Abstract
Walnut consumption improves cardiovascular disease risk; however, to our knowledge, the contribution of individual walnut components has not been assessed. This study evaluated the acute consumption of whole walnuts (85 g), separated nut skins (5.6 g), de-fatted nutmeat (34 g), and nut oil (51 g) on postprandial lipemia, endothelial function, and oxidative stress. Cholesterol efflux (ex vivo) was assessed in the whole walnut treatment only. A randomized, 4-period, crossover trial was conducted in healthy overweight and obese adults (n = 15) with moderate hypercholesterolemia. There was a treatment × time point interaction for triglycerides (P < 0.01) and increased postprandial concentrations were observed for the oil and whole walnut treatments (P < 0.01). Walnut skins decreased the reactive hyperemia index (RHI) compared with baseline (P = 0.02) such that a difference persisted between the skin and oil treatments (P = 0.01). The Framingham RHI was maintained with the oil treatment compared with the skins and whole nut (P < 0.05). There was a treatment effect for the ferric reducing antioxidant potential (FRAP) (P < 0.01), and mean FRAP was greater with the oil and skin treatments compared with the nutmeat (P < 0.01). Cholesterol efflux increased by 3.3% following whole walnut consumption in J774 cells cultured with postprandial serum compared with fasting baseline (P = 0.02). Walnut oil favorably affected endothelial function and whole walnuts increased cholesterol efflux. These 2 novel mechanisms may explain in part the cardiovascular benefits of walnuts.
Introduction
Cardiovascular disease continues to be the leading cause of morbidity and mortality in the United States (1). This has been the impetus for implementing new strategies to reduce the public health burden of cardiovascular disease. In 2010, the AHA issued the following Impact Goals: “By 2020, to improve the cardiovascular health of all Americans by 20% while reducing deaths from cardiovascular diseases and stroke by 20%” (2). The AHA recommends including ≥4 servings/wk of nuts, legumes, and seeds (2). Similarly, the 2010 Dietary Guidelines for Americans recommend a healthy eating pattern focused on nutrient-dense foods and advise a variety of protein foods, including nuts and seeds (3). These recommendations were established from a robust evidence base for nuts that has rapidly evolved over the last decade (4–6).
The putative health benefits of walnuts, including a cardioprotective effect, may be mediated by their constituent polyphenols, γ-tocopherol, α-linolenic acid (ALA)9, linoleic acid, and l-arginine (7, 8). Chronic and acute consumption of walnuts (42.5–85 g/d) lowers total cholesterol (TC) and LDL cholesterol concentrations (9, 10), decreases blood pressure (11), improves endothelial function (12, 13), may have beneficial effects on measures of oxidative stress (14), and reduces some inflammatory markers (12, 15–17). However, the contribution of different walnut components, i.e., skin, oil, and defatted nutmeat, to these biological effects has, to our knowledge, never been explored. Thus, the current study evaluated how whole walnuts and walnut components affect postprandial lipid/lipoprotein responses, endothelial function, and oxidative stress. In addition, we evaluated the effects of postprandial intake of whole walnuts, the most common form in which walnuts are consumed, on ex vivo cholesterol efflux.
Participants and Methods
Study population
Men and women (n = 15, 9 women and 6 men) aged 21–60 y who were free of any serious illness and did not use tobacco products were recruited for the study. Other inclusion criteria were BMI 25–39 kg/m2, LDL cholesterol ≥110 mg/dL (<95th percentile for age and gender based on NHANES data), and TG <350 mg/dL. Exclusion criteria included: alcohol consumption ≥21 or ≥28 servings/wk (female and male participants, respectively), intake of vitamin or mineral supplements within the past 3 wk, use of prescription cholesterol or blood pressure-lowering medications, intake of lipid-lowering supplements, use of antiinflammatory medications on a regular basis or an acute intake within 1 wk of a test day, vegetarian diet, weight gain/loss of ≥ 10% within the previous 6 mo, and/or pregnant or wishing to become pregnant 3 mo before or during the study. A complete blood count and standard chemistry panel were obtained at screening to rule out the presence of illness. Blood pressure was measured according to Joint National Committee 7 Guidelines (18).
Recruitment and ethical aspects
Participants were recruited through advertisements in the local newspaper and university e-mails; 246 individuals expressed interest in the study (Supplemental Fig. 1). Of those who were contacted and screened with the use of a semi-structured telephone interview, 50 qualified and were scheduled for clinic screening at the Penn State General Clinical Research Center. After written informed consent was obtained, a fasting blood sample was drawn for a complete blood count and general health profile (lipid panel, glucose, and liver and kidney function). Eligible participants were phoned and 2 visits were scheduled: first, to meet with a study dietitian at the study site (n = 18) or over the phone if they could not meet in person (n = 2) and second, for their first postprandial test visit. Twenty participants were randomized to the study; however, one participant withdrew at the first visit because of a vagal response following the first blood draw; one participant was excluded prior to their second visit due to noncompliance with the antioxidant and fasting requirements; and 3 participants each withdrew prior to their second visit due to nausea following the walnut meal, inability to consume the low antioxidant diet, and work commitments. The study protocol was approved by the Institutional Review Board of the Pennsylvania State University.
Design and intervention
A randomized, controlled, postprandial, 4-period crossover study with at least 1 wk separation between testing sessions was conducted. Each visit evaluated the oral ingestion of one treatment: 1) 85 g (3 oz) ground, whole walnuts; 2) 34 g ground, skinless, defatted nutmeat from walnuts; 3) 51 g oil from skinless walnuts; or 4) 5.6 g ground walnut skins.
The walnuts were unroasted English walnuts (Juglans regia) from a single lot and frozen upon delivery. Shelled whole walnuts were weighed to 85 g, blanched in hot water for 1 min, and then submerged in ice-cold water for 30 s. Trained research staff removed the skins with a large toothpick or knife. This procedure was repeated 5 times and the average weight of dry walnut skins was calculated using a balance accurate to 0.01 g. The research team carried out the walnut shelling, weighing, blanching, and skinning until sufficient skins had been obtained. The walnut skins were frozen until the day before use. The skinless walnuts were refrozen until they were fractionated into oil and defatted nutmeat. Frozen, skinless walnuts were thawed, ground with a mortar and pestle, and weighed to 85 g. Oil in the ground walnuts was then extracted with high purity n-hexane using a semicontinuous Soxhlet glass apparatus. For the defatted nutmeat, the residual hexane was extracted in n-hexane–free hexadecane and measured directly from the extracted oil. The samples were injected by static headspace techniques with the use of an internal standard. The residual hexane was <2 ppm, quantified using GC-MS. Following the extraction procedure, the defatted nutmeat and oil were refrozen until the day before use.
To facilitate consumption of the whole walnuts and the different walnut components, diet Jell-O was chosen to act as an inert food carrier. One-half cup of diet Jell-O (per 92-g serving: 7 kcal, 0 g fat, 1.3 g protein, 0 g sugar) was prepared according to packet instructions, portioned into an individual glass bowl, and combined with the thawed walnut component. The walnut-Jell-O meal was refrigerated and given to the participant for consumption following fasting measurements. The energy and macronutrient composition of each component are presented in Table 1.
TABLE 1.
Estimated nutrient composition for each walnut component derived from 85 g of whole walnuts
| Mass | Energy | Carbohydrate | Fat | Protein | |
| g | kJ | g | |||
| Whole walnut1 | 85 | 2330 | 12 | 55 | 13 |
| Oil1 | 51 | 1890 | 0 | 51 | 0 |
| Nutmeat2 | 34 | 440 | 12 | 4 | 13 |
| Skin | 5.6 | – | – | – | – |
Values obtained from the USDA Nutrient Database SR-24 (7).
Values calculated by subtracting walnut oil from whole walnut nutrient data.
Prior to starting the study and during each testing visit, a dietitian advised each participant on how to consume and incorporate low-antioxidant foods into their diet for 3 d prior to each test visit. The purpose of the low-antioxidant diet was to maximize the potential for observing effects with the high-antioxidant food (i.e., walnuts and walnut components). Participants were asked to keep a dietary record for the 3 low-antioxidant days and instructions on completing these records were explained. They also were asked to consume similar low-antioxidant foods for the 3 d before each of the 4 visits.
On the test morning, participants arrived at the General Clinical Research Center after a 12-h overnight fast and trained research staff measured their height and weight, obtained a 30-mL fasting blood sample, and conducted the endothelial function test. Participants then had 15 min to consume 1 of the 4 walnut-Jell-O test meals under study personnel supervision. Blood samples (∼30 mL) were subsequently taken at 30, 60, 120, 240, and 360 min following the meal and the endothelial function test was done immediately prior to the 240-min blood draw. Maximal changes in endothelial function and TG occur 3–4 h after a high-fat meal (19). For the 6-h postprandial time period, participants rested in an on-site room and were allowed to drink noncaloric, decaffeinated beverages (i.e., water and diet soda). Blood was centrifuged at 4°C for 15 min and aliquots of serum and plasma were stored in a −80°C freezer until further analyses.
Assay methods
Lipid profile.
TC and TG were determined by standard colorimetric and enzymatic procedures with commercially available kits (cholesterol and TG kits, Alfa Wassermann). HDL cholesterol was quantified according to the modified heparin-manganese precipitation procedure of Warnick and Albers (20). LDL cholesterol was determined using the Friedewald equation:
All samples from each participant were analyzed in the same batch. These assays were conducted at the General Clinical Research Center Core Laboratory at Hershey Medical Center.
Total thiols.
Total thiols in plasma were determined according to the spectrometric method of Hu (21). Briefly, an aliquot of EDTA plasma was mixed with Tris-EDTA buffer, followed by addition of 10 mmol/L 2,2-dithiobisnitrobenzoic acid and methanol. After incubation at room temperature for 15 min and centrifugation, the absorbance of the supernatant was measured at 412 nm (Shimadzu UV-1800 spectrophotometer). Plasma total thiols are expressed as mmol/L.
Ferric reducing antioxidant potential.
The ferric reducing antioxidant potential (FRAP) assay was used to determine the reducing ability of plasma in a redox-linked colorimetric reaction using the method of Benzie and Strain (22) with slight modifications. Briefly, plasma was incubated with the FRAP reagent at room temperature for 1 h and the absorbance at 593 nm was then recorded (Shimadzu UV-1800 spectrophotometer). Trolox was used as a reference to construct a standard curve to calculate the FRAP value of the samples (23). The FRAP assay was used to measure total antioxidant capacity, because it is responsive to lipophilic (tocopherols) and hydrophilic antioxidants (polyphenols), both of which are present in walnuts. FRAP is expressed as μmol/L Trolox equivalents.
Plasma malondialdehyde.
Plasma malondialdehyde (MDA) was measured by an Agilent 1100 HPLC system with fluorometric detection according to Behrens and Madère (24) with some modifications. Briefly, 0.1 mL plasma was saponified with 5.5 μL of 10 N NaOH at 60°C for 30 min, followed by the addition of 600 μL of 7.2% trichloroacetic acid. The resulting mixture (400 μL) was then incubated with 0.2 mL of 0.6% thiobarbituric acid in sodium acetate buffer (pH 3.5) and 20 μL of 0.031 mol/L FeCl3 at 95°C for 1 h to generate MDA-thiobarbituric acid conjugate. The MDA conjugate was eluted on a Varian Microsorb 100–5 C18 column (150 × 4.6 mm, 5 um, Agilent Technologies) with a mobile phase of 65% sodium phosphate buffer and 35% methanol at a 0.8-mL/min flow rate and monitored at 515/553 nm excitation/emission. The MDA concentration was calculated based on a standard curve constructed using an authentic standard, 1,1,3,3-tetraethoxypropane. MDA is expressed as μmol/L.
Vascular function.
Endothelial function [RHI, Framingham RHI (fRHI), augmentation index (AI), AI normalized to a heart rate of 75 beats/min (AI_75)] was measured using pulse amplitude tonometry (PAT) (Itamar Medical) as previously described (25). Heart rate was measured by the PAT device as beats/min.
Cholesterol efflux.
Cholesterol efflux was assayed using J774 macrophages as previously described (26). ApoB-depleted serum was prepared using the method previously described (27).
Statistical analyses
Statistical analyses were performed using SAS (version 9.2; SAS Institute). The mixed models procedure (PROC MIXED) was used to test the effects of treatment, time point, visit, and their interactions (i.e., treatment × time point and treatment × visit) on changes in metabolic and antioxidant outcomes following the test meal. For metabolic endpoints (e.g., TG), outcomes were modeled as doubly repeated measures with unstructured by compound symmetry for time point and visit, respectively. For antioxidant endpoints (e.g., FRAP), we imposed a compound symmetry structure by designating participant as random effect. PROC MIXED also was used to test the effects of treatment, visit, and their interaction on changes in endothelial function and heart rate outcomes following the test meal. For these endpoints, we imposed a compound symmetry structure by designating participant as random effect. Baseline values were included as covariates. No carryover effects were observed. For the whole walnut only analysis, PROC MIXED was used to test the effects of time point, visit, and their interaction on cholesterol efflux following the test meal. Participant was treated as a random effect and the remaining factors were fixed effects.
For all outcomes, model selection was based on optimizing fit statistics (evaluated as lowest Bayesian information criterion) and α was set at 0.05 for all tests. Post hoc analyses were adjusted for multiple comparisons. Unadjusted P values were multiplied by the number of respective comparisons to accommodate an overall α < 0.05 (i.e., Bonferroni adjustment) and these adjusted P values are reported in the text. Change scores were calculated by subtracting pre-meal baseline values (0 min) from each time point. AUC values were calculated with the trapezoidal rule, using the respective fasting baseline value as the line of reference (28). Means are reported as least squares means ± SEMs.
Correlational analysis (PROC CORR) also was performed in SAS to measure the association between time-matched variables by treatment group. A sample size of 15 was determined based on earlier studies (29, 30), which detected significant changes in measures of oxidative stress with α set to 0.05 and power set to 0.80. Participants were randomized to 1 of 24 possible treatment sequences, with no participants receiving an identical sequence.
Results
Participant characteristics.
The screening and treatment baseline characteristics of study participants are presented in Tables 2 and 3, respectively. Treatments did not differ at baseline (Table 3). Nine participants reported having loose stools/diarrhea between 1 and 9 h following the walnut oil treatment; however, they all remained in the study.
TABLE 2.
| Variable | Value |
| Age, y | 49 ± 2 |
| Weight, kg | 85 ± 4 |
| BMI, kg/m2 | 29 ± 1 |
| SBP, mm Hg | 121 ± 2 |
| DBP, mm Hg | 78 ± 2 |
| TC, mmol/L | 5.8 ± 0.2 |
| HDL cholesterol, mmol/L | 1.4 ± 0.1 |
| LDL cholesterol, mmol/L | 3.7 ± 0.2 |
| TG, mmol/L | 1.5 ± 0.2 |
| TC:HDL cholesterol | 4.3 ± 0.3 |
| Glucose, mmol/L | 5.3 ± 0.1 |
Values are means ± SEMs, n = 15. DBP, diastolic blood pressure; SBP, systolic blood pressure; TC, total cholesterol.
Blood was drawn after a 12-h fast.
TABLE 3.
Serum lipids, antioxidant status measures, and endothelial function outcomes in participants prior to meal consumption12
| Nutmeat | Oil | Skin | Whole nut | |
| TG, mmol/L | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| TC, mmol/L | 5.1 ± 0.2 | 5.0 ± 0.2 | 5.1 ± 0.2 | 5.0 ± 0.2 |
| LDL cholesterol, mmol/L | 3.4 ± 0.2 | 3.3 ± 0.2 | 3.5 ± 0.2 | 3.4 ± 0.2 |
| HDL cholesterol, mmol/L | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| TC:HDL cholesterol | 5.1 ± 0.4 | 5.0 ± 0.4 | 5.2 ± 0.4 | 4.9 ± 0.3 |
| FRAP, μmol TE/L | 1209 ± 55 | 1208 ± 78 | 1192 ± 56 | 1148 ± 50 |
| Total thiols, mmol/L | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.45 ± 0.03 | 0.40 ± 0.03 |
| MDA, μmol/L | 0.95 ± 0.13 | 0.85 ± 0.13 | 0.86 ± 0.07 | 0.75 ± 0.08 |
| RHI | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.2 |
| fRHI | 0.28 ± 0.07 | 0.25 ± 0.09 | 0.35 ± 0.09 | 0.43 ± 0.10 |
| AI | 5.7 ± 5.5 | 3.0 ± 5.5 | 5.1 ± 5.5 | 8.9 ± 5.5 |
| AI_75 | −5.1 ± 5.0 | −7.2 ± 5.0 | −4.3 ± 4.9 | −1.1 ± 4.9 |
| Heart rate, beats/min | 57.5 ± 1.7 | 58.2 ± 2.1 | 59.9 ± 2.1 | 59.1 ± 1.7 |
Values are means ± SEMs, n = 15. AI, augmentation index; AI_75, AI normalized to a heart rate of 75 beats/min; FRAP, ferric reducing antioxidant potential; fRHI, Framingham reactive hyperemia index; MDA, malondialdehyde; RHI, reactive hyperemia index; TC, total cholesterol; TE, Trolox equivalent.
Blood was drawn after a 12-h fast.
Postprandial lipid metabolism.
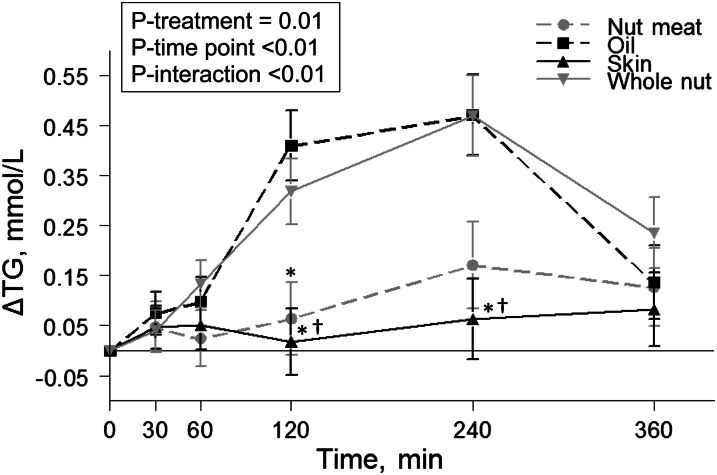
A mixed linear model demonstrated a treatment × time point interaction (P < 0.01) for TG. Peak TG responses for the oil and whole nut treatments were observed at 120 and 240 min postmeal (Fig. 1). At these time points, change in TG was greater following the oil (P < 0.01) and whole nut (P ≤ 0.03) treatments than the skin treatment. In addition, at 120 min, the TG response to the oil treatment was greater than that to nutmeat (P = 0.02). The significant treatment × time point interaction resulted in an 89% reduction in postprandial TG AUC for the skins relative to both the oil and whole nut treatments (P < 0.01). There were no treatment × time point interactions or treatment effects for TC, LDL-C, or HDL-C.
FIGURE 1.
Change in serum concentrations of TG after consumption of each test meal in healthy overweight and obese men and women. Values are least squares means ± SEMs, n = 15. Post hoc analyses at individual time points were conducted using the Bonferroni adjustment for multiple comparisons. *Compared with oil; †compared with whole nut, P < 0.05.
Oxidative stress.
The postmeal FRAP response differed by treatment (P < 0.01) (Supplemental Fig. 2). The nutmeat treatment tended to lower mean FRAP from baseline (P = 0.054) such that nutmeat differed from the oil and skin treatments (P < 0.01). Treatment × time interactions and treatment effects were not significant for plasma total thiols or MDA.
Endothelial function.
There were treatment effects for RHI and fRHI (P = 0.01 for both) (Table 4). The skin treatment reduced RHI compared with baseline (P = 0.02) such that the oil and skin treatments differed (P = 0.01). For fRHI, there were no changes from baseline; however, the oil treatment differed from the skin and whole nut treatments (P = 0.02 for both). There were no treatment effects for AI or AI_75 (Table 4).
TABLE 4.
Adjusted means and change scores for endothelial function outcomes after consumption of each treatment in overweight and obese adults1
| Nutmeat |
Oil |
Skin |
Whole nut |
||||||
| Mean2 | Δ3 | Mean | Δ | Mean | Δ | Mean | Δ | Treat P4 | |
| RHI | 1.80 ± 0.09a,b | −0.09 ± 0.09 | 1.96 ± 0.09a | 0.07 ± 0.09 | 1.64 ± 0.09b | −0.26 ± 0.09* | 1.70 ± 0.09a,b | −0.19 ± 0.09 | 0.01 |
| fRHI | 0.31 ± 0.07a,b | −0.02 ± 0.07 | 0.42 ± 0.07a | 0.09 ± 0.07 | 0.19 ± 0.07b | −0.14 ± 0.07 | 0.19 ± 0.07b | −0.14 ± 0.07 | 0.01 |
| AI | 1.48 ± 3.66 | −4.01 ± 3.66 | 2.87 ± 3.25 | −2.62 ± 3.25 | 4.10 ± 3.19 | −1.39 ± 3.19 | −1.08 ± 3.08 | −6.57 ± 3.08 | 0.44 |
| AI_75 | −7.61 ± 3.27 | −2.92 ± 3.27 | −4.74 ± 2.86 | −0.06 ± 2.86 | −5.88 ± 2.80 | −1.19 ± 2.80 | −8.69 ± 2.69 | −4.00 ± 2.69 | 0.62 |
Values are least squares means ± SEMs, n = 15. Labeled means in a row without a common letter differ, P < 0.05. *Represents a difference from 0 (baseline), P < 0.05. Post hoc analyses at individual time points were conducted using the Bonferroni adjustment for multiple comparisons. AI, augmentation index; AI_75, AI normalized to a heart rate of 75 beats/min; fRHI, Framingham reactive hyperemia index; RHI, reactive hyperemia index.
Means represent postprandial values (240 min).
Change scores were calculated by subtracting baseline values (0 min) from postprandial values (240 min).
P value for the main effect of treatment.
Heart rate.
Heart rate differed across treatments (P < 0.01). The postprandial heart rate was greater with the oil (63.1 ± 1.4 beats/min; P = 0.01) and whole nut (62.6 ± 1.4 beats/min; P = 0.02) treatments compared with the skin treatment (58.9 ± 1.4 beats/min). The oil and whole nut treatments also increased heart rate from baseline (P < 0.05 for both).
Whole walnut ex vivo cholesterol efflux.
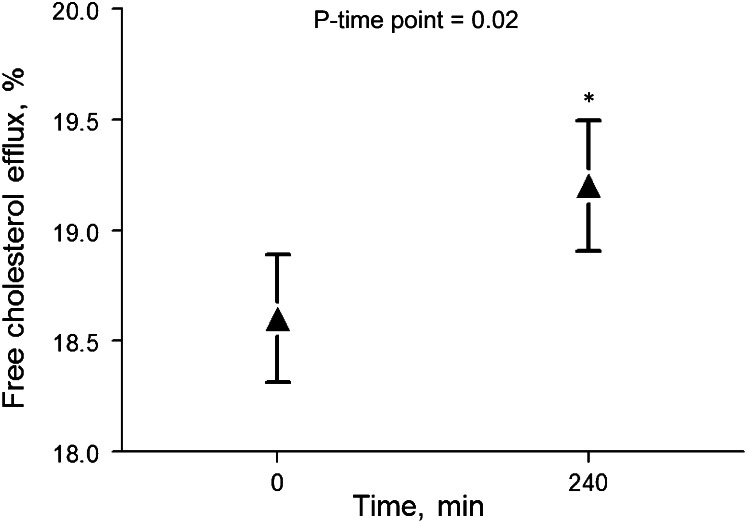
Cholesterol efflux increased by 3.3% in cells cultured with postprandial serum relative to fasting baseline (P = 0.02) (Fig. 2). Cholesterol efflux was positively correlated with plasma TC (r = 0.78), LDL cholesterol (r = 0.70), and HDL cholesterol (r = 0.43) concentrations (P ≤ 0.02 for all). In cells cultured with apoB-depleted serum, there were no significant changes in cholesterol efflux.
FIGURE 2.
Free cholesterol efflux was increased in cells cultured with postmeal serum relative to fasting baseline serum. Values are least squares means ± SEMs, n = 15. *Different from 0 min, P < 0.05.
Discussion
The present study is the first to our knowledge to evaluate the cardioprotective mechanisms of individual walnut components (i.e., skin, defatted nutmeat, and oil) compared with whole walnuts. Herein, we report significant effects for some of these walnut components on TG, heart rate, endothelial function (RHI and fRHI), and FRAP. Importantly, we also observed that whole walnuts enhanced ex vivo cholesterol efflux, a measure of reverse cholesterol transport.
Acute consumption of walnut oil preserved endothelial function compared with whole walnuts and walnut skins. Walnuts are a rich source of ALA (∼13% of total lipids) and γ-tocopherol (20 mg/100 g) and contain phytosterols (164 mg/100 g), which may explain the positive effects of the walnut oil treatment (8). This is consistent with the findings of West et al. (11) that a chronic diet high in ALA (6.5% of kcal provided by 37 g walnuts, 15 g walnut oil, and 19 g flaxseed oil) increased flow-mediated dilation (FMD) compared with the average American diet in hypercholesterolemic participants. In the current study, the effect of walnut oil on endothelial function may be due in part to ALA and/or its greater bioavailability (in a postprandial model) than the whole walnut. Support for this is suggested by the subtle differences in the TG response curve; walnut oil elicited a more rapid increase than did whole walnuts. Furthermore, postprandial endothelial function is impaired after consumption of a high-fat meal (31); thus, greater bioavailability of nutrients in the oil portion (i.e., ALA and γ-tocopherol) may have blunted this response and contributed to the differential effects observed between whole walnuts and walnut oil (32). Moreover, in an almond study conducted by Ellis et al. (33), the bioaccessibility of almond fat was hampered by cell wall integrity such that mechanical manipulation and chewing did not enable complete release of intracellular lipids for enzymatic digestion and absorption. Further studies are warranted to examine the potential for the difference in bioavailability of ALA in a more accessible food form compared with that in a complex food matrix, such as whole walnuts. That endothelial function decreased with walnut skin consumption could be due to a lack of antioxidant absorption, perhaps because of the food matrix used for their delivery or the lack of ALA in the skins.
Another explanation for the lack of a whole walnut effect on endothelial function could reflect the participant population studied. In a study conducted by Cortés et al. (13), acute consumption of walnuts (40 g) ingested with a high-fat meal increased FMD (24%) in hypercholesterolemic adults (TC: 250 ± 25 mg/dL) compared with the same meal with olive oil (25 g) but not in adults with normal TC concentrations (TC: 185 ± 27 mg/dL). In the present study, participants had TC concentrations that were comparable with the normocholesterolemic participants in the Cortés et al. (13) study, which may explain why we also observed no postprandial change in endothelial function after whole walnut consumption. Ros et al. (12) reported improved endothelium-dependent vasodilation in hypercholesterolemic men and women after 8 wk of a walnut diet that provided 32% of energy from walnuts compared with the control Mediterranean diet (5.9 ± 3.3% vs. 3.6 ± 3.3%, respectively). In this study, endothelial function was assessed after consumption of a high-fat meal enriched with either olive oil or walnuts within the context of the respective test diet. Another explanation for our findings compared with those reported by Ros et al. (12) could reflect the acute postprandial study design we used as well as the delivery mode of the walnut components. Finally, as suggested by Moerland et al. (34), the PAT technique may measure physiologically different outcomes than conventional methods (i.e., FMD) and may not be suitable for assessing acute changes in endothelial function (34).
Arterial stiffness, as measured by the PAT AI and AI_75, is increased in patients with chronic kidney disease and in patients with type 2 diabetes; however, it is unaffected by acute vascular injury, such as smoking and glucose overloading interventions (34). There were no treatment effects for arterial stiffness in our study, which is consistent with the results of Din et al. (35), who demonstrated in a randomized, single-blind, crossover study that dietary walnut supplementation (15 g/d) for 4 wk did not affect AI using pulse waveform analysis.
Our findings for biomarkers of oxidative stress are consistent with those reported by others (12–14). In a 6-wk, free-living study, no chronic effects of walnut consumption (21 and 42 g/d) on antioxidant activity (e.g., MDA, FRAP, and total thiols) were observed (14). Ros et al. (12) found oxidized LDL and MDA remained unchanged for both the Mediterranean diet with and without walnuts. Similarly, Cortés et al. (13) reported in vitro resistance of LDL against oxidation was unaffected by acute walnut consumption. The observed differences in plasma FRAP may be attributed to the lack of bioactive components in the nutmeat compared with the oil, skin, and whole walnut treatments. Although walnuts contain numerous phenolic and other antioxidant constituents, the null effects on antioxidant measures in our study may be attributed to a relatively small increase in their plasma concentration against an already sufficient antioxidant defense status in this moderately healthy, albeit overweight/obese, cohort.
This study is the first to our knowledge to report increased ex vivo cholesterol efflux following postprandial consumption of whole walnuts. Cholesterol efflux is an important antiatherogenic step in the reverse cholesterol transport pathway and is a novel measure of HDL functionality. Independent of HDL cholesterol and apoA-1, cholesterol efflux from macrophages is a strong predictor of both carotid intima-media thickness and coronary disease (36). Zhang et al. (37, 38) reported that ALA and walnut oil increased in vitro cholesterol efflux from macrophage-derived foam cells. However, the treatment response was dependent on the C-reactive protein (CRP) concentration; when participants were stratified on the basis of high and low CRP concentrations, the serum of the low CRP group enhanced ex vivo cholesterol efflux by 17%, whereas the high CRP group showed no effect (37). Based on the results reported herein, acute consumption of walnuts increased ex vivo cholesterol efflux, suggesting a novel mechanism by which walnut consumption may reduce cardiovascular risk.
A major strength of this study is its original design, which directly compares whole walnuts with individual walnut components. In addition, we demonstrated metabolic effects of some walnut constituents as measured by changes in TG and heart rate. Another strength is the postprandial study design that allowed us to characterize the metabolic time course of walnut and walnut component consumption. Finally, novel outcome measures employed in this study that had positive effects included endothelial function and ex vivo cholesterol efflux. These findings extend our understanding of how walnuts may elicit a cardioprotective effect.
A limitation of this study is the small sample size (n = 15). The results from the current study need to be verified in a larger and more diverse population. Another limitation is the lack of a control group, e.g., a Jell-O meal without any walnuts or walnut components, which would have provided a standard of comparison. Other potential shortcomings of our study include measurement timing, oil and nutmeat extraction method, and/or nutrient bioavailability. For example, the time points we chose to draw blood and measure endpoints may not have been representative of the entire postprandial period, the oil and nutmeat extraction method may have contributed residuals and/or adversely affected walnut bioactives, and the polyphenols in walnut skins may have been only partially absorbed because of their high fiber content. In a previous postprandial study (13), each treatment was consumed in the context of a high-fat meal, which may have increased absorption and bioavailability of lipophilic nutrients. Finally, cholesterol efflux was quantified only in the whole walnut group as an exploratory analysis, which precluded comparison among treatments. An effect of walnuts on ex vivo cholesterol efflux was observed with whole serum but not apoB-depleted serum; in addition to HDL, LDL can serve as an acceptor of free cholesterol in the reverse cholesterol transport pathway (39), indicating that walnuts may have a greater effect on global cholesterol efflux than HDL-specific efflux. It will be important to further evaluate cholesterol efflux in response to acute and chronic walnut consumption.
In conclusion, we showed that acute consumption of walnut oil favorably affects endothelial function compared with whole walnuts and walnut skins. We also demonstrated novel effects of whole walnuts on reverse cholesterol transport. Therefore, frequent consumption of walnuts and/or walnut oil (which typically is how walnut products are consumed) may improve cardiovascular risk via mechanisms that extend beyond their established cholesterol-lowering action.
Supplementary Material
Acknowledgments
The authors are grateful for use of the General Clinical Research Center of the Pennsylvania State University. P.M.K.-E., S.G.W., and J.B.B. designed the research; J.A.G. and C.E.B. conducted the research; C-Y.O.C. and J.B.B. selected and performed the MDA, FRAP, and thiol measures; G.H.R. and S.S. selected and performed the ex vivo cholesterol efflux measures; C.E.B. and S.G.W. performed the statistical analysis; and C.E.B. and P.M.K.-E. wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AI, augmentation index; AI_75, augmentation index normalized to a heart rate of 75 beats/min; ALA, α-linolenic acid; CRP, C-reactive protein; FMD, flow-mediated dilation; FRAP, ferric reducing antioxidant potential; fRHI, Framingham reactive hyperemia index; MDA, malondialdehyde; PAT, pulse amplitude tonometry; RHI, reactive hyperemia index; TC, total cholesterol.
Literature Cited
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 3.Dietary Guidelines for Americans 2010 [cited 2011 Feb 7]. Available from: http://www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm.
- 4.Kris-Etherton PM, Hu FB, Ros E, Sabate J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138:S1746–51. [DOI] [PubMed] [Google Scholar]
- 5.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–7. [DOI] [PubMed] [Google Scholar]
- 6.Griel AE, Kris-Etherton PM. Tree nuts and the lipid profile: a review of clinical studies. Br J Nutr. 2006;96:S68–78. [DOI] [PubMed] [Google Scholar]
- 7.USDA, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page; 2012 [cited 2012 July 9]. Available from: http://www.nal.usda.gov/fnic/foodcomp/search/.
- 8.Robbins KS, Shin E-C, Shewfelt RL, Eitenmiller RR, Pegg RB. Update on the healthful lipid constituents of commercially important tree nuts. J Agric Food Chem. 2011;59:12083–92. [DOI] [PubMed] [Google Scholar]
- 9.Olmedilla-Alonso B, Granado-Lorencio F, Herrero-Barbudo C, Blanco-Navarro I, Blázquez-García S, Pérez-Sacristán B. Consumption of restructured meat products with added walnuts has a cholesterol-lowering effect in subjects at high cardiovascular risk: a randomised, crossover, placebo-controlled study. J Am Coll Nutr. 2008;27:342–8. [DOI] [PubMed] [Google Scholar]
- 10.Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Baré M, Kennedy M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27:2777–83. [DOI] [PubMed] [Google Scholar]
- 11.West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M, Bagshaw DM, Wagner P, Ceballos RM, Holub BJ, et al. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr. 2010;29:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects. Circulation. 2004;109:1609–14. [DOI] [PubMed] [Google Scholar]
- 13.Cortés B, Núñez I, Cofán M, Gilabert R, Pérez-Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–71. [DOI] [PubMed] [Google Scholar]
- 14.McKay DL, Chen C-YO, Yeum K-J, Matthan NR, Lichtenstein AH, Blumberg JB. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J. 2010;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiménez-Gómez Y, López-Miranda J, Blanco-Colio LM, Marín C, Pérez-Martínez P, Ruano J, Paniagua JA, Rodríguez F, Egido J, Pérez-Jiménez F. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009;204:e70–6. [DOI] [PubMed] [Google Scholar]
- 16.Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–7. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:385–91. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 19.de Koning EJ, Rabelink TJ. Endothelial function in the post-prandial state. Atheroscler Suppl. 2002;3:11–6. [DOI] [PubMed] [Google Scholar]
- 20.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 21.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–5. [DOI] [PubMed] [Google Scholar]
- 22.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. [DOI] [PubMed] [Google Scholar]
- 23.Chen CYO, Blumberg JB. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J Agric Food Chem. 2008;56:4427–34. [DOI] [PubMed] [Google Scholar]
- 24.Behrens WA, Madère R. Malonaldehyde determination in tissues and biological fluids by ion-pairing high-performance liquid chromatography. Lipids. 1991;26:232–6. [DOI] [PubMed] [Google Scholar]
- 25.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancey PG, Asztalos BF, Stettler N, Piccoli D, Williams DL, Connelly MA, Rothblat GH. SR-BI- and ABCA1-mediated cholesterol efflux to serum from patients with Alagille syndrome. J Lipid Res. 2004;45:1724–32. [DOI] [PubMed] [Google Scholar]
- 27.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–53. [DOI] [PubMed] [Google Scholar]
- 28.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, Sies H. Flavanol-rich cocoa drink lowers plasma F2-isoprostane concentrations in humans. Free Radic Biol Med. 2004;37:411–21. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins DJA, Kendall CWC, Josse AR, Salvatore S, Brighenti F, Augustin LSA, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136:2987–92. [DOI] [PubMed] [Google Scholar]
- 31.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–4. [DOI] [PubMed] [Google Scholar]
- 32.Mah E, Noh SK, Ballard KD, Park HJ, Volek JS, Bruno RS. Supplementation of a γ-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J Nutr Biochem. 2013;24:196–203. [DOI] [PubMed] [Google Scholar]
- 33.Ellis PR, Kendall CWC, Ren Y, Parker C, Pacy JF, Waldron KW, Jenkins DJA. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am J Clin Nutr. 2004;80:604–13. [DOI] [PubMed] [Google Scholar]
- 34.Moerland M, Kales AJ, Schrier L, van Dongen MGJ, Bradnock D, Burggraaf J. Evaluation of the endoPAT as a tool to assess endothelial function. Int J Vasc Med. 2012;2012:904141. [DOI] [PMC free article] [PubMed]
- 35.Din JN, Aftab SM, Jubb AW, Carnegy FH, Lyall K, Sarma J, Newby DE, Flapan AD. Effect of moderate walnut consumption on lipid profile, arterial stiffness and platelet activation in humans. Eur J Clin Nutr. 2011;65:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Grieger JA, Kris-Etherton PM, Thompson JT, Gillies PJ, Fleming JA, Vanden Heuvel JP. Walnut oil increases cholesterol efflux through inhibition of stearoyl CoA desaturase 1 in THP-1 macrophage-derived foam cells. Nutr Metab (Lond). 2011;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Kris-Etherton PM, Thompson JT, Hannon DB, Gillies PJ, Vanden Heuvel JP. Alpha-linolenic acid increases cholesterol efflux in macrophage-derived foam cells by decreasing stearoyl CoA desaturase 1 expression: evidence for a farnesoid-X-receptor mechanism of action. J Nutr Biochem. 2012;23:400–9. [DOI] [PubMed] [Google Scholar]
- 39.Tréguier M, Moreau M, Sposito A, Chapman MJ, Huby T. LDL particle subspecies are distinct in their capacity to mediate free cholesterol efflux via the SR-BI/Cla-1 receptor. Biochim Biophys Acta. 2007;1771:129–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.