Abstract
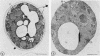
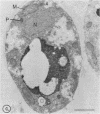
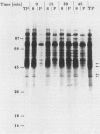
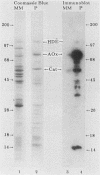
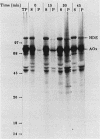
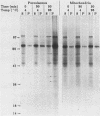
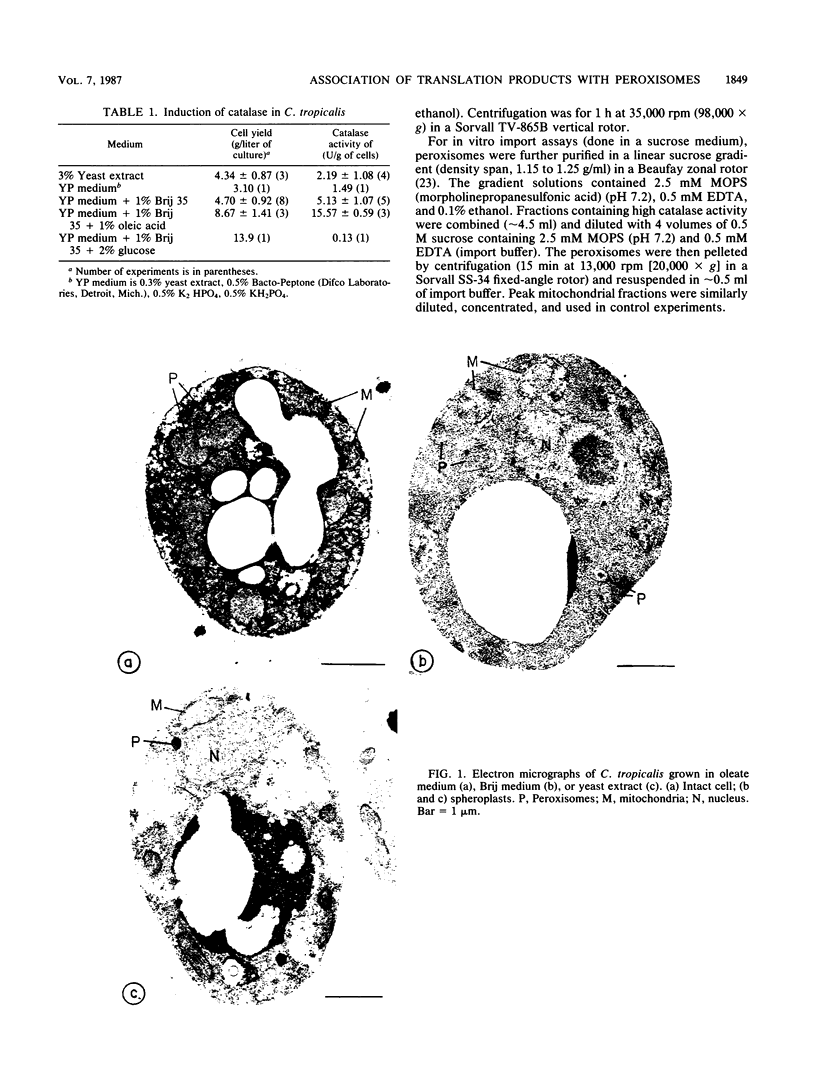
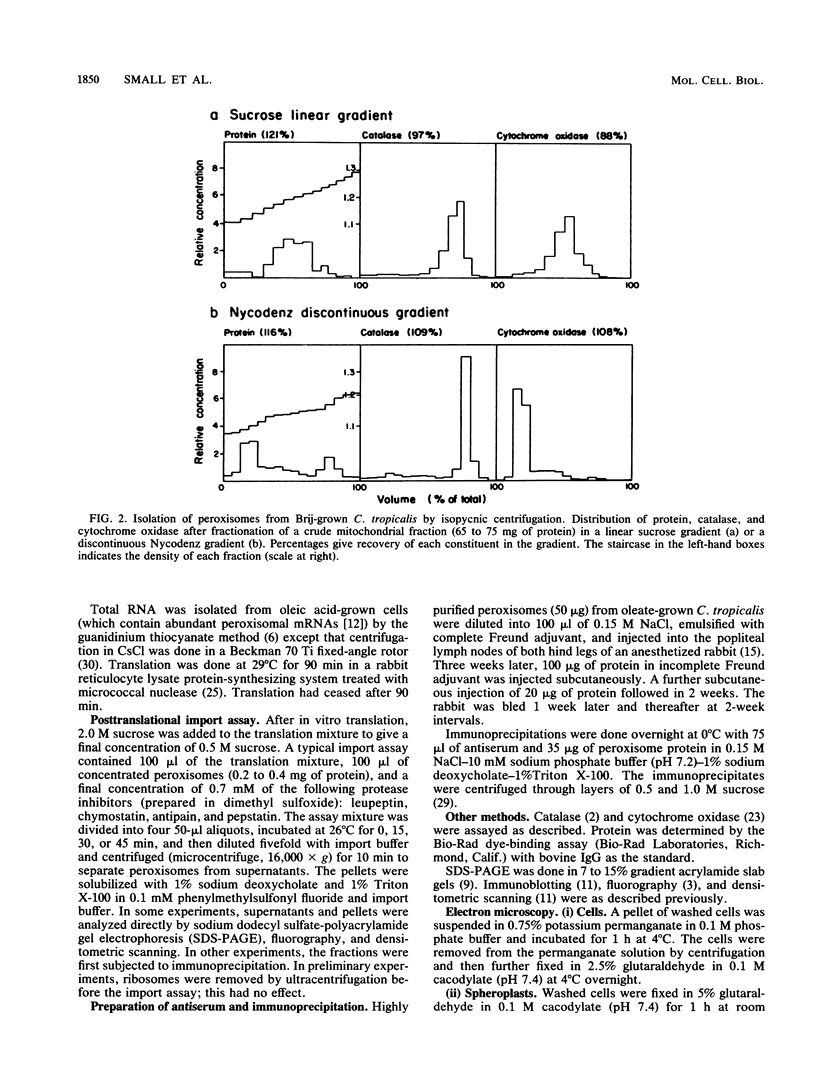
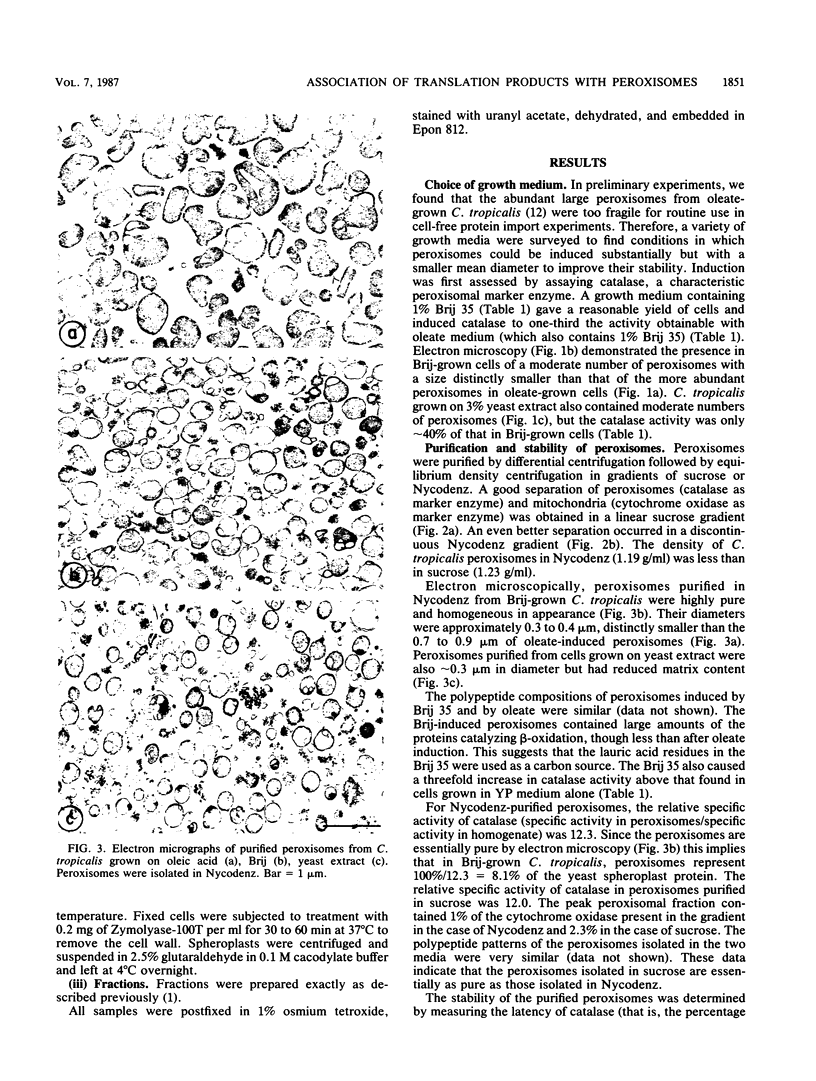
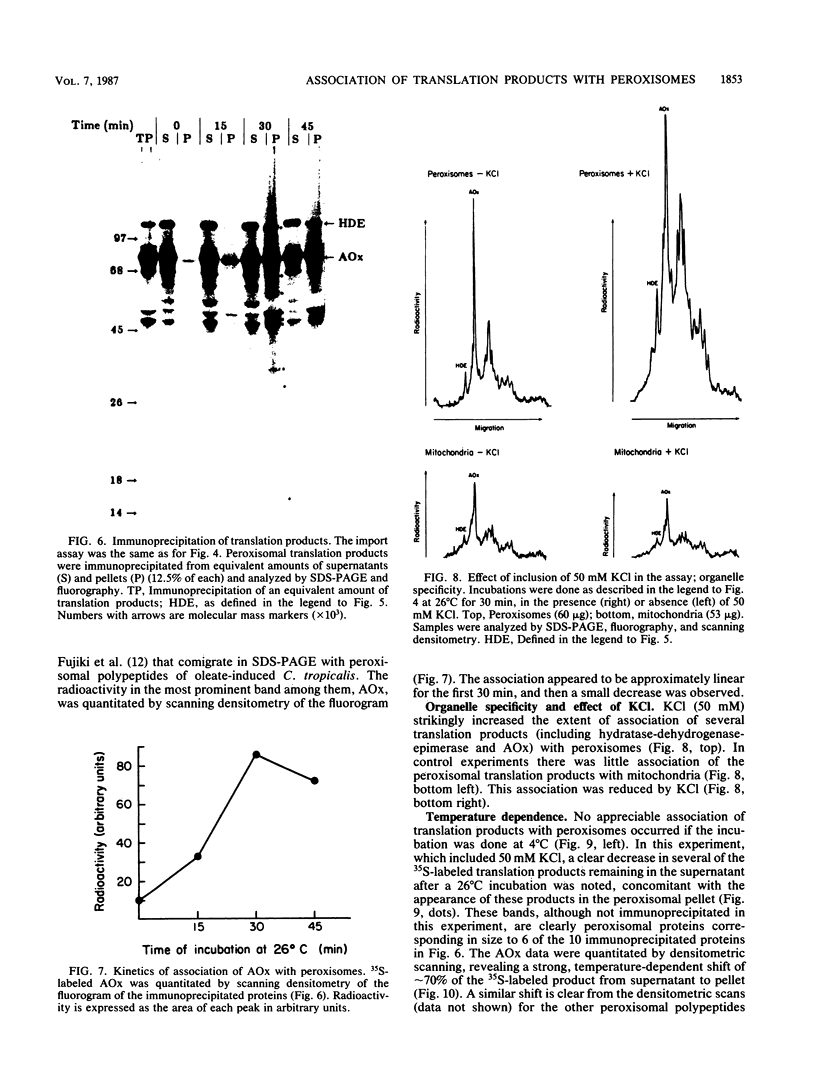
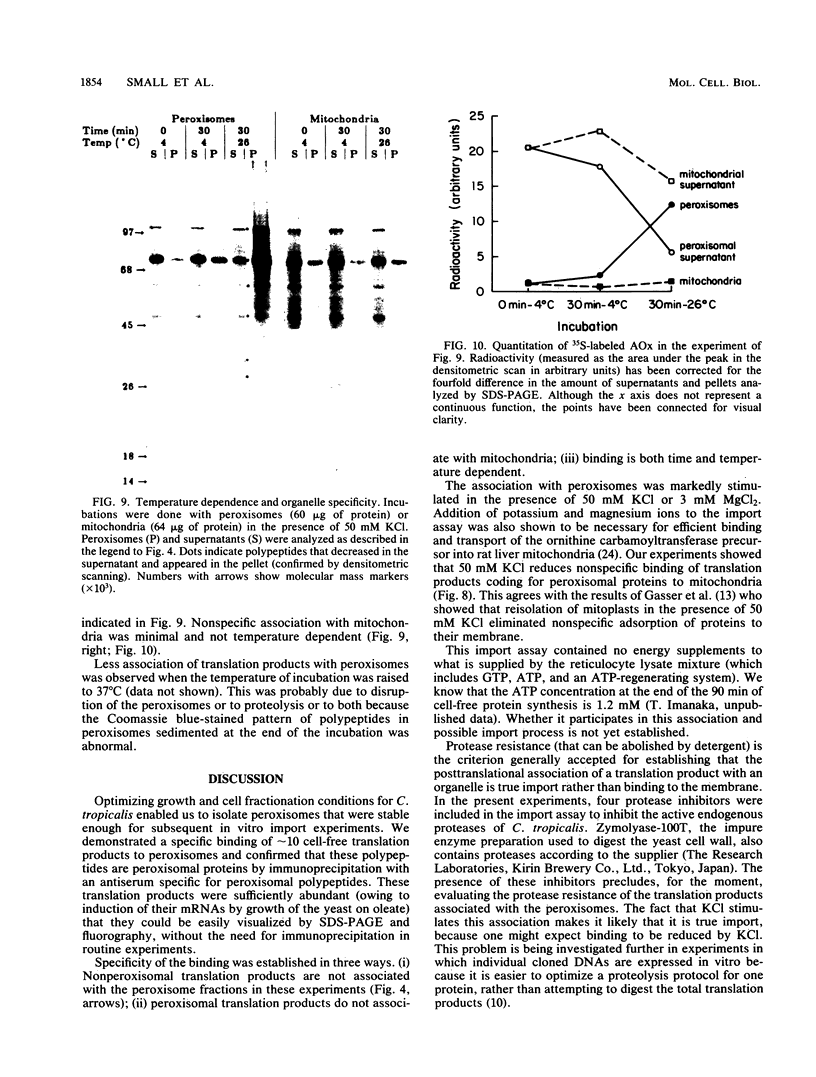
Newly synthesized peroxisomal proteins enter preexisting peroxisomes posttranslationally in vivo, generally without proteolytic processing. An efficient reconstitution of this process in vitro together with cloned DNAs for peroxisomal proteins would make possible investigation of the molecular information that targets proteins to peroxisomes. We have previously reported the isolation of clones for Candida tropicalis peroxisomal proteins; here we describe the association (and possible import) of peroxisomal proteins with peroxisomes in vitro. C. tropicalis was grown in a medium containing Brij 35, resulting in the induction of a moderate number of medium-sized peroxisomes. These peroxisomes, isolated in a sucrose gradient, had a catalase latency of 54% and were sufficiently stable to be concentrated and used in an import assay. The reticulocyte lysate translation products of total RNA from oleate-grown cells were incubated with the peroxisomes at 26 degrees C in the presence of 50 mM KCl, protease inhibitors, 0.5 M sucrose, 2.5 mM MOPS (morpholinepropanesulfonic acid) (pH 7.2), and 0.5 mM EDTA. Ten major translation products (which could be immunoprecipitated with antiserum against peroxisomal protein) became progressively associated with the peroxisomes during the first 30 min of incubation (some up to approximately 70%). These include acyl coenzyme A oxidase and the trifunctional protein hydratase-dehydrogenase-epimerase. This association did not occur at 4 degrees C nor did it occur if the peroxisomes were replaced with mitochondria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexson S. E., Fujiki Y., Shio H., Lazarow P. B. Partial disassembly of peroxisomes. J Cell Biol. 1985 Jul;101(1):294–304. doi: 10.1083/jcb.101.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borst P. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim Biophys Acta. 1986 May 5;866(4):179–203. doi: 10.1016/0167-4781(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dommes P., Dommes V., Kunau W. H. beta-Oxidation in Candida tropicalis. Partial purification and biological function of an inducible 2,4-dienoyl coenzyme A reductase. J Biol Chem. 1983 Sep 25;258(18):10846–10852. [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Lazarow P. B. Post-translational import of fatty acyl-CoA oxidase and catalase into peroxisomes of rat liver in vitro. J Biol Chem. 1985 May 10;260(9):5603–5609. [PubMed] [Google Scholar]
- Fujiki Y., Rachubinski R. A., Lazarow P. B. Synthesis of a major integral membrane polypeptide of rat liver peroxisomes on free polysomes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7127–7131. doi: 10.1073/pnas.81.22.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Rachubinski R. A., Zentella-Dehesa A., Lazarow P. B. Induction, identification, and cell-free translation of mRNAs coding for peroxisomal proteins in Candida tropicalis. J Biol Chem. 1986 Nov 25;261(33):15787–15793. [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Goudie R. B., Horne C. H., Wilkinson P. C. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966 Dec 3;2(7475):1224–1226. doi: 10.1016/s0140-6736(66)92305-1. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate reductase into the mitochondrial matrix. EMBO J. 1984 Dec 20;3(13):3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Nozaki C., Tanaka A., Fukui S. Fatty acid beta-oxidation system in microbodies of n-alkane-grown Candida tropicalis. Eur J Biochem. 1978 Feb;83(2):609–613. doi: 10.1111/j.1432-1033.1978.tb12130.x. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Tatibana M. Transport of ornithine carbamoyltransferase precursor into mitochondria. Stimulation by potassium ion, magnesium ion, and a reticulocyte cytosolic protein(s). J Biol Chem. 1983 Jun 10;258(11):6671–6674. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rachubinski R. A., Fujiki Y., Lazarow P. B. Cloning of cDNA coding for peroxisomal acyl-CoA oxidase from the yeast Candida tropicalis pK233. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3973–3977. doi: 10.1073/pnas.82.12.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Synthesis of rat liver albumin in a rabbit reticulocyte cell-free protein-synthesizing system. J Biol Chem. 1973 Nov 25;248(22):7661–7668. [PubMed] [Google Scholar]
- Zimmermann R., Neupert W. Biogenesis of glyoxysomes. Synthesis and intracellular transfer of isocitrate lyase. Eur J Biochem. 1980 Nov;112(2):225–233. doi: 10.1111/j.1432-1033.1980.tb07198.x. [DOI] [PubMed] [Google Scholar]