Abstract
In the present paper we apply a new neurophysiological technique to make single-electrode, dual loose-patch recordings from pairs of neuronal elements in the cerebellar cortex in vivo. The analyzed cell pairs consisted of an inhibitory molecular layer interneuron and a Purkinje cell (PC) or a Golgi cell and a granule cell, respectively. To detect the magnitude of the unitary inhibitory synaptic inputs we used histograms of the spike activity of the target cell, triggered by the spikes of the inhibitory cell. Using this analysis, we found that single interneurons had no detectable effect on PC firing, which could be explained by an expected very low synaptic weight of individual interneuron-PC connections. However, interneurons did have a weak delaying effect on the overall series of interspike intervals of PCs. Due to the very high number of inhibitory synapses on each PC, a concerted activation of the interneurons could still achieve potent PC inhibition as previously shown. In contrast, in the histograms of the Golgi cell-granule cell pairs, we found a weak inhibitory effect on the granule cell but only at the time period defined as the temporal domain of the slow IPSP previously described for this connection. Surprisingly, the average granule cell firing frequency sampled at one second was strongly modulated with a negative correlation to the overall firing level of the Golgi cell when the latter was modified through current injection via the patch pipette. These findings are compatible with that tonic inhibition is the dominant form of Golgi cell-granule cell inhibition in the adult cerebellum in vivo.
Introduction
Analysis of neuron-to-neuron connections in vivo is an important approach since it provides information on the potency of single synaptic inputs on a target neuron, which in turn is crucial for our understanding of how the neuronal microcircuitry could work. For the molecular layer interneuron input to Purkinje cells (PCs), it is estimated that there are several thousand inhibitory interneuron synapses on each PC (Palay and Chan-Palay, 1974). In vivo, these interneurons have a spontaneous activity exceeding 10 Hz (Jorntell et al., 2010), and are firing at several hundreds of Hz during appropriate input (Jorntell and Ekerot, 2002, 2003). Since the summed activity of the interneurons would translate to at least 10 kHz of inhibitory synaptic inputs in the PC, it seems unlikely that the individual interneuron-to-PC synapses have any substantial weight. For example, if each synapse generated an average inhibitory postsynaptic current (IPSC) of 10 pA for 10 ms (Kano et al., 1996; Kondo and Marty, 1998), then even at rest the PC would be constantly depressed by a current of several thousand pA, which would be comparable only to the magnitude of the current generated by the climbing fiber synapse (Silver et al., 1998). In a study using dual patch clamp recordings in vitro, the interneuron-to-PC IPSP was indeed found to be quite weak (range 0.05–0.61 mV) (Hausser and Clark, 1997). For many reasons, these amplitudes may be expected to be much lower in vivo (see discussion).
How can we find connected pairs of interneurons and PCs? In the cerebellum, the classical concept is that interneuronal inhibition is organized according to the so called parallel fiber beams where the activity in one beam triggers inhibition in neurons located “off-beam” (Eccles et al., 1967), also illustrated in more recent studies (Gao et al., 2006; Dizon and Khodakhah, 2011). However, intracellular recordings both in vivo and in vitro have shown that electrical or optical activation of a beam of parallel fibers also evokes powerful inhibition in neurons located on-beam, although this is also mixed with on-beam excitation (Jorntell and Ekerot, 2003; Mittmann and Hausser, 2007; Jorntell et al., 2010; Dizon and Khodakhah, 2011). Anatomical analysis of interneurons, either in dedicated studies (Sultan and Bower, 1998) or by examples of morphologically recovered interneurons published in papers with other main purposes (Christie and Jahr, 2008; Pugh and Jahr, 2011; Hull and Regehr, 2012), show that the branching of the axon and the number of synaptic boutons have the highest densities nearby the soma. Paired recordings of adjacent basket cells and Purkinje cells also directly demonstrated that on-beam inhibition exists between these two types of neurons when they are both located on-beam (Hull and Regehr, 2012). These anatomical and physiological findings indicate that the inhibitory interneurons have strong on-beam effects and that interneurons and PCs located adjacently to each other should have a good probability of being synaptically connected.
In contrast to the interneuron to PC synaptic communication, there exist no good indications of how many Golgi cells may innervate each granule cell. The closest estimate seem to stem from the ultrastructural study of the glomerulus made by (Jakab and Hamori, 1988). In this paper, the number of Golgi cell axons per glomerulus is estimated to 1-2. For a granule cell with four dendrites (Cathala et al., 2003), this would amount to a total of 4–8 Golgi cell inputs per granule cell. The major source of uncertainty is the estimate of Golgi cell inputs to the single glomerulus since Jakab and Hamori (1988) investigated only two glomeruli. However, it is clear that the granule cell receives synaptic inputs from very few Golgi cells.
In contrast to the fast, classical inhibitory response in interneuron-to-Purkinje cell synapses, the Golgi cell to granule cell inhibition has a prominent tonic inhibitory component (Brickley et al., 1996; Wall and Usowicz, 1997; Chadderton et al., 2004; Jorntell and Ekerot, 2006; Duguid et al., 2012). For this connection, there exists little doubt that vicinity means connectivity: the Golgi cell issues an axon that branch profusely to cover the volume around its soma with densely packed synaptic terminals (Eccles et al., 1967; Holtzman et al., 2006).
In the present paper, we draw advantage of a recently described technique for loose-patch dual extracellular recordings (Bengtsson and Jorntell, 2009) to describe the cross-correlations between such neuron pairs in the non-anesthetized decerebrated cat preparation. For this purpose, the choice of preparation is important as most known anesthetics potentiate inhibition (Bengtsson and Jorntell, 2007). We find that single interneurons have only little effect on the PC simple spike firing, probably due to the shunting of small individual IPSPs by a massive background of other inputs in vivo. In contrast, our data on the Golgi cell to granule cell connection indicates a comparably strong inhibitory effect partly at the time window of slow IPSPs in vivo (Jorntell and Ekerot, 2006) but in particular across longer time periods (second-by-second).
MATERIALS AND METHODS
PREPARATION
Adult cats were prepared as previously described (Jorntell and Ekerot, 2002, 2003). Briefly, following an initial anesthesia with propofol (Diprivan® Zeneca Ltd, Macclesfi eld Cheshire, UK), the animals were decerebrated at the intercollicular level and the anesthesia was discontinued. The animals were artificially ventilated and the end-expiratory CO2, blood pressure and rectal temperature were continuously monitored and maintained within physiological limits. Mounting in a stereotaxic frame, drainage of cerebrospinal fluid, pneumothorax and clamping the spinal processes of a few cervical and lumbar vertebral bodies served to increase the mechanical stability of the preparation. Our EEG recordings were characterized by a background of periodic 1—4 Hz oscillatory activity, periodically interrupted by large-amplitude 7–14 Hz spindle oscillations lasting for 0.5 s or more. These forms of EEG activities are normally associated with deep stages of sleep (Niedermayer and Lopes da Silva, 1993). The pattern of EEG activity and the blood pressure remained stable, also on noxious stimulation, throughout experiments.
RECORDINGS AND STIMULATION
The initial delineation of the forelimb area of the C3 zone in the cerebellar anterior lobe and the continuous monitoring of the general condition in the sensitive mossy fiber-to-granule cell-to-parallel fiber pathway were performed as described previously (Ekerot and Jorntell, 2001; Jorntell and Ekerot, 2002). In vivo patch clamp recordings were made in the lower molecular layer, the Purkinje cell layer and the granule cell layer in the tip of the folia accessible from the surface of the cerebellum. We used patch pipettes pulled to 6—14 MOhm (potassium-gluconate-based internal solution) on a Sutter micropipette puller (P-97, Sutter Instruments Co., USA). Loose patch recordings were obtained on a routine basis as a result of failed attempts to obtain giga-Ohm seals. The present analysis was confined to the rare cases in which two distinct unitary spikes could be detected in the same recording. This method is described in further detail in (Bengtsson and Jorntell, 2009).
ANALYSIS
Using home-made software and the Data Translation 3010 A/D board, all recordings were continuously sampled and digitized at 100 kHz. Off-line analysis of spike times was made in another custom-made software. For estimation of cross-correlations, one of the unitary two spikes recorded (in this paper the spike identified as an interneuron or a Golgi cell) was set as the trigger ('primary' spike). The software then identified the relative times of the other unitary spike ('secondary' spike) over a 2-s time window that straddled the time point of the trigger spike. In cases where the two spikes coincided or nearly coincided, the time was determined by close inspection of the trace. Typically, when spikes partly overlapped in time, they generated a voltage trace that could be accounted for by simple addition of the waveforms of the two spikes (Bengtsson and Jorntell, 2009) and the start point of a spike was still easy to identify by a distinct break in the normal time course of the other spike. The procedure was repeated for every primary spike encountered and a frequency distribution histogram of the spike times of the secondary spike was created. For each cell pair analyzed, the number of primary spikes was more than 1,000 (with one exception for an Int-PC pair with only 400 primary spikes) in some cases more than 10,000. From these histograms, we subtracted the baseline activity of the secondary spike and calculated the net, average change in this spike's activity for the time windows 1–25 and 26–200 ms, respectively, after the primary spike. The time windows were chosen to fit the expected time courses of fast (Hausser and Clark, 1997) and slow IPSPs (Jorntell and Ekerot, 2006), respectively.
All experiments were approved in advance by the local Swedish Animal Ethics Committee.
Results
Using the dual loose-cell patch technique (Bengtsson and Jorntell, 2009), we recorded from 23 pairs of molecular layer interneurons and PCs as well as 17 pairs of Golgi cells and granule cells. Of these recordings, sufficient amount of data was obtained only from 8 pairs of interneurons and PCs, and from 6 pairs of Golgi cells and granule cells.
Interneuron and PC pairs
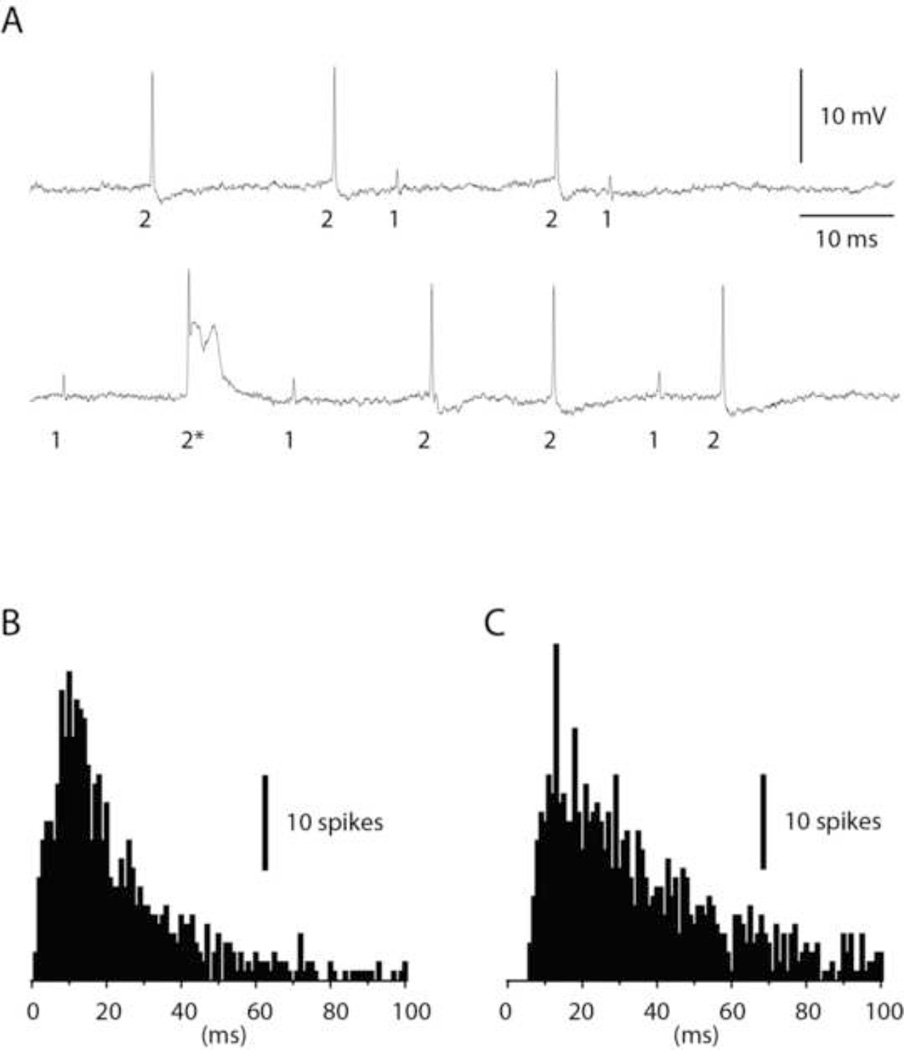
Dual recordings of interneurons and PCs were obtained in the lowermost part of the molecular layer and in the PC layer. Due to the recording depth most of the recorded interneurons are likely to have been basket cells or basket cell axons (Southan and Robertson, 1998). Figure 1A illustrates an example of a dual interneuron and PC recording. In this case, the spike amplitude of the interneuron was substantially smaller than that of the PC. Across the material, the ratio between the spike amplitudes of the PC and the interneuron could vary between 1:10 to 10:1, approximately. The PC was defined as having a characteristic complex spike evoked by the climbing fiber (asterisk in Figure 1A), a feature that was absent in the interneuron (although climbing fiber responses exist also in this type of neuron (Jorntell and Ekerot, 2011). To further differentiate interneurons and PCs, and to differentiate the interneuron located in the vicinity of the Purkinje cell layer from Golgi cells, we also checked the interspike intervals of the recorded neurons. Interneurons are characterized by an irregular but consistent spontaneous firing rate on which is superimposed occasional spontaneous spike bursts of 2–4 spikes at 200–500 Hz (Jorntell et al., 2010; Jorntell and Ekerot, 2011), a feature that is absent in Purkinje cells (Fig. 1B,C) as well as Golgi cells (see below). The general spike firing patterns and a consistency of the interspike interval frequency histograms for each cell type were important verifications that the cells were relatively unperturbed by the patch pipette, both for interneuron-PC pairs and for Golgi cell-granule cell pairs.
Figure 1.
Paired recording of a molecular layer interneuron and a PC. (A) Raw traces illustrating the spikes of the interneuron ('1') and the PC ('2'). The PC was also characterized by spontaneous complex spikes ('2*'). (B) Frequency distribution of the interspike intervals of the interneuron. (C) Frequency distribution of the PC simple spikes. In both (B) and (C), data are truncated at 100 ms. Bin width 1 ms.
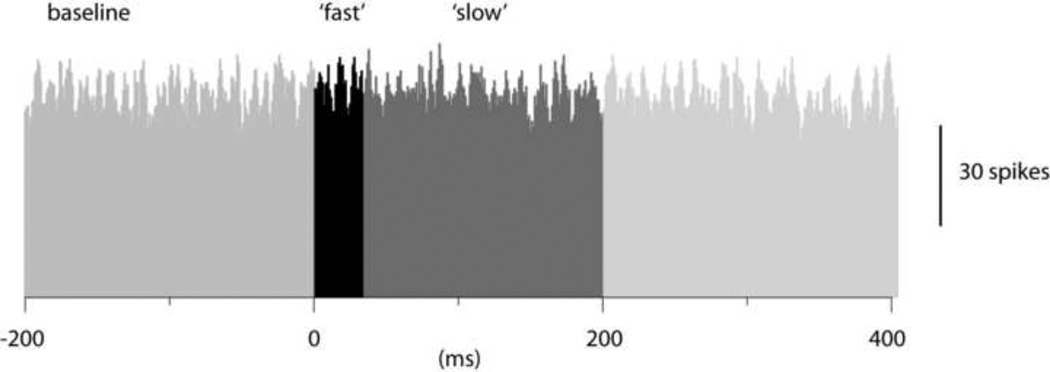
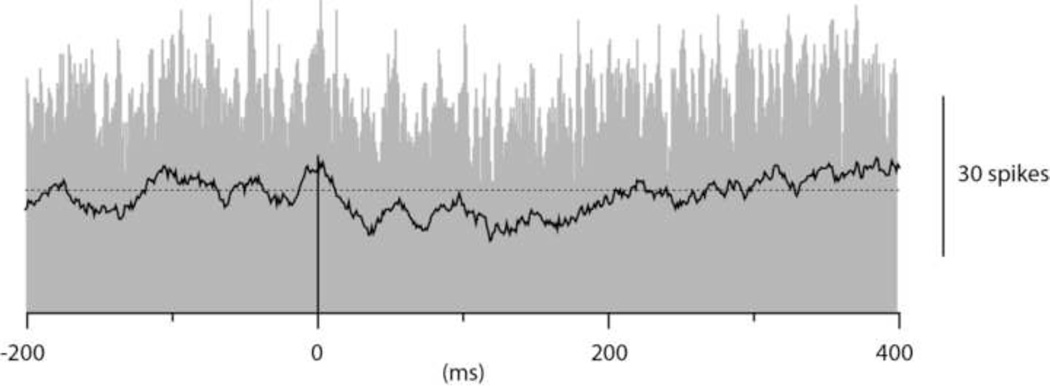
Figure 2 illustrates an example of a histogram of PC simple spike activity triggered by spontaneous interneuron spikes. As shown in this example, the PC spike activity did not seem to change at all in relation to the time point of the interneuron spikes, a pattern that was consistent across the population of interneuron to PC pairs. To quantify the data obtained from 8 interneuron-PC pairs, we designated a period that preceded the interneuron spike by 200 ms as baseline activity. We next defined a time period (1–25 ms after the interneuron spike) as the time window of fast inhibitory effects and a second time period (26–200 ms) as the time window of slow inhibitory effects (see differently shaded areas in Fig. 2). For interneuron and PC pairs, there was essentially no effect, neither during the 'fast' time period (net change in activity of the PC spike: −0.5+/−1.3% (mean+/−standard deviation) (N=8)) nor during the 'slow' time period (change in PC spike activity +0.8+/−1.5% (N=8)).
Figure 2.
Cross-correlation between the spikes of a molecular layer interneuron and PC simple spike firing in a dual recording. The spontaneous spikes of the interneuron was used to trigger the PC simple spikes. In the peristimulus histogram, the pretrigger 'baseline' time period as well as the time periods of the expected 'fast' and 'slow' inhibitory effects are indicated in different shades of grey. Bin width 1 ms.
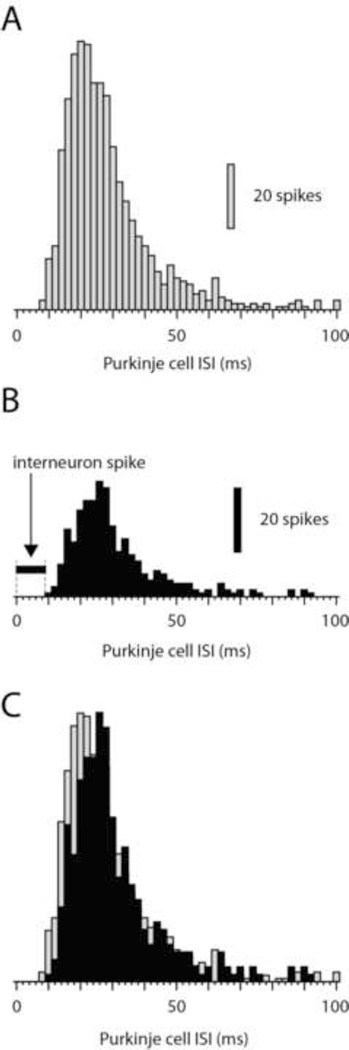
In a related study in vitro, Häusser and Clark (1997) made two-electrode dual patch clamp recordings from interneurons and PCs and used the delay in the interspike intervals of PCs triggered by the interneuron spike as an indicator of the efficacy of the interneuron to PC synapse. Figure 3 illustrates a similar analysis applied to our material. Figure 3A illustrates a normal interspike interval (IS) histogram of the PC simple spike whereas Fig. 3B shows the distribution of ISIs in the same cell when we selectively sorted out the ISIs in which the PC spike at time zero (from which the ISI was measured) was succeeded be an interneuron spike within a time window of 1–9 ms (time interval indicated by horizontal bar in Fig. 3B). In Fig. 3C, these two histograms are normalized with respect to their peak values and superimposed. As this panel rather clearly illustrates, the PC ISIs tended to become longer, i.e. there was a rightward shift of the bulk of the ISIs. This effect was weak but consistent, with an average ISI increase of 1.8+/−0.95 ms (N=6), i.e. the effect in vivo was about one order of magnitude lower than that in vitro (Hausser and Clark, 1997).
Figure 3.
Effect of interneuron spike on PC interspike intervals (ISIs). (A) Frequency distribution of the PC simple spike in the control condition, i.e. no attention paid to the occurrence of interneuron spikes. (B) Frequency distribution of the PC ISIs in the same cell, but in this case only including ISIs when the interneuron spike occurred within a time window of 1–9 ms after the reference simple spike (from which the ISI to the next spike was measured). (C) Superimposition of the histograms in A and B after normalization of the peak amplitudes in the histograms. Bin width in all histograms, 2 ms.
Still, the consistent effect on the PC ISI triggered by single interneuron spikes was surprising given the absence of effects in the spike-triggered histograms (Fig. 2). We speculated that the effect could possibly be due to that interneurons in vivo seem to be subjected to a number of network mechanisms that make their spikes preferably fire in relative synchrony or in concert (reviewed in Jörntell et al, 2010). Hence, in the case of Figure 3C, this could mean that even though we recorded the occurrence of a spike from one interneuron, neighboring interneurons innervating the same PC could have a strong tendency to fire within the same time window. The total inhibitory input to the PC during this time window would then be much more powerful than that inferred by the single interneuron spike we recorded. The only test that we could do in this set of experiments was to explore the influence of the climbing fiber response in the PC (i.e. the complex spike) on the inhibitory effect recorded. Since climbing fibers generate quite powerful responses in the interneurons (Jorntell and Ekerot, 2003; Jorntell et al., 2010; Jorntell and Ekerot, 2011), spontaneous climbing fiber responses could tend to synchronize the spikes of the local interneurons. Hence, by excluding interneuron spikes that occurred within the time window of the complex spike (−10 to +30 ms of the occurrence of the complex spike in the PC) we could test whether this potential synchronization mechanism was behind the increase in PC ISIs shown in Fig. 3C. However, in contrast to the expected outcome, this step actually resulted in a further increase in the average PC ISIs (by about 1 ms, tested in 6 cells, data not shown).
Golgi cell and granule cell pairs
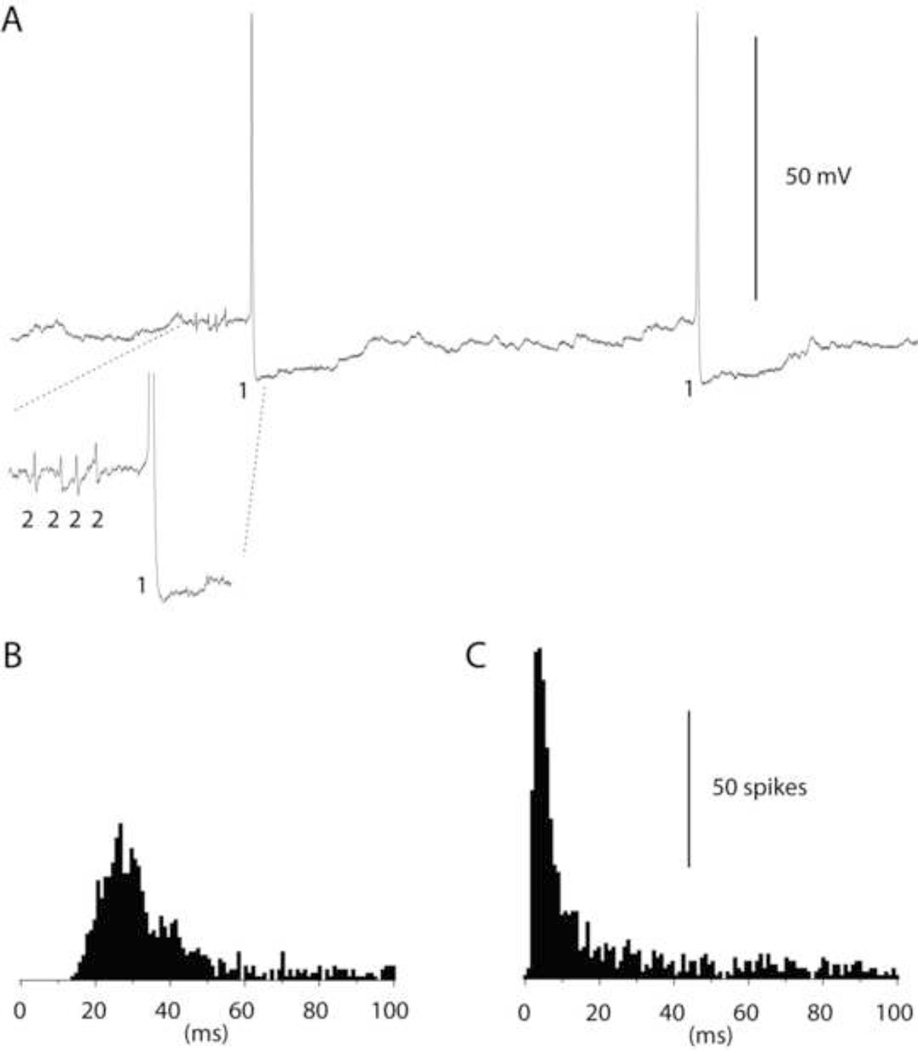
Figure 4A illustrates an example of a dual Golgi cell and granule cell recording. The Golgi cell is recorded essentially intracellularly with full-amplitude spikes and spike after hyperpolarizations (Forti et al., 2006). The granule cell, recorded extracellularly, was characterized by an overall low spontaneous activity but also by occasional high-frequency bursts well beyond 500 Hz (magnified portion in Fig. 4A). The difference between the autocorrelograms of the Golgi cells and granule cells was very clearly manifested as a reflection of the tendency in the granule cell to burst, which is lacking in Golgi cells (Fig. 4B,C).
Figure 4.
Paired recording of a Golgi cell and a granule cell. (A) Raw traces illustrating the spikes of the Golgi cell ('1') and the granule cell ('2'). Inset illustrates the intensity in the spontaneous granule cell spike bursts. The time period of the magnified portion is 15 ms. (B) Frequency distribution of the interspike intervals of the Golgi cell. (C) Frequency distribution of the granule cell spikes. In both (B) and (C), data are truncated at 100 ms. Bin width 1 ms.
Figure 5 illustrates a cross-correlogram for the same Golgi cell-granule cell pair. In contrast to the interneuron-PC pair, in this case there was a tendency for the spontaneous Golgi cell spikes to depress the granule cell spike activity, in particular during the time period of the slow IPSP (Jorntell and Ekerot, 2006). This may not be directly obvious against the background of high bin-to-bin variance, so a separate line illustrating the moving average of 20 adjacent bins is superimposed on the histogram in Figure 5. In summary, Golgi cell to granule cell pairs were characterized by an absence of fast inhibitory effects (net change −0.8+/−2.5% (N=6)) but a weak slow inhibitory effect (−5.3+/−3.6%(N=6)).
Figure 5.
Cross-correlation between the spikes of a Golgi cell and granule cell spike firing. Similar display as in Fig. 2. To facilitate the illustration of the underlying trend in the depression of the granule cell spike firing, a moving average of 20 bins is superimposed on the peristimulus histogram. Bin width 1 ms.
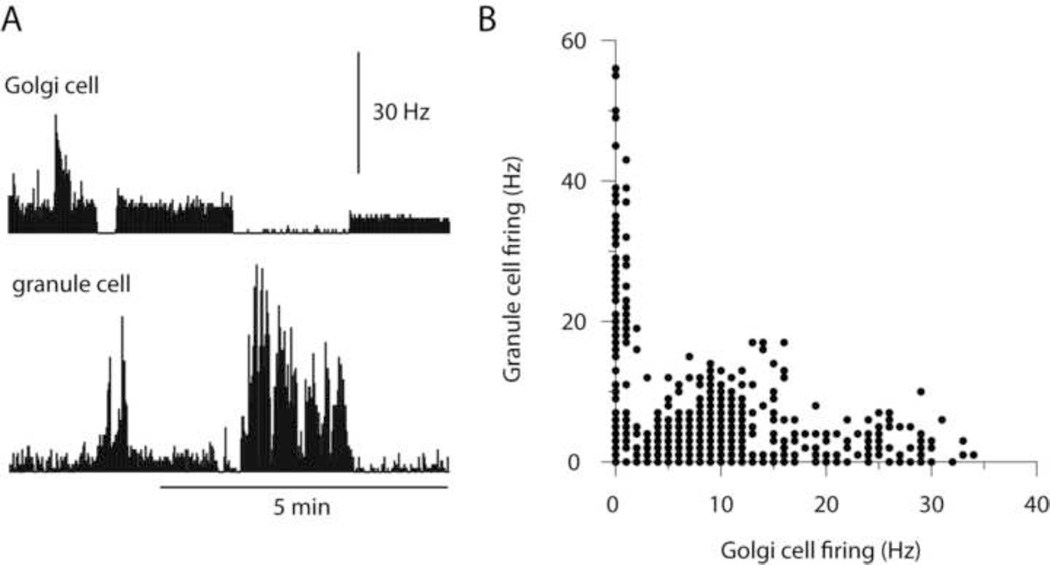
Apart from the slow inhibitory effect, when viewed over longer time periods, changes in the Golgi cell spiking activity induced by current injection was clearly reflected in the spike activity of the granule cell (Figure 6A). In fact, a higher spontaneous activity in the granule cell essentially only occurred when the Golgi cell was made almost entirely quiet (Figure 6B). This relationship was clear in all Golgi cell-granule cell pairs analyzed in this respect, and first of all provided evidence that the recorded cell pairs really were synaptically connected. But more importantly, these findings suggest that the main time constant over which the Golgi cell inhibition is exerted in granule cells is counted in seconds rather than 100's or 10's of ms.
Figure 6.
Long-term relationship between Golgi cell and granule cell spike activity. (A) The normally quite regular Golgi cell spike firing frequency was altered to a few different levels using current injection. The simultaneously recorded granule cell substantially changed its firing frequency in response to these manipulations. Bin width, 1 s. (B) Scatter plot of the relationship between the spontaneous firing frequencies of the two cells. Note in particular the close connection between high granule cell activity and low Golgi cell activity.
Discussion
In this paper we applied a novel technique for dual loose-patch recordings (Bengtsson and Jorntell, 2009) to analyze the weight of individual inhibitory synaptic connections between interneuron-PC pairs or from Golgi cell-granule cell pairs. Since a single electrode is used to record from two neuronal units, it is given that the recorded neurons are located adjacently to each other, presumably separated by less than 10 um. The inhibitory effect from single interneurons to PCs was weak. In contrast, the influence of single Golgi cells on the overall firing frequency of granule cells was remarkably potent, even though we could not detect any effect of fast inhibitory IPSPs and only moderate inhibition during the time window of slow IPSPs.
The present findings suggest that unitary connections between molecular layer interneurons and PCs have only small effects on the PC simple spike firing in vivo. The fact that we did record an effect (a rightward shift in the histograms plotting the frequency distribution of the ISIs) suggests that the recorded neurons were indeed synaptically connected. Notably, however, it does not prove a connection, since interneurons in vivo may be subjected to a number of network mechanisms that tend to synchronize the local interneurons (see account in Results and (Jorntell et al., 2010)). However, a number of observations strengthen the logic of weak inhibitory synapses in this link. First of all, it has already been demonstrated that in the juvenile rat cerebellum in vitro this synaptic connection is quite weak, with the peak amplitudes of the IPSPs being a few tenths of millivolts (Hausser and Clark, 1997). In the adult cat in vivo, substantially higher background synaptic activity and the larger size of the PCs will result in that the input resistance becomes substantially lower (by up to a factor 20) - this has so far been shown to be the case for interneurons (cf. (Hausser and Clark, 1997) and (Jorntell and Ekerot, 2003) and deep cerebellar nuclear neurons (Bengtsson et al., 2011). This would be expected to result in that the unitary IPSPs have a correspondingly smaller amplitude and influence on the PC. In addition, PC firing in vivo is irregular, making it more difficult to detect small inhibitory effects.
As accounted for in the introduction, nearby interneurons and PCs are expected to have a high probability of being connected. This raises the issue of how such weak synaptic connections can make any difference on the processing of the PCs, which is in contrast to quite powerful inhibitory responses in PCs in vivo (Ito, 1984; Edgley and Lidierth, 1988; Jorntell and Ekerot, 2002; Pasalar et al., 2006). The answer to this question may be that the circuitry is arranged to concert the activity of a large number of interneurons impinging on a PC, so that they are activated by the same or closely related inputs. This arrangement can be achieved since the interneurons respect the same microzonal organization as that defined for PCs (see (Dean et al., 2010; Jorntell et al., 2010)). Presumable as a consequence of the associated learning mechanisms, interneurons within a microzone learn to give large weight to functionally correlated mossy fiber inputs, which in the case of cutaneous input means that they are activated from the same small skin area (Ekerot and Jorntell, 2001, 2003; Dean et al., 2010) or, in the case of cyclic movements, can be expected to be activated during the same phase of that movement (Jorntell et al., 2010). Since PCs are mainly inhibited by interneurons from within the same microzone (Ekerot and Jorntell, 2001; Jorntell and Ekerot, 2002; Ekerot and Jorntell, 2003; Jorntell et al., 2010), the PCs would be expected to receive a massive, concerted inhibitory synaptic input during the appropriate part of the controlled movement.
In contrast, the recorded pairs of Golgi cells and granule cells overall indicated a weak, but demonstrable inhibition in the time window defined as the time period of the slow IPSP observed in this synaptic junction in the adult (Rossi and Hamann, 1998; Jorntell and Ekerot, 2006). The fact that we could detect this input using this analysis, as opposed to the spike-triggered histograms of the interneuron-to-PC inhibitory synaptic input, is most likely due to the much fewer Golgi cells expected to provide input to the granule cell, whereby each input presumably has to be more potent. But the most intriguing effect observed was the substantially more potent granule cell modulation obtained by more long-term changes in Golgi cell firing (Figure 5), which would seem to be related to the relatively powerful tonic inhibition observed in the slice (Brickley et al., 1996; Wall and Usowicz, 1997) and in vivo (Chadderton et al., 2004; Duguid et al., 2012). In vivo, this tonic conductance can account for more than half of the steady state conductance (Chadderton et al., 2004), which naturally could explain the quite dramatic effects observed in the present study when the Golgi cell was made to stop firing for a prolonged period of time. This finding, which was made in the face of an evident lack of fast inhibitory effects, could potentially have major consequences for our understanding of the cerebellar granule layer and thereby for models of cerebellar function in general.
ACKNOWLEDGEMENTS
This study was supported by grants from NINDS/NIH (ROl NS040863), The Hand Embodied (THE) (an Integrated Project funded by the EU under FP7, project no. 248587) EU FP6 (SENSOPAC) and the Swedish Research Council (VR Medicine).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copy editing, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bengtsson F, Ekerot CF, Jorntell H. In vivo analysis of inhibitory synaptic inputs and rebounds in deep cerebellar nuclear neurons. PloS one. 2011;6:el8822. doi: 10.1371/journal.pone.0018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Jorntell H. Ketamine and xylazine depress sensory-evoked parallel fiber and climbing fiber responses. J Neurophysiol. 2007;98:1697–1705. doi: 10.1152/jn.00057.2007. [DOI] [PubMed] [Google Scholar]
- Bengtsson F, Jorntell H. Climbing fiber coupling between adjacent purkinje cell dendrites in vivo. Front Cell Neurosci. 2009;3:7. doi: 10.3389/neuro.03.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Brickley S, Cull-Candy S, Farrant M. Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse. Journal of Neuroscience. 2003;23:6074–6085. doi: 10.1523/JNEUROSCI.23-14-06074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Christie JM, Jahr CE. Dendritic NMDA receptors activate axonal calcium channels. Neuron. 2008;60:298–307. doi: 10.1016/j.neuron.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Porrill J, Ekerot CF, Jorntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43. doi: 10.1038/nrn2756. [DOI] [PubMed] [Google Scholar]
- Dizon MJ, Khodakhah K. The role of interneurons in shaping Purkinje cell responses in the cerebellar cortex. J Neurosci. 2011;31:10463–10473. doi: 10.1523/JNEUROSCI.1350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid I, Branco T, London M, Chadderton P, Hausser M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci. 2012;32:11132–11143. doi: 10.1523/JNEUROSCI.0460-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Springer-Verlag: Berlin; 1967. [Google Scholar]
- Edgley SA, Lidierth M. Step-related discharges of Purkinje cells in the paravermal cortex of the cerebellar anterior lobe in the cat. J Physiol. 1988;401:399–415. doi: 10.1113/jphysiol.1988.sp017169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H. Parallel fibre receptive fields of Purkinje cells and interneurons are climbing fibre-specific. Eur. J. Neurosci. 2001;13:1303–1310. doi: 10.1046/j.0953-816x.2001.01499.x. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H. Parallel fiber receptive fields: a key to understanding cerebellar operation and learning. Cerebellum. 2003;2:101–109. doi: 10.1080/14734220309411. [DOI] [PubMed] [Google Scholar]
- Forti L, Cesana E, Mapelli J, D'Angelo E. Ionic mechanisms of autorhythmic firing in rat cerebellar Golgi cells. J.Physiol. 2006;574:711–729. doi: 10.1113/jphysiol.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Chen G, Reinert KC, Ebner TJ. Cerebellar cortical molecular layer inhibition is organized in parasagittal zones. J Neurosci. 2006;26:8377–8387. doi: 10.1523/JNEUROSCI.2434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol. 2006;574:491–507. doi: 10.1113/jphysiol.2006.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Regehr WG. Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity. Neuron. 2012;73:149–158. doi: 10.1016/j.neuron.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Jakab RL, Hamori J. Quantitative morphology and synaptology of cerebellar glomeruli in the rat. Anatomy and embryology. 1988;179:81–88. doi: 10.1007/BF00305102. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Bengtsson F, Schonewille M, De Zeeuw CI. Cerebellar molecular layer interneurons - computational properties and roles in learning. Trends Neurosci. 2010;33:524–532. doi: 10.1016/j.tins.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. Journal of Neuroscience. 2003;23:9620–9631. doi: 10.1523/JNEUROSCI.23-29-09620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. Journal of Neuroscience. 2006;26:11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Receptive Field Remodeling Induced by Skin Stimulation in Cerebellar Neurons in vivo. Front Neural Circuits. 2011;5:3. doi: 10.3389/fncir.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. Journal of Neuroscience. 2006;26:11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Fukunaga K, Konnerth A. Ca(2+)-induced rebound potentiation of gamma-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc Natl Acad Sci U S A. 1996;93:13351–13356. doi: 10.1073/pnas.93.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Marty A. Synaptic currents at individual connections among stellate cells in rat cerebellar slices. J Physiol. 1998;509(Pt 1):221–232. doi: 10.1111/j.1469-7793.1998.221bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann W, Hausser M. Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells. J Neurosci. 2007;27:5559–5570. doi: 10.1523/JNEUROSCI.5117-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermayer E, Lopes da Silva F. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Baltimore: Williams and Wilkins; 1993. [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. Cytology and Organization. Berlin, Heidelberg, New York: Springer-Verlag; 1974. [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–1411. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Jahr CE. NMDA receptor agonists fail to alter release from cerebellar basket cells. J Neurosci. 2011;31:16550–16555. doi: 10.1523/JNEUROSCI.3910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Silver RA, Momiyama A, Cull-Candy SG. Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre-Purkinje cell synapses. J.Physiol. 1998;510(Pt 3):881–902. doi: 10.1111/j.1469-7793.1998.881bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J Neurosci. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan F, Bower JM. Quantitative Golgi study of the rat cerebellar molecular layer interneurons using principal component analysis. J Comp Neural. 1998;393:353–373. [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]