indicating that during morphological changes they follow different pathways toward the surface of metacyclic trypomastigotes.
Methods
Ethics statement
This study was carried out in accordance with recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on Animal Experiment Ethics of Universidade Federal de São Paulo (Protocol Number: CEP09555-07).
Parasites and in vitro metacyclogenesis
T. cruzi G strain [31] was maintained alternately in mice and in liver infusion tryptose (LIT) medium, containing 10% fetal bovine serum at 28°C. Metacyclogenesis was induced according to the procedure described by Contreras et al.[5]. Briefly, epimastigotes were grown to stationary phase, collected by centrifugation, washed once in triatomine artificial urine (TAU) medium (190 mM NaCl, 17 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 8 mM sodium phosphate buffer, pH 6.0) and diluted to 5 × 108 cells/mL in the same medium. After 2 h at 28°C, parasites were diluted 100 fold in TAU supplemented with 50 mM sodium glutamate, 10 mM L-proline, 2 mM sodium aspartate and 10 mM glucose (TAU3AAG), allowed to attach to cell culture flasks and maintained afterwards at 28°C. Attached parasites were collected 24 and 48 h later by removing the supernatant, washing the attached cells once with TAU, and vigorously shaking the parasites with TAU medium. Metacyclic trypomastigotes were obtained from culture supernatants from TAU3AAG and purified by anion-exchange chromatography using DEAE-cellulose as previously described [18,31]. Briefly, parasites were washed twice with phosphate-buffered saline containing 5.4% glucose (PSG) pH 8.0, followed by passage through a DEAE-cellulose column packed in a 20 mL plastic syringe and elution with PSG.
RNA extraction, cDNA synthesis and quantitative real-time PCR
Real-time PCR was performed to assess the expression of GP82 and GP90 mRNAs during metacyclogenesis. Total RNA was isolated from 5 × 107 parasites using Trizol reagent (Invitrogen) and treated with RNAse-free DNase (Invitrogen). Two micrograms of total RNA was used for cDNA synthesis using the ThermoScript Preamplification System according to the manufacturer’s instructions (Invitrogen). Quantitative real-time PCR was mainly conducted as described earlier [29]. Briefly, reactions were carried out with 12.5 μL of SYBR-Green PCR master mix (Applied Biosystems), 1.6 μL of cDNA and primers at a final concentration of 200 nM in a final volume of 20 μL. PCR was conducted in the ABI Prism 7500 (Applied Biosystems) and analyzed with ABI Prism 7500 SDS version 2.0 software. cDNA from exponentially growing epimastigotes were used as a control for comparison purposes. All quantifications were normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH, GenBank: X52898). It is worth mentioning that RT-PCR primers specifically detected GP82 and GP90 family members and results shown in here are derived from a subset of members sharing high sequence similarity.
Western blot
Total proteins were extracted from 2.5 × 106 parasites with a solution containing Tris-HCl 62 mM, 10% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.01% bromophenol blue and boiled prior to sample loading on 8% SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes, blocked with 5% non-fat milk for 1 h at room temperature (RT) and incubated for 1 h at RT with monoclonal antibody (mAb) 3F6 or 1G7 against GP82 and GP90, respectively [18]. Membranes were washed four times for 5 min each with phosphate-buffered saline (PBS) containing 0.05% Tween 20, incubated with anti-mouse IgG coupled to horseradish peroxidase (Sigma) for 1 h at RT, washed again in the same conditions and visualized by chemiluminescence.
Flow cytometry
Live parasites (4 × 107) were incubated for 30 min on ice with mAbs 3F6 or 1G7 diluted in 1% bovine serum albumin (BSA)/PBS. After washings in PBS, cells were fixed with 4% paraformaldehyde (PFA) in PBS for 15 min. The fixative was washed out and the parasites were incubated with Alexa Fluor 488-conjugated anti-mouse IgG diluted in 1% BSA/PBS for 1 h at RT. After two more washes, the fluorescence was determined on a FACSCalibur II cytometer (Becton Dickinson) and data analysis performed using CellQuest software. Assays with permeabilized parasites were carried out as follows: fixation with 4% PFA, washes in PBS, incubation with 50 mM ammonium chloride for 15 min, washes in PBS, treatment with 0.1% saponin in 1% BSA/PBS at RT for 30 min, washes in PBS, incubation with mAb 3F6 or 1G7 diluted in 1% BSA/PBS for 1 h at RT, washes in PBS, incubation with Alexa Fluor 488-conjugated antibody and final washes in PBS.
Immunofluorescence
Parasites were fixed with 4% PFA, incubated with 50 mM ammonium chloride, washed with PBS and let attach to glass slides pretreated with 0.01% poly-L-lysine. Parasites were then blocked and permeabilized with a solution containing 0.5% saponin and 1% BSA for 1 h. Cells were incubated with mAbs 3F6 or 1G7 diluted in 1% BSA and 0.1% saponin for 1 h at RT or with rabbit polyclonal antibody anti-cruzipain provided by Dr. Ana Paula C. A. Lima, UFRJ, Brazil. The preparations were washed with PBS, incubated with anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 568 (Invitrogen) diluted in 1% BSA and 0.1% saponin for 1 h, together with 10 μM of DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes) to visualize the nucleus and kinetoplast. Images were acquired on an Olympus BX51 fluorescence microscope coupled to an Olympus DP71 digital camera using Image-Pro 6.2 software. It is worth mentioning that mAb 3F6 and 1G7 are specific to a subset of GP82 and GP90 family members sharing high sequence similarity.
Statistical analysis
Student’s t-test was used to determine the significance in real-time PCR experiments in which GP82 and GP90 mRNA levels of exponentially growing epimastigotes were compared to intermediate and metacyclic forms and in flow cytometry assays where the fluorescence intensity was compared between live and permeabilized parasites.
Results
Expression of GP82 and GP90 during metacyclogenesis
In vitro differentiation was carried out using TAU3AAG medium, which allows parasites to attach to cell culture flasks while undergoing differentiation. Parasite forms that are close to completing differentiation into metacyclic trypomastigotes detach from culture flasks and stay in the supernatant while other forms that are still differentiating remain attached to culture flasks [6]. Thus, TAU3AAG medium is a good choice for quantitative analysis as it allows isolation of intermediate forms undergoing differentiation, with a minor contamination of metacyclic forms.
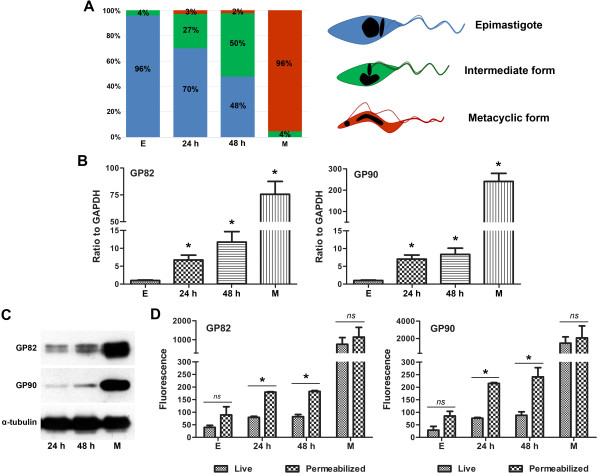
The following developmental forms were analyzed: exponentially growing epimastigotes (E) obtained from LIT; intermediate forms attached to culture flasks 24 (24 h) and 48 h (48 h) after inoculum in TAU3AAG; and metacyclic trypomastigotes (M) obtained from TAU3AAG supernatant and purified by DEAE-cellulose. To distinguish T. cruzi developmental stages, DAPI staining and phase contrast were used to identify nucleus/kinetoplast and flagellum position. Based on parasite’s morphology, the relative number of epimastigotes, intermediate forms and metacyclic forms were estimated in each sample according to Ferreira et al. [13]. Epimastigote forms contain a spherical nucleus with a flagellum protruding from the anterior portion of the cell body near to the disk-shaped kinetoplast. Intermediate forms have a somewhat elongated nucleus with the kinetoplast varying in position relative to the nucleus, either anterior, at the middle or posterior. Metacyclic trypomastigotes have a fully elongated nucleus with a round kinetoplast at the posterior end of the parasite (see Figure 1A). Upon seeding in TAU3AAG medium for differentiation, the percentage of intermediate forms increased from the initial 4% in epimastigote cultures to 50% in samples collected at 48 h (Figure 1A), showing that this time point is enriched in intermediate forms with a minimal contamination of metacyclic trypomastigotes (2%). Such contamination did not interfere significantly in posterior analyses as according to flow cytometry results there is a shift in fluorescence intensity in the whole parasite population obtained at 24 h and 48 h compared to epimastigote sample, which would not be observed if the results obtained were driven by the 2-3% metacyclic forms (Additional file 1).
Figure 1.
Expression of GP82 and GP90 at different time-points during T. cruzi metacyclogenesis. (A) Relative number of epimastigotes (blue), intermediate forms (green) and metacyclic trypomastigotes (red) in analyzed samples. Numbers are derived from three independent experiments where 200 cells were analyzed. A representative drawing of each parasite form is shown on the right. (B) Quantitative real-time PCR for determination of transcript levels. Means and standard deviations are derived from three independent experiments. The difference between epimastigotes and other forms was significant (P <0.05). (C) SDS-PAGE followed by Western blot showing the presence of GP82 and GP90 at 24 and 48 h. Total protein extract from 2.5 × 106 parasites were used and anti-α-tubulin mAb was used as loading control. Experiments shown were carried out three times with similar results. (D) Live or permeabilized parasites were reacted with mAb 3F6 or 1G7 and processed for flow cytometry analysis. Values represent fluorescence means and standard deviation of independent experiments that were normalized by the fluorescence of parasites incubated only with secondary antibody. The difference between live and permeabilized parasites was significant for 24 and 48 h samples (P <0.05) and not significant (ns) for epimastigotes and metacyclic forms.
GP82 and GP90 mRNA are enriched in metacyclic trypomastigotes when compared to exponentially growing epimastigotes [22]. To investigate whether GP82 and GP90 mRNAs start to be stabilized in intermediate forms undergoing differentiation, total RNA was extracted and analyzed by quantitative real-time PCR. Results revealed that GP82 and GP90 mRNA levels are increased in parasites undergoing metacyclogenesis (24 and 48 h) when compared to exponentially growing epimastigotes (E) (Figure 1B). Intermediate forms collected at 48 h showed 11-fold and 8-fold increase in GP82 and GP90 mRNA levels when compared to epimastigotes, suggesting that mRNA stabilization occurs before the differentiation to metacyclic trypomastigotes (Figure 1B). Thus, GP82 and GP90 transcript levels increase during the differentiation process, reaching higher levels in metacyclic forms.
Total protein extracts of parasites attached to culture flasks at 24 and 48 h were analyzed by Western blot, revealing that GP82 and GP90 are already translated in intermediate forms (Figure 1C). To determine whether GP82 and GP90 were located in the plasma membrane surface or in intracellular compartments, live and permeabilized parasites were incubated with mAbs 3F6 and 1G7 and processed for flow cytometry (Figure 1D). The fluorescence signal was lower in live intermediate forms compared to permeabilized ones, indicating that GP82 and GP90 are mainly located in intracellular compartments. Statistical significant differences were observed between live and permeabilized parasites at 24 and 48 h, but no significant difference was observed for epimastigotes or metacyclic forms (Figure 1D). Together, these data show that the expression of GP82 and GP90 starts in intermediate stages during metacyclogenesis and reaches the highest level in fully differentiated metacyclic forms.
Cellular localization of GP82 and GP90 during metacyclogenesis
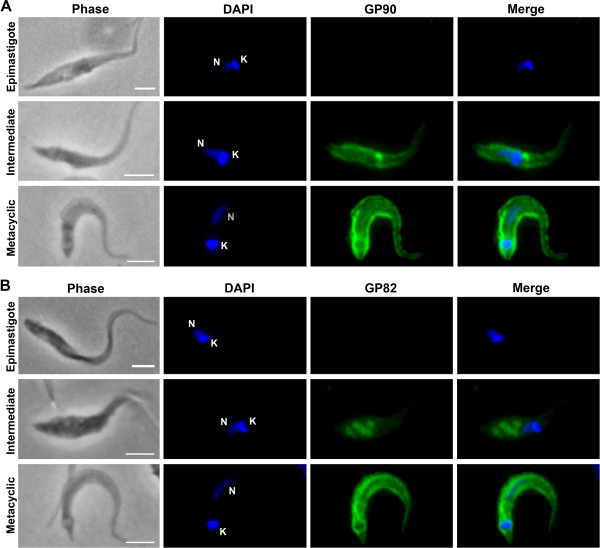
In metacyclic trypomastigotes, GP82 and GP90 are found mainly at the plasma membrane [18]. Since both molecules are also expressed in intermediate forms, but their localizations appear to be predominantly intracellular (Figure 1D), indirect immunofluorescence was performed to assess GP82 and GP90 cellular localization. Distinct from exponentially growing epimastigotes that did not exhibit fluorescence signal and from metacyclic trypomastigotes that displayed a membrane labeling pattern for both GP82 and GP90; in intermediate forms, GP82 localized mainly in vesicular structures at the parasite posterior region, while GP90 localized at the plasma membrane and in the region close to the kinetoplast, where the Golgi complex and the flagellar pocket are located (Figure 2A and B). These labeling patterns, easily detected in parasites attached to culture flasks at 48 h, could be seen in a minor portion of the parasite population as soon as 12 h after nutritional stress (data not shown). In intermediate forms at 48 h, labeling with anti-GP82 mAb was detected in vesicular structures in 68 ± 6.5% of the cells, while anti-GP90 mAb labeled the region close to kinetoplast in 30 ± 1.5% of the cells (three independent experiments).
Figure 2.
Differential cellular localization of GP82 and GP90 in parasites undergoing metacyclogenesis. Immunofluorescence was performed using exponentially growing epimastigotes, parasites attached to culture flasks at 48 h after nutritional stress and metacyclic trypomastigotes purified by DEAE-cellulose. Cells were fixed, permeabilized with 0.5% saponin, reacted with mAb 1G7 (A) or 3F6 (B) and incubated with secondary antibody Alexa Fluor 488. DAPI was used to stain nucleus (N) and kinetoplast (K). Scale bar = 10 μm.
GP82 and GP90 genes encode an N-terminal signal peptide and a C-terminal GPI anchor signal, which address these proteins to the endoplasmic reticulum (ER) and Golgi complex for addition of GPI anchor [32-34] and glycosylation [35,36]. In T. cruzi, the processes of protein secretion and trafficking to the plasma membrane occur mainly via the flagellar pocket, the only region where the parasite’s body is not covered by subpellicular microtubules [37]. Since GP82 and GP90 are glycoproteins located at the plasma membrane, they were expected to localize in the Golgi complex and flagellar pocket of intermediate forms, as observed for GP90 (Figure 2A). However, in contrast to GP90 localization, GP82 was detected in vesicular structures at the posterior region of intermediate forms (Figure 2B), indicating that the trafficking of these proteins occurs through distinct pathways. To investigate whether these vesicular structures correspond to late endosomes named reservosomes or lysosomes-related organelles (LROs), which are localized at T. cruzi posterior region [38,39], assays were performed to assess the colocalization with the cysteine proteinase cruzipain that is abundant in LROs [40]. Figure 3 shows GP82 colocalization with cruzipain at the posterior region of attached intermediate forms (Figure 3A), as well as in structures close to the kinetoplast in a small portion of the population (Figure 3B). Moreover, GP82 colocalized with cruzipain later in the differentiation process, when parasites isolated from culture supernatant at 48 h were analyzed (Figure 3C-D). These results suggest that GP82 accumulates in LROs of intermediate forms, which are then directed to the flagellar pocket during differentiation to metacyclic forms, delivering GP82 to the plasma membrane.
Figure 3.
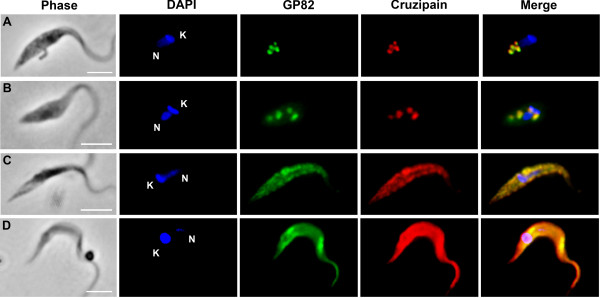
Colocalization of GP82 with cruzipain during metacyclogenesis. Immunofluorescence showing intermediate forms attached to culture flasks at 48 h (A-B) and late intermediate forms and fully differentiated metacyclic forms in culture supernatant (C-D) at 48 h after nutritional stress. Cells were fixed, permeabilized with 0.5% saponin and submitted to immunofluorescence using mAb 3F6 and rabbit polyclonal antibody against T. cruzi cruzipain. DAPI was used to stain nucleus (N) and kinetoplast (K). Scale bar = 10 μm.
Discussion
Metacyclogenesis is a process whereby T. cruzi transforms from noninfective epimastigotes into infective metacyclic trypomastigotes and comprises a progressive morphological transformation, including transitional forms described as intermediate [13]. In this study, we demonstrated that GP82 and GP90 transcript levels increase in parasites forms undergoing differentiation and this increase is accompanied by translation of GP82 and GP90 proteins in intermediate forms. Several studies have shown that GP82 and GP90 are expressed by metacyclic trypomastigotes, but not by epimastigotes [18,19,21,22,41]. These results are in agreement with proteomic and transcriptomic analyzes of T. cruzi life-cycle stages, which revealed that GP82 and GP90 are up-regulated in metacyclic forms [42,43]. Despite those studies, little information was available about the expression of GP82 and GP90 during metacyclogenesis. In a previous work, different time points during metacyclogenesis were studied by proteomic analysis, revealing the presence of GP90 in parasites attached to culture flasks 24 h after nutritional stress [12]; however, this study was not able to detect GP82. In addition, although with no statistical significance, another quantitative proteomic study of T. cruzi metacyclogenesis identified different members of the trans-sialidase family expressed at higher levels in intermediate forms compared to epimastigotes [44].
In trypanosomes, gene expression is regulated at the posttranscriptional level mostly via mRNA stability and at least three known factors modulate mRNAs steady-state levels: cis-acting elements, trans-acting factors and the apparatus involved in mRNA turnover and degradation [45]. We have previously shown that GP82 transcripts accumulate in metacyclic forms and that treatment with translation inhibitors increased GP82 mRNA half-life in epimastigotes, suggesting that protein factors act by destabilizing transcripts in the epimastigote stage [22,46]. Thus, our findings support the notion that trans-acting factors that regulate GP82 and GP90 mRNAs steady-state levels are already operating in intermediate forms. Furthermore, it has been reported that transcriptional activity is constant during metacyclogenesis, but it is reduced in fully differentiated metacyclic trypomastigotes, when RNA Pol II disassembles from the nucleus [13]. Distinct from epimastigotes that replicate within the insect vector, metacyclic trypomastigotes are non-dividing forms that rapidly differentiate into amastigotes upon transmission to mammalian host, so it makes sense that these forms present a reduced transcriptional activity. Thus, we speculate that the majority of GP82 and GP90 mRNAs are transcribed and stabilized in intermediated forms and then accumulate in metacyclic forms.
The mechanisms involved in exocytosis, endocytosis and recycling in T. cruzi are still poorly understood compared to mammalian cells or to the closely related organism Trypanosoma brucei[47,48]. Most of what is known comes from structural and biochemical studies focused on morphology and localization of enzymes and endocytic markers [38-40,49-52]. Attempts to characterize and localize protein markers of intracellular compartments, e.g. Rab5, Rab7, Rab11 and Rho1, were unsuccessful and/or not fully explored [53-56]. We found that during nutritional stress GP82 and GP90 accumulate in distinct compartments in intermediate forms, despite their localization on the cell surface of metacyclic forms, indicating the existence of different control mechanisms to access the cell surface. The basis for such a difference is unclear, but it may be related to specific differences in the primary amino acid sequence and/or to posttranslational modifications, for instance, in the GPI anchor [57] and/or other lipid modifications [58]. Further studies will be needed to identify the mechanism responsible for delivering GP82 into LROs of intermediate forms and for the difference in protein localization observed between intermediate and metacyclic forms.
An increasing number of proteins with varied functions have being identified to localize in LROs, such as 52-kDa protein homologue to glutathione-S-transferase (Tc52) [59], farnesylated protein tyrosine phosphatase [60], β-tubulin [61], and RNA-binding protein (TcRBP40) [62]. In addition, subcellular proteomic analysis of reservosomes (LROs) fractions revealed a great variety of proteins with different functions; among them were surface proteins such as protease GP63, dispersed gene family protein-1, procyclic surface glycoprotein and kinetoplast membrane protein-11 [63]. Cunha-e-Silva et al. [64] suggested that LROs could be a heterogeneous population of organelles with different purposes, presenting storing, recycling and lysosome functions according to their maturation state. Our results are in agreement with this multipurpose hypothesis and also support the notion that the parasite nutritional state and developmental form influence the function of LROs. As endocytosis is very low or absent in metacyclic trypomastigotes compared to epimastigotes [65], our results suggest that LROs play a different role during metacyclogenesis and in metacyclic trypomastigotes compared to epimastigotes. Further characterization of the LROs population will help to clarify these findings.
The finding that GP82 colocalizes with cruzipain in LROs of intermediate forms, and that both proteins are directed to the plasma membrane with metacyclogenesis progression, may be relevant for the infective properties of metacyclic trypomastigotes. In preliminary experiments, we have found that cruzipain is involved in metacyclic trypomastigotes host cell invasion through a mechanism distinct from that described for tissue culture trypomastigotes (TCT) (unpublished data). Therefore, we suppose that upon GP82-mediated binding to target cells, cruzipain contributes for effective parasite internalization through a mechanism as yet to be elucidated.
Conclusions
This study discloses new aspects of protein expression and trafficking during T. cruzi differentiation by showing that the machinery involved in GP82 and GP90 gene expression starts to operate early in the differentiation process and that different secretion pathways are responsible for delivering these glycoproteins toward the cell surface (Figure 4).
Figure 4.
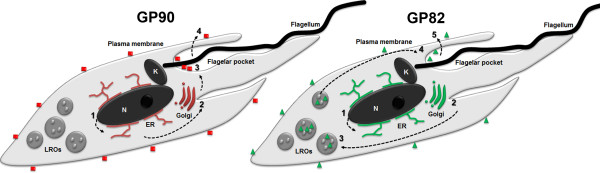
Schematic representation of GP82 and GP90 pathways toward the cell surface during differentiation. Intermediate forms in which these proteins start to be expressed are represented. GP90 (red squares) mRNAs sequences encode an N-terminal signal peptide and a C-terminal signal anchor driving polypeptide through the ER-Golgi secretory pathway (1-2) to be glycosylated and receive GPI anchor. GP90 proteins leave the Golgi complex in secretory vesicles that fuse with the flagellar pocket membrane (3) and are distributed along the parasite plasma membrane (4). GP82 (green triangles) mRNAs also encode N-terminal and C-terminal signal sequences that drive polypeptide through the ER-Golgi pathway (1-2) to receive post-translational modifications (glycosylation and GPI anchor). However, instead of being addressed straight to the plasma membrane via flagellar pocket, GP82 leave the Golgi complex in vesicles that concentrate in lysosome-related organelles (LROs) (3). Further in the differentiation process, sorting mechanisms occurring at LROs and organelle repositioning allow vesicles carrying GP82 to fuse with the flagellar pocket membrane (4) and distribute the protein along the plasma membrane (5). N: nucleus, K: kinetoplast.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The authors EBS and JFS idealized the project, contributed to the experimental design and wrote the manuscript. EBS performed the experiments and analyzed the data. NLCS and NY helped with discussions and manuscript elaboration. All authors read and approved the final version of the manuscript.
Supplementary Material
Flow cytometry analysis of parasite populations undergoing differentiation. Histograms showing there is a shift in fluorescence intensity in the whole parasite population obtained at 24 h and 48 h compared with epimastigote sample, indicating that intermediate forms are responsible for the signal and not the 2-3% metacyclic forms contamination.
Contributor Information
Ethel Bayer-Santos, Email: ethel.bs@gmail.com.
Narcisa Leal Cunha-e-Silva, Email: narcisacunha@gmail.com.
Nobuko Yoshida, Email: nyoshida@unifesp.br.
José Franco da Silveira, Email: jose.franco@unifesp.br.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to JFS. EBS was awarded a doctoral fellowship by CNPq. We would like to thank Dr. Ana Paula C. A. Lima (Universidade Federal do Rio de Janeiro) for the anti-cruzipain antibody.
References
- Boker CA, Schaub GA. Scanning electron microscopic studies of Trypanosoma cruzi in the rectum of its vector Triatoma infestans. Z Parasitenkd. 1984;70(4):459–469. doi: 10.1007/BF00926686. [DOI] [PubMed] [Google Scholar]
- de Souza W. Cell biology of Trypanosoma cruzi. Int Rev Cytol. 1984;86:197–283. doi: 10.1016/s0074-7696(08)60180-1. [DOI] [PubMed] [Google Scholar]
- Kollien AH, Schmidt J, Schaub GA. Modes of association of Trypanosoma cruzi with the intestinal tract of the vector Triatoma infestans. Acta Trop. 1998;70(2):127–141. doi: 10.1016/S0001-706X(97)00117-4. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Tyler KM, Llewellyn MS. Invasion mechanisms among emerging food-borne protozoan parasites. Trends Parasitol. 2011;27(10):459–466. doi: 10.1016/j.pt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Contreras VT, Araujo-Jorge TC, Bonaldo MC, Thomaz N, Barbosa HS, Meirelles Mde N, Goldenberg S. Biological aspects of the Dm 28c clone of Trypanosoma cruzi after metacyclogenesis in chemically defined media. Mem Inst Oswaldo Cruz. 1988;83(1):123–133. doi: 10.1590/S0074-02761988000100016. [DOI] [PubMed] [Google Scholar]
- Bonaldo MC, Souto-Padron T, de Souza W, Goldenberg S. Cell-substrate adhesion during Trypanosoma cruzi differentiation. J Cell Biol. 1988;106(4):1349–1358. doi: 10.1083/jcb.106.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-Perdomo M, Romero P, Goldenberg S. Cyclic AMP and adenylate cyclase activators stimulate Trypanosoma cruzi differentiation. Exp Parasitol. 1988;66(2):205–212. doi: 10.1016/0014-4894(88)90092-6. [DOI] [PubMed] [Google Scholar]
- Wainszelbaum MJ, Belaunzaran ML, Lammel EM, Florin-Christensen M, Florin-Christensen J, Isola EL. Free fatty acids induce cell differentiation to infective forms in Trypanosoma cruzi. Biochem J. 2003;375(Pt 3):705–712. doi: 10.1042/BJ20021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MC, da Cunha JP, de Faria FP, Mortara RA, Freymuller E, Schenkman S. Morphological events during the Trypanosoma cruzi cell cycle. Protist. 2007;158(2):147–157. doi: 10.1016/j.protis.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Di Noia JM, D’Orso I, Sanchez DO, Frasch AC. AU-rich elements in the 3'-untranslated region of a new mucin-type gene family of Trypanosoma cruzi confers mRNA instability and modulates translation efficiency. J Biol Chem. 2000;275(14):10218–10227. doi: 10.1074/jbc.275.14.10218. [DOI] [PubMed] [Google Scholar]
- Avila AR, Dallagiovanna B, Yamada-Ogatta SF, Monteiro-Goes V, Fragoso SP, Krieger MA, Goldenberg S. Stage-specific gene expression during Trypanosoma cruzi metacyclogenesis. Genet Mol Res. 2003;2(1):159–168. [PubMed] [Google Scholar]
- Parodi-Talice A, Monteiro-Goes V, Arrambide N, Avila AR, Duran R, Correa A, Dallagiovanna B, Cayota A, Krieger M, Goldenberg S. Proteomic analysis of metacyclic trypomastigotes undergoing Trypanosoma cruzi metacyclogenesis. J Mass Spectrom. 2007;42(11):1422–1432. doi: 10.1002/jms.1267. [DOI] [PubMed] [Google Scholar]
- Ferreira LR, Dossin Fde M, Ramos TC, Freymuller E, Schenkman S. Active transcription and ultrastructural changes during Trypanosoma cruzi metacyclogenesis. An Acad Bras Cienc. 2008;80(1):157–166. doi: 10.1590/s0001-37652008000100011. [DOI] [PubMed] [Google Scholar]
- Contreras VT, Morel CM, Goldenberg S. Stage specific gene expression precedes morphological changes during Trypanosoma cruzi metacyclogenesis. Mol Biochem Parasitol. 1985;14(1):83–96. doi: 10.1016/0166-6851(85)90108-2. [DOI] [PubMed] [Google Scholar]
- Goldenberg S, Salles JM, Contreras VT, Lima Franco MP, Katzin AM, Colli W, Morel CM. Characterization of messenger RNA from epimastigotes and metacyclic trypomastigotes of Trypanosoma cruzi. FEBS Lett. 1985;180(2):265–270. doi: 10.1016/0014-5793(85)81083-8. [DOI] [PubMed] [Google Scholar]
- Esteves MG, Gonzales-Perdomo M, Alviano CS, Angluster J, Goldenberg S. Changes in fatty acid composition associated with differentiation of Trypanosoma cruzi. FEMS Microbiol Lett. 1989;50(1–2):31–34. doi: 10.1016/0378-1097(89)90453-9. [DOI] [PubMed] [Google Scholar]
- de Andrade AF, Esteves MJ, Angluster J, Gonzales-Perdomo M, Goldenberg S. Changes in cell-surface carbohydrates of Trypanosoma cruzi during metacyclogenesis under chemically defined conditions. J Gen Microbiol. 1991;137(12):2845–2849. doi: 10.1099/00221287-137-12-2845. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, Yoshida N. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibodies. Mol Biochem Parasitol. 1986;18(3):271–282. doi: 10.1016/0166-6851(86)90085-X. [DOI] [PubMed] [Google Scholar]
- Araya JE, Cano MI, Yoshida N, da Silveira JF. Cloning and characterization of a gene for the stage-specific 82-kDa surface antigen of metacyclic trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1994;65(1):161–169. doi: 10.1016/0166-6851(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Carmo MS, Araya JE, Ramirez MI, Boscardin S, Cano MI, Baida RP, Ruiz RC, Santos MR, Chiurillo MA, Ramirez JL. Organization and expression of a multigene family encoding the surface glycoproteins of Trypanosoma cruzi metacyclic trypomastigotes involved in the cell invasion. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):169–171. doi: 10.1590/s0074-02761999000700022. [DOI] [PubMed] [Google Scholar]
- do Carmo MS, dos Santos MR, Cano MI, Araya JE, Yoshida N, da Silveira JF. Expression and genome-wide distribution of the gene family encoding a 90 kDa surface glycoprotein of metacyclic trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 2002;125(1-2):201–206. doi: 10.1016/S0166-6851(02)00212-8. [DOI] [PubMed] [Google Scholar]
- Gentil LG, Cordero EM, do Carmo MS, dos Santos MR, da Silveira JF. Posttranscriptional mechanisms involved in the control of expression of the stage-specific GP82 surface glycoprotein in Trypanosoma cruzi. Acta Trop. 2009;109(2):152–158. doi: 10.1016/j.actatropica.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Dorta ML, Ferreira AT, Oshiro ME, Yoshida N. Ca2+ signal induced by Trypanosoma cruzi metacyclic trypomastigote surface molecules implicated in mammalian cell invasion. Mol Biochem Parasitol. 1995;73(1–2):285–289. doi: 10.1016/0166-6851(94)00123-5. [DOI] [PubMed] [Google Scholar]
- Ruiz RC, Favoreto S Jr, Dorta ML, Oshiro ME, Ferreira AT, Manque PM, Yoshida N. Infectivity of Trypanosoma cruzi strains is associated with differential expression of surface glycoproteins with differential Ca2+ signalling activity. Biochem J. 1998;330(Pt 1):505–511. doi: 10.1042/bj3300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez M, Atayde V, Yoshida N. Host cell invasion mediated by Trypanosoma cruzi surface molecule gp82 is associated with F-actin disassembly and is inhibited by enteroinvasive Escherichia coli. Microbes Infect. 2006;8(6):1502–1512. doi: 10.1016/j.micinf.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Martins RM, Alves RM, Macedo S, Yoshida N. Starvation and rapamycin differentially regulate host cell lysosome exocytosis and invasion by Trypanosoma cruzi metacyclic forms. Cell Microbiol. 2011;13(7):943–954. doi: 10.1111/j.1462-5822.2011.01590.x. [DOI] [PubMed] [Google Scholar]
- Tardieux I, Webster P, Ravesloot J, Boron W, Lunn JA, Heuser JE, Andrews NW. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71(7):1117–1130. doi: 10.1016/S0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- Malaga S, Yoshida N. Targeted reduction in expression of Trypanosoma cruzi surface glycoprotein gp90 increases parasite infectivity. Infect Immun. 2001;69(1):353–359. doi: 10.1128/IAI.69.1.353-359.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero EM, Gentil LG, Crisante G, Ramirez JL, Yoshida N, Anez N, Franco da Silveira J. Expression of GP82 and GP90 surface glycoprotein genes of Trypanosoma cruzi during in vivo metacyclogenesis in the insect vector Rhodnius prolixus. Acta Trop. 2008;105(1):87–91. doi: 10.1016/j.actatropica.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Contreras VT, Salles JM, Thomas N, Morel CM, Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Yoshida N. Surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi. Infect Immun. 1983;40(2):836–839. doi: 10.1128/iai.40.2.836-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S, Yoshida N, Cardoso de Almeida ML. Glycophosphatidylinositol-anchored proteins in metacyclic trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1988;29(2–3):141–151. doi: 10.1016/0166-6851(88)90069-2. [DOI] [PubMed] [Google Scholar]
- de Almeida ML, Heise N. Proteins anchored via glycosylphosphatidylinositol and solubilizing phospholipases in Trypanosoma cruzi. Biol Res. 1993;26(1–2):285–312. [PubMed] [Google Scholar]
- Guther ML, de Almeida ML, Yoshida N, Ferguson MA. Structural studies on the glycosylphosphatidylinositol membrane anchor of Trypanosoma cruzi 1G7-antigen. The structure of the glycan core. J Biol Chem. 1992;267(10):6820–6828. [PubMed] [Google Scholar]
- Ramirez MI, Ruiz Rde C, Araya JE, Da Silveira JF, Yoshida N. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect Immun. 1993;61(9):3636–3641. doi: 10.1128/iai.61.9.3636-3641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Blanco SA, Araguth MF, Russo M, Gonzalez J. The stage-specific 90-kilodalton surface antigen of metacyclic trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1990;39(1):39–46. doi: 10.1016/0166-6851(90)90006-8. [DOI] [PubMed] [Google Scholar]
- Souza W. Structural organization of Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):89–100. doi: 10.1590/s0074-02762009000900014. [DOI] [PubMed] [Google Scholar]
- Soares MJ, De Souza W. Cytoplasmic organelles of trypanosomatids: a cytochemical and stereological study. J Submicrosc Cytol Pathol. 1988;20(2):349–361. [PubMed] [Google Scholar]
- Sant’Anna C, Parussini F, Lourenco D, de Souza W, Cazzulo JJ, Cunha-e-Silva NL. All Trypanosoma cruzi developmental forms present lysosome-related organelles. Histochem Cell Biol. 2008;130(6):1187–1198. doi: 10.1007/s00418-008-0486-8. [DOI] [PubMed] [Google Scholar]
- Souto-Padron T, Campetella OE, Cazzulo JJ, de Souza W. Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite-host cell interaction. J Cell Sci. 1990;96(Pt 3):485–490. doi: 10.1242/jcs.96.3.485. [DOI] [PubMed] [Google Scholar]
- Franco FR, Paranhos-Bacalla GS, Yamauchi LM, Yoshida N, da Silveira JF. Characterization of a cDNA clone encoding the carboxy-terminal domain of a 90-kilodalton surface antigen of Trypanosoma cruzi metacyclic trypomastigotes. Infect Immun. 1993;61(10):4196–4201. doi: 10.1128/iai.61.10.4196-4201.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood JA 3rd, Weatherly DB, Minning TA, Bundy B, Cavola C, Opperdoes FR, Orlando R, Tarleton RL. The Trypanosoma cruzi proteome. Science. 2005;309(5733):473–476. doi: 10.1126/science.1110289. [DOI] [PubMed] [Google Scholar]
- Minning TA, Weatherly DB, Atwood J 3rd, Orlando R, Tarleton RL. The steady-state transcriptome of the four major life-cycle stages of Trypanosoma cruzi. BMC Genomics. 2009;10:370. doi: 10.1186/1471-2164-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Godoy LM, Marchini FK, Pavoni DP, Rampazzo Rde C, Probst CM, Goldenberg S, Krieger MA. Quantitative proteomics of Trypanosoma cruzi during metacyclogenesis. Proteomics. 2012;12(17):2694–2703. doi: 10.1002/pmic.201200078. [DOI] [PubMed] [Google Scholar]
- Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol Biochem Parasitol. 2007;156(2):93–101. doi: 10.1016/j.molbiopara.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bayer-Santos E, Gentil LG, Cordero EM, Correa PR, da Silveira JF. Regulatory elements in the 3' untranslated region of the GP82 glycoprotein are responsible for its stage-specific expression in Trypanosoma cruzi metacyclic trypomastigotes. Acta Trop. 2012;123(3):230–233. doi: 10.1016/j.actatropica.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Field MC, Carrington M. Intracellular membrane transport systems in Trypanosoma brucei. Traffic. 2004;5(12):905–913. doi: 10.1111/j.1600-0854.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- Field MC, Lumb JH, Adung’a VO, Jones NG, Engstler M. Macromolecular trafficking and immune evasion in african trypanosomes. Int Rev Cell Mol Biol. 2009;278:1–67. doi: 10.1016/S1937-6448(09)78001-3. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Souto-Padron T, De Souza W. Identification of a large pre-lysosomal compartment in the pathogenic protozoon Trypanosoma cruzi. J Cell Sci. 1992;102(Pt 1):157–167. doi: 10.1242/jcs.102.1.157. [DOI] [PubMed] [Google Scholar]
- Porto-Carreiro I, Attias M, Miranda K, De Souza W, Cunha-e-Silva N. Trypanosoma cruzi epimastigote endocytic pathway: cargo enters the cytostome and passes through an early endosomal network before storage in reservosomes. Eur J Cell Biol. 2000;79(11):858–869. doi: 10.1078/0171-9335-00112. [DOI] [PubMed] [Google Scholar]
- Sant’Anna C, de Souza W, Cunha-e-Silva N. Biogenesis of the reservosomes of Trypanosoma cruzi. Microsc Microanal. 2004;10(5):637–646. doi: 10.1017/S1431927604040863. [DOI] [PubMed] [Google Scholar]
- Sant’Anna C, Pereira MG, Lemgruber L, de Souza W, Cunha e Silva NL. New insights into the morphology of Trypanosoma cruzi reservosome. Microsc Res Tech. 2008;71(8):599–605. doi: 10.1002/jemt.20592. [DOI] [PubMed] [Google Scholar]
- de Mendonca Mauricio SM, da Silva Nepomuceno JL, Cunha e Silva N, de Souza W, Gazos Lopes U. Characterization of a Rab11 homologue in Trypanosoma cruzi. Gene. 2000;243(1-2):179–185. doi: 10.1016/S0378-1119(99)00480-1. [DOI] [PubMed] [Google Scholar]
- Araripe JR, Cunha e Silva NL, Leal ST, de Souza W, Rondinelli E. Trypanosoma cruzi: TcRAB7 protein is localized at the Golgi apparatus in epimastigotes. Biochem Biophys Res Commun. 2004;321(2):397–402. doi: 10.1016/j.bbrc.2004.06.159. [DOI] [PubMed] [Google Scholar]
- Araripe JR, Ramos FP, Cunha e Silva NL, Urmenyi TP, Silva R, Leite Fontes CF, da Silveira JF, Rondinelli E. Characterization of a RAB5 homologue in Trypanosoma cruzi. Biochem Biophys Res Commun. 2005;329(2):638–645. doi: 10.1016/j.bbrc.2005.02.021. [DOI] [PubMed] [Google Scholar]
- de Melo LD, Nepomuceno-Silva JL, Sant’Anna C, Eisele N, Ferraro RB, Meyer-Fernandes JR, de Souza W, Cunha-e-Silva NL, Lopes UG. TcRho1 of Trypanosoma cruzi: role in metacyclogenesis and cellular localization. Biochem Biophys Res Commun. 2004;323(3):1009–1016. doi: 10.1016/j.bbrc.2004.08.197. [DOI] [PubMed] [Google Scholar]
- Fujita M, Kinoshita T. GPI-anchor remodeling: Potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta. 2012;1821(8):1050–1058. doi: 10.1016/j.bbalip.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 1999;18(8):2057–2065. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaissi MA, Dubremetz JF, Schoneck R, Fernandez-Gomez R, Gomez-Corvera R, Billaut-Mulot O, Taibi A, Loyens M, Tartar A, Sergheraert C. Trypanosoma cruzi: a 52-kDa protein sharing sequence homology with glutathione S-transferase is localized in parasite organelles morphologically resembling reservosomes. Exp Parasitol. 1995;81(4):453–461. doi: 10.1006/expr.1995.1138. [DOI] [PubMed] [Google Scholar]
- Cuevas IC, Rohloff P, Sanchez DO, Docampo R. Characterization of farnesylated protein tyrosine phosphatase TcPRL-1 from Trypanosoma cruzi. Eukaryot Cell. 2005;4(9):1550–1561. doi: 10.1128/EC.4.9.1550-1561.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo A, Oliveira CR, Wurtele M, Chung J, Hilpert K, Schenkman S. A novel monoclonal antibody against the C-terminus of beta-tubulin recognizes endocytic organelles in Trypanosoma cruzi. Protein Pept Lett. 2012;19(6):636–643. doi: 10.2174/092986612800494075. [DOI] [PubMed] [Google Scholar]
- Guerra-Slompo EP, Probst CM, Pavoni DP, Goldenberg S, Krieger MA, Dallagiovanna B. Molecular characterization of the Trypanosoma cruzi specific RNA binding protein TcRBP40 and its associated mRNAs. Biochem Biophys Res Commun. 2012;420(2):302–307. doi: 10.1016/j.bbrc.2012.02.154. [DOI] [PubMed] [Google Scholar]
- Sant’Anna C, Nakayasu ES, Pereira MG, Lourenco D, de Souza W, Almeida IC, Cunha ESNL. Subcellular proteomics of Trypanosoma cruzi reservosomes. Proteomics. 2009;9(7):1782–1794. doi: 10.1002/pmic.200800730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-e-Silva N, Sant’Anna C, Pereira MG, Porto-Carreiro I, Jeovanio AL, de Souza W. Reservosomes: multipurpose organelles? Parasitol Res. 2006;99(4):325–327. doi: 10.1007/s00436-006-0190-3. [DOI] [PubMed] [Google Scholar]
- de Souza W, Sant’Anna C, Cunha-e-Silva NL. Electron microscopy and cytochemistry analysis of the endocytic pathway of pathogenic protozoa. Prog Histochem Cytochem. 2009;44(2):67–124. doi: 10.1016/j.proghi.2009.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometry analysis of parasite populations undergoing differentiation. Histograms showing there is a shift in fluorescence intensity in the whole parasite population obtained at 24 h and 48 h compared with epimastigote sample, indicating that intermediate forms are responsible for the signal and not the 2-3% metacyclic forms contamination.