Abstract
Background: Postpartum weight retention (PPWR) can contribute to obesity development in women of reproductive age. Few studies have examined the association between postnatal diet and PPWR.
Objective: We examined both PPWR and substantial PPWR (≥4.55 kg) in association with the following dietary patterns: the alternate Mediterranean Diet Score (aMED) and the Alternative Healthy Eating Index-2010 (AHEI-2010).
Design: Women (n = 1136) in the Infant Feeding Practices Study II (2005–2007) self-reported their prepregnancy and postpartum weights at ∼4, 7, 10, and 14 mo. Dietary patterns were calculated from a food-frequency questionnaire administered ∼4 mo postpartum. Linear mixed models and generalized estimating equations for repeated measurements were used to examine PPWR and substantial PPWR, respectively, in association with the dietary patterns with adjustment for energy intake, breastfeeding, age, education, smoking, and marital status.
Results: At 14 mo postpartum, the mean (±SD) PPWR was 1.1 ± 6.7 kg, and 22.4% of women had substantial PPWR. Although the change in PPWR over time seemed to differ by diet quality 4–7 mo postpartum, no differences were ultimately observed in the total mean PPWR or probability of substantial PPWR across aMED and AHEI-2010 categories during the rest of the follow-up (P > 0.12). Instead, PPWR and substantial PPWR were associated with total energy intake (at ∼7–14 mo postpartum: 0.97 kg/1000 kcal (95% CI: 0.40, 1.55 kg/1000 kcal); OR: 1.25/1000 kcal (95% CI: 1.03, 1.52/1000 kcal), respectively].
Conclusions: Postpartum diet quality assessed by 2 patterns was not associated with weight retention. Total energy intake, regardless of the diet composition, plays a more important role in weight retention.
INTRODUCTION
Obesity has become a major concern in all populations worldwide, including in women of reproductive age (1). In the United States, ∼32% of women of reproductive age (20–39 y) are obese [BMI (kg/m2) ≥30] (2). In women of reproductive age, pregnancy has been identified as a trigger for the development of obesity because of excessive weight gain and long-term weight retention (3, 4). Although average estimates of weight retention have been modest, ranging from 1 to 2 kg at 6–18 mo postpartum (5–7), the proportion of women with substantial weight retention (≥4.55 kg) ranges from 14% to 25% (5–7). This retained weight is potentially harmful as a result of its centralized distribution because evidence has suggested a preferential accumulation of adipose tissue in the visceral compartment, which is an independent risk factor for a wide range of chronic diseases (8–10).
Factors associated with postpartum weight retention (PPWR)4 include excessive gestational weight gain (GWG), breastfeeding, cigarette smoking, maternal income, marital status, age, energy intake, and exercise (3, 7, 11–15). Very few studies have examined the association between individual dietary components and patterns of overall dietary intake and PPWR (14). An examination of dietary patterns, rather than individual nutrients or foods, can capture overall patterns and interactions between separate dietary components (16). Numerous studies, including clinical trial evidence, have demonstrated the importance of diet quality on weight status in men and nonpregnant women (17–19). Thus, we report on the association of 2 such validated patterns of dietary quality as follows: the alternate Mediterranean Diet Score (aMED) and the Alternative Healthy Eating Index-2010 (AHEI-2010) with PPWR in the Infant Feeding Practices Study II (IFPS II).
SUBJECTS AND METHODS
Overall study design and participants
The IFPS II (2005–2007) was a longitudinal survey of mothers from pregnancy through their infant's first birthday (20). All women identified from a consumer-opinion panel during their third trimester (weeks 32–40) of pregnancy were invited to participate in a US national study of infant feeding practices. After delivery, a birth screener identified eligible participants as adult mothers (≥18 y) of healthy, term, or near-term (≥35 wk) singletons who weighed ≥2.27 kg (5 lb) and were not in an intensive care unit for >3 d. In addition, mothers with medical problems (eg, hypothyroidism and pituitary dysfunction) that affected infant feeding were excluded.
Data collected longitudinally included one prenatal mailed questionnaire sent around the third trimester of pregnancy, a short telephone interview close to the time of the infant's birth, and 10 mailed questionnaires about infant feeding, health, and related topics over the 12-mo postpartum period. The Food and Drug Administration's Research Involving Human Subjects Committee approved the IFPS II study protocol.
Dietary assessment
Diet History Questionnaire
Two maternal dietary assessments, one during late pregnancy and one ∼4 mo postpartum, were conducted in a subsample of IFPS II respondents by using a modified version of the National Cancer Institute's (NCI's) Diet History Questionnaire (DHQ) (20). The DHQ has been previously validated against four 24-h dietary recalls in a nationally representative sample (21) and captures the consumption of over 100 food items including portion-size information. Modifications of the DHQ included changing the time span of the foods consumed from the past year to the past month and inclusion of food items relevant to pregnant and lactating women such as specific types of fish and certain dietary supplements (20). Thus, the prenatal DHQ mailed in the third trimester (weeks 32–40) represented intake between 28 and 36 wk, whereas the postpartum DHQ reflected intake between 3–4 mo after delivery. The NCI's Diet*Calc software (version 1.4.3), which produces estimates of nutrients, total energy intake, food categories, and glycemic load estimates, was used to process DHQ assessments. From the originally mailed sample, a total of 1483 (83%) respondents completed the postpartum DHQ. The NCI excluded data for women who were in the top 2% or bottom 1% of energy intake (which corresponded to women with a caloric intake >4539 and <606 kcal, respectively). With the use of DHQ data from 1420 women, the aMED was the primary dietary pattern evaluated. Secondarily, the AHEI-2010 was an additional dietary pattern evaluated.
aMED
To examine postpartum adherence to the Mediterranean diet, the aMED (22), which is a scale adapted from the traditional Mediterranean diet score developed by Trichopoulou et al (23), was used. This score (range: 0–9) is based on the dietary intake of 9 components with greater adherence implied by higher scores. Each of the beneficial dietary components, including vegetables (excluding potatoes), legumes, fruit, nuts, whole grains, fish, and the ratio of monounsaturated fat to saturated fat, receives a score of 1 if consumption exceeds the median intake. One point is scored if alcohol intake is between 5 and 15 g/d. For presumed nonbeneficial dietary components, including red and processed meats, one point is scored if intake is less than the median. Median intakes are derived from the distribution of dietary components of the cohort under study. Because this index has been previously developed for the nonpregnant and nonlactating population, we excluded alcohol consumption from the index, and thus, the total aMED score ranged between 0 (least adherence) and 8 (highest adherence). The aMED score was categorized to examine 3 groups with low (≤3 points), moderate (4–5), and high (6–8) adherence.
AHEI-2010
The AHEI-2010 is a recent update to the Alternative Healthy Eating Index, which is a diet quality index that is based on foods and nutrients recommended by the US National Dietary Guidelines for Americans (24). The AHEI-2010 score is based on the dietary intake of 11 components that range between 0 (worst) and 10 (best) with better adherence implied by a higher total AHEI-2010 score (0: nonadherence; 110: perfect adherence). The intake of dietary components with positive health effects included vegetables (0 points for 0 servings/d and 10 points for ≥5 servings/d) (excluding potatoes), whole fruit (0 points for 0 servings/d and 10 points for ≥4 servings/d), whole grains (0 points for 0 g/d and 10 points for 75 g/d), nuts and legumes (0 points for 0 servings/d and 10 points for ≥1 servings/d), the percentage of energy intake from PUFAs (0 points for ≤2% and 10 points for ≥10%), and long-chain (n−3) fats (EPA + DHA) (0 points for 0 mg/d and 10 points for 250 mg/d) (24). The intake of nonbeneficial dietary components, including sugar-sweetened beverages and fruit juice (0 points for ≥1 servings/d and 10 points for 0 servings/d), red and processed meat (0 points for ≥1.5 servings/d and 10 points for 0 servings/d), the percentage of energy intake from trans fat (0 points for ≥4% and 10 points for ≤0.5%), and sodium (0 points for highest–sodium-intake group and 10 points for lowest group; participants divided into 11 groups) resulted in lower adherence scores (24). Similar to the alcohol component in the aMED, we excluded alcohol consumption from the total score, and thus, the total AHEI-2010 score ranged between 0 and 100. The AHEI-2010 was examined in tertiles.
PPWR
PPWR in kilograms was defined as the difference between self-reported prepregnancy body weight, as reported in the prenatal questionnaire, and self-reported weight as obtained from 3-, 6-, 9-, and 12-mo postpartum questionnaires. The 3-, 6-, 9-, and 12-mo questionnaires were intended to target mothers with infants of a specific age. However, the date of completion of questionnaires did not always match the targeted infant's age. As a consequence, the time frame for reported weights in the postpartum period ranged between 11 and 73 wk (11–62 wk: 99.8%). After the exclusion of 20 women with implausible postpartum weights, a total of 1136 mothers had available data on prepregnancy weight, dietary data, and at least one self-reported postpartum weight assessment. We also excluded 8 observations beyond 62 wk postpartum to restrict the analysis duration between 11 and 62 wk after delivery. Postpartum time was categorized to represent relatively equally spaced time intervals and to reduce multiple measures of weight assessment per woman per time interval. Time categories included 11–20 wk (1037 observations), 21–34 wk (948 observations), 35–48 wk (888 observations), and 49–62 wk (811 observations). PPWR was first examined as a continuous outcome. We also defined substantial PPWR as retaining ≥4.55 kg above the prepregnancy weight. This definition (≥4.55 kg) was chosen for comparison purposes with previous research (6, 7).
Covariate assessment
Demographic data were either available directly from the Panel Demographic Questionnaire or collected through a short demographic questionnaire. Maternal education was categorized as high school or less, some college, college graduate, and postgraduate. The ratio of annual household income to appropriate poverty-threshold values used by the US Census Bureau was categorized as <185% and ≥185% with low-income families indicated by <185% of the federal poverty. Maternal race and ethnicity were categorized as non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Pacific Islander/other. Parity was examined as primiparous or multiparous. GWG, which was obtained from the self-reported neonatal questionnaires, was categorized as below, within or above the 2009 Institute of Medicine guidelines for GWG (25). Postpartum smoking, which was repeatedly reported at the time of postpartum weight assessment, was categorized as smoker or nonsmoker at any time during the postpartum period.
The exclusive breastfeeding duration was extracted from each monthly postpartum questionnaire to ≤10 mo during which the mother indicated whether she provided only breast milk without any feedings of liquids or foods. The duration of exclusive breastfeeding was set to be equal to the midpoint of infant age at the last questionnaire in which the mother reported exclusive breastfeeding and infant age on the first questionnaire in which she indicated that she stopped breastfeeding exclusively. If a mother was still breastfeeding at the last questionnaire, the variable was set to be equal to the postpartum time of her last weight measurement. In concordance with previous research, we created a scale that depicted both the intensity and duration of breastfeeding to reflect the energy cost of full and partial lactation (11). Women were assigned a score of 1 point/wk for full breastfeeding and 0.5 points/wk for partial breastfeeding up until the infant was 1 y old. Beyond 1 y, women scored 0.25 points/wk for any continued breastfeeding, which indicated the decrease in energy costs of lactation because of the introduction of other foods to the infant's diet (11).
Statistical analyses
Linear mixed models for repeated measurements with an unstructured error term were used to examine the association between dietary patterns and longitudinally measured PPWR. The odds of substantial weight retention (≥4.55 kg) were also examined by using generalized estimating equations. From these models, the probability of retaining ≥4.55 kg was estimated. Initially, we examined the association between PPWR and aMED categories (0–3, 4–5, and 6–8), with adjustment for energy intake and interactions with time (11–20, 21–34, 35–48, and 49–62 wk) for both aMED categories and energy intake. The interaction between time and aMED reflected whether mothers with different aMED adherence scores retained weight at the same rate over time. Subsequent models were adjusted for covariates significantly associated with aMED categories and weight retention and identified by using Pearson's chi-square analysis or ANOVA. The choice of covariates was based a priori on what was previously reported in the literature. In separate models we also examined aMED categories in a continuous fashion to test for a linear trend. Separate models were also adjusted for BMI, GWG, and both BMI and GWG. Similar analyses were conducted to examine the association with AHEI-2010 categories. Spearman's correlation coefficients were used to examine correlations between dietary patterns. All levels of association are presented with their 95% CIs and P values based on a 2-sided test with significance at P < 0.05. Repeated-measurement analyses were performed with Proc Mixed and Proc Genmod procedures in SAS software (version 9.2; SAS Institute Inc).
RESULTS
Of the total 1136 women with available data on prepregnancy weight, dietary data and at least one self-reported postpartum weight assessment, the mean (±SD) age was 29.2 ± 5.3 y, the mean prepregnancy BMI was 26.1 ± 6.2, and the majority of the women had some college education or higher (82%), were non-Hispanic white (86%), and married (83%) (Table 1). At the end of the follow-up period (49–62 postpartum weeks), mean weight retention was 1.1 ± 6.7 kg with 22.4% of women who reported substantial weight retention (≥4.55 kg).
TABLE 1.
Maternal characteristics according to adherence to the alternate Mediterranean Diet in the IFPS II (2005–2007)1
| Total cohort (n = 1136) | Low (scores: 0–3; n = 465) | Moderate (scores: 4–5; n = 431) | High (scores: 6–8; n = 240) | P | |
| Demographics | |||||
| Age (y) | 29.2 ± 5.32 | 28.4 ± 5.2 | 29.6 ± 5.1 | 30.2 ± 5.4 | <0.001 |
| Education [n (%)] | <0.001 | ||||
| High school or less | 192 (17.7) | 103 (23.0) | 60 (14.7) | 29 (12.7) | |
| Some college | 411 (37.9) | 191 (42.6) | 151 (37.0) | 69 (30.1) | |
| College degree | 358 (33.0) | 116 (25.9) | 144 (35.3) | 98 (42.8) | |
| Graduate degree | 124 (11.4) | 38 (8.5) | 53 (13.0) | 33 (14.4) | |
| Race-ethnicity [n (%)] | 0.87 | ||||
| Non-Hispanic white | 963 (86.0) | 403 (87.4) | 361 (85.1) | 199 (84.7) | |
| Non-Hispanic black | 39 (3.5) | 15 (3.3) | 14 (3.3) | 10 (4.3) | |
| Hispanic | 58 (5.2) | 22 (4.8) | 25 (5.9) | 11 (4.7) | |
| Asian/Pacific Islander/other | 60 (5.4) | 21 (4.6) | 24 (5.7) | 15 (6.4) | |
| Married [n (%)] | 896 (82.7) | 345 (77.0) | 349 (85.8) | 202 (88.6) | <0.001 |
| Poverty level (<185% of the federal poverty level) [n (%)] | 402 (35.4) | 188 (40.4) | 139 (32.3) | 75 (31.3) | 0.012 |
| Nulliparous [n (%)] | 331 (29.8) | 137 (30.4) | 128 (30.3) | 66 (27.9) | 0.76 |
| Behavioral characteristics | |||||
| Smoker during pregnancy [n (%)] | 89 (7.9) | 59 (12.7) | 21 (4.9) | 9 (3.8) | <0.001 |
| Smoker postpartum [n (%)]3 | 175 (16.6) | 109 (25.2) | 50 (12.6) | 16 (7.1) | <0.001 |
| Alcohol intake [n (%)] | 524 (46.2) | 215 (46.3) | 207 (48.1) | 102 (42.5) | 0.37 |
| Beer | 266 (23.4) | 100 (21.5) | 113 (26.3) | 53 (22.1) | 0.21 |
| Wine | 356 (31.5) | 135 (29.2) | 139 (32.5) | 82 (34.2) | 0.34 |
| Liquor | 243 (21.4) | 103 (22.2) | 94 (21.9) | 46 (19.2) | 0.63 |
| Breastfeeding score | |||||
| 11–20 wk | 6.9 ± 4.7 | 5.8 ± 4.7 | 7.5 ± 4.4 | 8.3 ± 4.7 | <0.001 |
| 21–34 wk | 12.0 ± 8.7 | 9.4 ± 8.5 | 13.0 ± 8.2 | 14.9 ± 8.4 | <0.001 |
| 35–48 wk | 15.0 ± 11.2 | 11.6 ± 10.8 | 16.6 ± 10.8 | 18.0 ± 11.1 | <0.001 |
| 49–62 wk | 17.2 ± 13.4 | 13.4 ± 13.0 | 18.9 ± 12.7 | 21.1 ± 13.7 | <0.001 |
| Anthropometric measures [n (%)] | |||||
| Prepregnancy BMI | 0.62 | ||||
| <25 kg/m2 | 598 (52.7) | 233 (50.2) | 231 (53.6) | 134 (56.1) | |
| 25 to <30 kg/m2 | 284 (25.0) | 124 (26.7) | 103 (23.9) | 57 (23.9) | |
| ≥30 kg/m2 | 252 (22.2) | 107 (23.1) | 97 (22.5) | 48 (20.1) | |
| Gestational weight gain | 0.53 | ||||
| Less than IOM guidelines | 205 (18.1) | 92 (19.8) | 74 (17.2) | 39 (16.3) | |
| Within IOM guidelines | 418 (36.9) | 161 (34.7) | 160 (37.1) | 97 (40.6) | |
| Greater than IOM guidelines | 511 (45.1) | 211 (45.5) | 197 (45.7) | 103 (43.1) | |
| Weight retention ≥4.55 kg | |||||
| 11–20 wk | 324 (31.2) | 120 (28.1) | 132 (33.7) | 72 (33.0) | 0.19 |
| 21–34 wk | 240 (25.3) | 100 (26.2) | 93 (26.1) | 47 (22.5) | 0.57 |
| 35–48 wk | 203 (22.9) | 92 (26.4) | 69 (20.4) | 42 (20.9) | 0.12 |
| 49–62 wk | 182 (22.4) | 75 (23.4) | 68 (22.1) | 39 (21.3) | 0.84 |
| Dietary characteristics | |||||
| Total energy (kcal) | 1889.0 ± 717.3 | 1646.7 ± 662.9 | 1965.0 ± 681.2 | 2221.7 ± 720.8 | <0.001 |
| Proteins (percentage of kcal) | 16.0 ± 3.3 | 15.4 ± 3.3 | 16.5 ± 3.3 | 16.4 ± 3.1 | <0.001 |
| Carbohydrates (percentage of kcal) | 51.8 ± 7.9 | 51.7 ± 8.2 | 51.4 ± 7.9 | 52.6 ± 7.2 | 0.17 |
| Fat (percentage of kcal) | 33.5 ± 6.2 | 33.7 ± 6.1 | 33.4 ± 6.3 | 33.0 ± 6.0 | 0.30 |
| SFA (percentage of kcal) | 11.6 ± 2.8 | 12.5 ± 2.8 | 11.3 ± 2.6 | 10.5 ± 2.4 | <0.001 |
| MUFA (percentage of kcal) | 12.6 ± 2.6 | 12.6 ± 2.6 | 12.6 ± 2.7 | 12.6 ± 2.6 | 0.92 |
| PUFA (percentage of kcal) | 6.7 ± 1.9 | 6.2 ± 1.7 | 6.9 ± 1.9 | 7.3 ± 1.8 | <0.001 |
Information was missing for age (n = 2), education (n = 51), race-ethnicity (n = 16), marital status (n = 53), parity (n = 25), prenatal smoking (n = 5), postpartum smoking (n = 80), alcohol (n = 2), beer (n = 1), wine (n = 5), liquor (n = 2), prepregnancy BMI (n = 2), and gestational weight gain (n = 2). Statistical tests by Pearson chi-square for categorical variables or by ANOVA for continuous variables. IFPS II, Infant Feeding Practices Study II; IOM, Institute of Medicine.
Mean ± SD (all such values).
Smoked postpartum anytime during the 11–62-wk interval.
The mean (±SD) of the aMED was 4.0 ± 1.8, with 41% of women who reported a low score (0–3) and 21% of women who reported a high score (6–8). Older, more-educated, and married women reported a higher aMED than did their counterparts (P < 0.001). Behavioral characteristics, including postpartum smoking and lower breastfeeding scores, were associated with a low score (P < 0.001). Energy intake increased with increasing aMED. Of the macronutrients, a higher aMED was associated with a greater protein intake but no difference in carbohydrate intake.
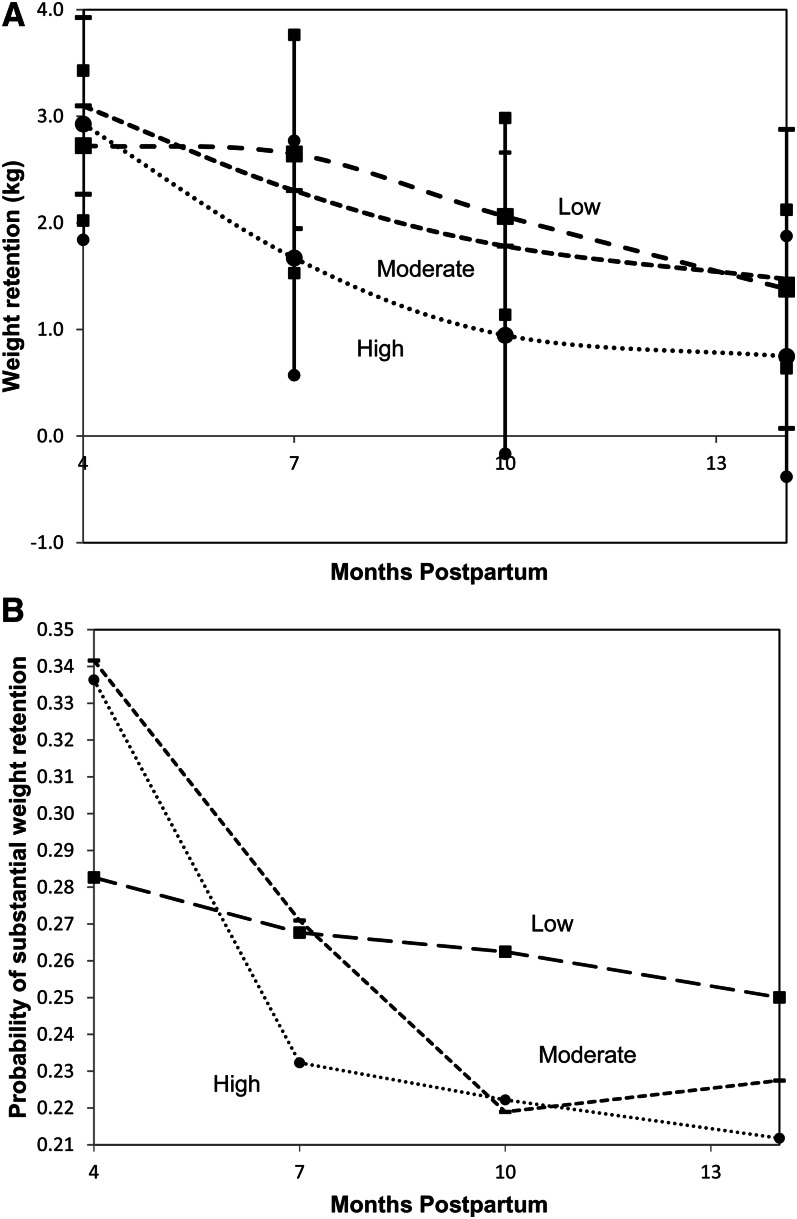
A significant interaction (continuous PPWR: P = 0.02; substantial PPWR: P = 0.007) between aMED categories and time after adjustment for energy intake was identified whereby women with better adherence to the aMED showed an apparent different trajectory of weight change than did women with lower adherence (continuous PPWR and 95% CIs are shown in Figure 1A; the probability of substantial PPWR by aMED categories is shown in Figure 1B). Empirically, the interaction between aMED and time was due to the first time interval, and curves appeared to be parallel when the first time interval was excluded. In support of this observation, we tested for this interaction after the exclusion of the first postpartum interval and showed that the interaction term was NS. Thus, subsequent models with the aMED excluded the first postpartum interval, and we report on the rest of the follow-up phase (21–62 wk).
FIGURE 1.
A: PPWR with 95% CIs (vertical lines) by aMED (square marker: low aMED; dash marker: moderate aMED; circle marker: high aMED) by using linear mixed models. B: Probability of substantial postpartum weight retention by aMED (square marker: low aMED; dash marker: moderate aMED; circle marker: high aMED) by using generalized estimating equations. The model examined was as follows: PPWR (or substantial PPWR ≥4.55 kg) = β0 + β1 × time + β2 × aMED categories + β3 × time × aMED categories + β4 × energy intake + β5 × time × energy intake. Significant interaction was noted for the first time interval (4–7 mo) between aMED categories and time: PPWR P = 0.02 and substantial PPWR P = 0.007. Midpoints of postpartum intervals are plotted. aMED, alternate Mediterranean Diet Score; PPWR, postpartum weight retention.
The results of the multivariable longitudinal model for the association between PPWR and aMED are shown in Table 2. Being married, breastfeeding for a longer duration, and a lower energy intake were significantly associated with lower PPWR [−1.92 kg for being married (95% CI: −3.03, −0.81 kg); −0.054 kg for each unit increase in breastfeeding score (95% CI: −0.082, −0.026 kg), and −0.97 kg for each 1000-kcal decrease (95% CI: −0.40, −1.55 kg)]. No significant differences were detected in PPWR across different aMED categories.
TABLE 2.
Linear mixed models that examined factors associated with risk of postpartum weight retention in the IFPS II (2005–2007)1
| Weight retention | P | |
| kg | ||
| Model with aMED2 | ||
| Postpartum time | ||
| 49–62 compared with 21–34 wk | −0.67 (−0.97, −0.37) | <0.001 |
| 35–48 compared with 21–34 wk | −0.34 (−0.61, −0.075) | 0.012 |
| Education | ||
| Some college compared with high school or less | −0.85 (−1.98, 0.28) | 0.14 |
| College degree compared with high school or less | −0.58 (−1.80, 0.63) | 0.35 |
| Graduate degree compared with high school or less | −0.67 (−2.20, 0.85) | 0.39 |
| Married compared with not | −1.92 (−3.03, −0.81) | <0.001 |
| Maternal age (per 1-y increase) | −0.014 (−0.093, 0.065) | 0.72 |
| Postpartum nonsmoker compared with smoker | 0.60 (−0.56, 1.76) | 0.31 |
| Breastfeeding score (per 1-unit increase) | −0.054 (−0.082, −0.026) | <0.001 |
| Energy intake (per 1000-kcal increase) | 0.97 (0.40, 1.55) | <0.001 |
| aMED score | ||
| Moderate (4–5) compared with low (0–3) | 0.41 (−0.49, 1.30) | 0.37 |
| High (6–8) compared with low (0–3) | −0.26 (−1.36, 0.84) | 0.65 |
| Model with AHEI-201023 | ||
| AHEI-2010 score | ||
| Moderate (31.94–40.51) compared with low (16.21–31.92) | 0.24 (−0.70, 1.18) | 0.62 |
| High (40.53–70.39) compared with low (16.21–31.92) | 0.64 (−0.34, 1.61) | 0.20 |
All values are mean differences; 95% CIs in parentheses. Means (±SDs) of diet scores were 4.0 ± 1.8 for the aMED and 36.9 ± 9.6 for the AHEI-2010. Median values for calculating aMED-component scores were as follows: vegetables excluding potatoes, 2.410 servings/d; legumes, 0.060 servings/d; fruit, 1.680 servings/d; nuts, 0.150 servings/d; whole grains, 1.020 servings/d; red meat and products, 1.830 servings/d; fish and seafood, 0.270 servings/d; and MUFA:SFA ratio, 1.088. Mean (±SD) scores of AHEI-2010 components were as follows: vegetables excluding potatoes, 5.32 ± 2.80 servings/d; fruit, 2.91 ± 2.59 servings/d; whole grains, 2.28 ± 1.53 g/d; nuts and legumes, 3.58 ± 2.94 servings/d; PUFA percentage of energy intake, 5.85 ± 2.13%; long-chain fats, 2.75 ± 2.51 mg/d; sugar-sweetened beverages and fruit juice, 2.83 ± 3.73 servings/d; red meat and products, 1.41 ± 2.38 servings/d; trans fat percentage of energy intake, 5.08 ± 1.82%; and sodium decile, 5.0 ± 3.16. The examined linear mixed model was as follows: PPWR = β0 + β1 × time + β2 × education + β3 × marital status + β4 × maternal age + β5 × smoking status + β6 × breastfeeding score + β7 × energy intake + β8 × diet-quality categories. AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternate Mediterranean Diet score; IFPS II, Infant Feeding Practices Study II; PPWR, postpartum weight retention.
Postpartum time includes 21–62 wk.
Adjusted for postpartum time, education, marital status, maternal age, smoking status, breastfeeding score, and energy intake.
For substantial PPWR (≥4.55 kg), we similarly identified the energy intake, breastfeeding score, and marital status as significant contributors (Table 3). For every 1000-kcal increase in consumption, odds of substantial weight retention increased by 25% (OR: 1.25; 95% CI: 1.03, 1.52). Being married was associated with reduced odds of substantial PPWR (OR: 0.62; 95% CI: 0.43, 0.90). Breastfeeding was also associated with reduced odds (OR: 0.97; 95% CI: 0.96, 0.98). The aMED and postpartum time did not have significant effects on substantial PPWR. No qualitative changes were observed in aMED estimates after adjustment for prepregnancy BMI or GWG or both prepregnancy BMI and GWG (data not shown).
TABLE 3.
Generalized estimating equations that examined factors associated with odds of substantial postpartum weight retention (≥4.55 kg) in the IFPS II (2005–2007)1
| Weight retention (≥4.55 kg) | P | |
| Model with aMED2 | ||
| Postpartum time | ||
| 49–62 wk compared with 21–34 wk | 1.00 (0.83, 1.20) | 1.0 |
| 35–48 wk compared with 21–34 wk | 0.97 (0.84, 1.12) | 0.68 |
| Education | ||
| Some college compared with high school or less | 0.91 (0.63, 1.32) | 0.62 |
| College degree compared with high school or less | 0.78 (0.52, 1.19) | 0.25 |
| Graduate degree compared with high school or less | 0.80 (0.46, 1.38) | 0.42 |
| Married compared with not | 0.62 (0.43, 0.90) | 0.011 |
| Maternal age (per 1-y increase) | 0.99 (0.96, 1.01) | 0.30 |
| Postpartum nonsmoker compared with smoker | 0.84 (0.57, 1.23) | 0.37 |
| Breastfeeding score (per 1-wk increase) | 0.97 (0.96, 0.98) | <0.001 |
| Energy intake (per 1000-kcal increase) | 1.25 (1.03, 1.52) | 0.021 |
| aMED score | ||
| Moderate (4–5) compared with low (0–3) | 1.14 (0.84, 1.57) | 0.40 |
| High (6–8) compared with low (0–3) | 1.09 (0.74, 1.60) | 0.68 |
| Model with AHEI-201023 | ||
| AHEI-2010 score | ||
| Moderate (31.94–40.51) compared with low (16.21–31.92) | 0.99 (0.73, 1.33) | 0.94 |
| High (40.53–70.39) compared with low (16.21–31.92) | 1.28 (0.94, 1.74) | 0.12 |
All values are ORs; 95% CIs in parentheses. Means (±SDs) of diet scores were 4.0 ± 1.8 for the aMED and 36.9 ± 9.6 for the AHEI-2010. Median values for calculating aMED-component scores were as follows: vegetables excluding potatoes, 2.410 servings/d; legumes, 0.060 servings/d; fruit, 1.680 servings/d; nuts, 0.150 servings/d; whole grains, 1.020 servings/d; red meat and products, 1.830 servings/d; fish and seafood, 0.270 servings/d; and MUFA:SFA ratio, 1.088. Mean (±SD) scores of AHEI-2010 components were as follows: vegetables excluding potatoes, 5.32 ± 2.80 servings/d; fruit, 2.91 ± 2.59 servings/d; whole grains, 2.28 ± 1.53 g/d; nuts and legumes, 3.58 ± 2.94 servings/d; PUFA percentage of energy intake, 5.85 ± 2.13%; long-chain fats, 2.75 ± 2.51 mg/d; sugar-sweetened beverages and fruit juice, 2.83 ± 3.73 servings/d; red meat and products, 1.41 ± 2.38 servings/d; trans fat percentage of energy intake, 5.08 ± 1.82%; and sodium decile, 5.0 ± 3.16. The examined generalized estimating equations model was as follows: substantial PPWR ≥4.55 kg = β0 + β1 × time + β2 × education + β3 × marital status + β4 × maternal age + β5 × smoking status + β6 × breastfeeding score + β7 × energy intake + β8 × diet-quality categories. AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternate Mediterranean Diet score; IFPS II, Infant Feeding Practices Study II; PPWR, postpartum weight retention.
Postpartum time includes 21–62 wk.
Adjusted for postpartum time, education, marital status, maternal age, smoking status, breastfeeding score, and energy intake.
Results that examined the association between AHEI-2010 and PPWR retention were very similar to aMED results. The mean (±SD) of the AHEI-2010 was 36.9 ± 9.6. For substantial weight retention, the interaction between AHEI-2010 and time was borderline significant (P = 0.09). The consistency of the associations between PPWR and the 2 dietary scores aMED and AHEI-2010 may have been due to the correlation between the 2 dietary scores (correlation coefficient: 0.61; P < 0.0001). Results of the linear mixed and generalized estimating equation models that examined PPWR and AHEI-2010, with the exclusion of the first postpartum period, are reported in Tables 2 and 3.
DISCUSSION
Despite the apparent difference in the trajectory of weight change by diet quality as assessed by aMED or AHEI-2010 in the early part of the postpartum period, neither total mean weight retention nor the probability of substantial weight retention differed through the end of follow-up. Instead, the total energy intake was a significant contributor of substantial weight retention as were breastfeeding and marital status. These findings suggested that the quantity, regardless of the quality, of food consumption after pregnancy may play a more important role in PPWR.
The average amount of weight retained in our study was similar to that reported in other studies with a similar follow-up period (5–7). In the final phase of the follow-up period in our study, women retained on average of 1.1 ± 6.7 kg, whereas 22.4% of women retained ≥4.55 kg. In a prospective, observational cohort study of women in upstate New York, Olson et al (7), reported an average weight retention of 1.5 ± 6.0 kg at 1 y postpartum with nearly 25% of subjects who had major weight retention. The significant associations we observed between PPWR, breastfeeding, marital status, and energy intake have also been previously reported (7, 11, 12). Although the effect of marital status on PPWR is not fully understood, being married might serve as a proxy measure for social support that explains part of this association (12).
Postpartum weight retained may be particularly harmful compared with weight gained because of age alone in nulliparous women. Evidence has shown that childbearing is associated with a preferential centralized distribution of adipose tissue, with data that suggested an increased accumulation of visceral fat compared with abdominal subcutaneous fat (8–10). This weight gain can ultimately influence maternal health by increasing mother's risk of developing major chronic diseases including type 2 diabetes and cardiovascular disease (26, 27). For mothers who are entering into a new pregnancy, the postpartum weight retained carries additional risk with major public health implications. In the final follow-up phase of our study, the overall median weight retained in major weight retainers (22.4%) was 9 kg. This substantial weight retained from one pregnancy to the next is associated with increased risk of maternal and neonatal complications (28). This problem underlines the significance of identifying potential modifiable risk factors for PPWR.
As such, the focus of this study was to identify dietary influences on PPWR. Although the examination of individual nutrients and foods has been quite useful, an examination of dietary patterns provides a more holistic view of the total diet. Accordingly, dietary patterns are currently on the frontier of nutritional epidemiology in the examination of the association between diet and the risk of chronic diseases (16). Previous studies have reported extensively on the beneficial effects of the Mediterranean diet and weight change, albeit not in a lactating population (17). The Healthy Eating Index has also been associated with obesity (29, 30). The lack of association between PPWR and the examined dietary patterns might have been due to the relatively short follow-up period after delivery or because of the importance of diet quantity rather than quality as depicted in our current findings. Few previous studies have examined PPWR in relation to diet, and the majority of these studies have focused on diet quantity or energy intake rather than diet quality. For example, Olson et al (7) identified that women who reported a lower amount of food intake at 6–12 mo postpartum than in the first 6 mo postpartum were less likely to retain a major amount of weight (≥4.55 kg), whereas Boardley et al (31) did not find a relation between absolute energy intake in the postpartum period and postpartum weight change. Oken et al (14) examined dietary components, including intakes of fiber, total fat, saturated fat, and trans fat and the glycemic index, in 902 women enrolled in Project Viva and reported an association with weight retention ≥5 kg at 1 y postpartum for trans fat only [OR: 1.33 (95% CI: 1.09, 1.62) per 0.5% increment in daily energy intake from trans fat]. We did not see such an association with trans fat in the IFPS II (data not shown).
The findings of our current study should be assessed in light of the strengths and limitations of the data. Available data were based on self-reported weights, which might have introduced some bias because women of higher BMI have a tendency to underreport their weights (32). However, any bias reported in the prepregnancy weight should be similar to the bias in the reporting of the postpartum weight, such that the difference between prepregnancy and postpartum weights should be relatively unbiased (14, 15, 33). Nevertheless, we could not completely rule out that any bias in recall of prepregnancy weight in the prenatal period may have differed from any reporting bias of postpartum body weight in real time. The IFPS II also did not collect data on physical activity. In addition, our study population was predominantly white (86%) compared with the national estimate of 72% in 2010 (34), which restricted the generalizability of our findings to other racial-ethnic groups. Moreover, diet was assessed only once over the period of follow-up with a DHQ, and women might have made dietary changes to lose their pregnancy weight. Such changes might have confounded the association between the dietary patterns and PPWR and biased our estimates toward the null. Strengths of the present study included a relatively large sample size and the examination of a wide range of potential behavioral and socioeconomic covariates that might have confounded the association between dietary patterns and PPWR. In addition, the longitudinal analytic methodology used offered a greater precision and power to identify potential differences in PPWR between different dietary adherence levels than is the case with cross-sectional study designs. We also had a good retention rate in the final phase of the follow-up period (71%).
In conclusion, we showed no differences in the mean weight retained and probability of substantial weight retention by dietary patterns as captured by the aMED and the AHEI-2010. A future understanding of dietary predictors of PPWR is warranted to improve future maternal health and subsequent pregnancy outcomes.
Acknowledgments
The authors’ responsibilities were as follows—NSB and EHY: study concept and design, drafting of the manuscript, and responsibility for the final content of the manuscript; NSB, EHY, and PSA: analysis and interpretation of data; NSB and PSA: statistical analysis; and all authors: critical revision of the manuscript for important intellectual content. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternate Mediterranean Diet Score; DHQ, Diet History Questionnaire; GWG, gestational weight gain; IFPS II, Infant Feeding Practices Study II; NCI, National Cancer Institute; PPWR, postpartum weight retention.
REFERENCES
- 1.Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–43 [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7 [DOI] [PubMed] [Google Scholar]
- 3.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med 2003;26:149–59 [DOI] [PubMed] [Google Scholar]
- 4.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol 2002;100:245–52 [DOI] [PubMed] [Google Scholar]
- 5.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev 2000;22:261–74 [DOI] [PubMed] [Google Scholar]
- 6.Lipsky LM, Strawderman MS, Olson CM. Maternal weight change between 1 and 2 years postpartum: the importance of 1 year weight retention. Obesity (Silver Spring) 2012;20:1496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord 2003;27:117–27 [DOI] [PubMed] [Google Scholar]
- 8.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA). Int J Obes Relat Metab Disord 2004;28:525–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunderson EP, Sternfeld B, Wellons MF, Whitmer RA, Chiang V, Quesenberry CP, Jr, Lewis CE, Sidney S. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 2008;16:1078–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA 1994;271:1747–51 [PubMed] [Google Scholar]
- 11.Baker JL, Gamborg M, Heitmann BL, Lissner L, Sorensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr 2008;88:1543–51 [DOI] [PubMed] [Google Scholar]
- 12.Janney CA, Zhang D, Sowers M. Lactation and weight retention. Am J Clin Nutr 1997;66:1116–24 [DOI] [PubMed] [Google Scholar]
- 13.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr 2011;94:1225–31 [DOI] [PubMed] [Google Scholar]
- 14.Oken E, Taveras EM, Popoola FA, Rich-Edwards JW, Gillman MW. Television, walking, and diet: associations with postpartum weight retention. Am J Prev Med 2007;32:305–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen P, Baker JL, Henriksen TB, Lissner L, Heitmann BL, Sorensen TI, Nohr EA. Influence of psychosocial factors on postpartum weight retention. Obesity (Silver Spring) 2011;19:639–46 [DOI] [PubMed] [Google Scholar]
- 16.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9 [DOI] [PubMed] [Google Scholar]
- 17.Buckland G, Bach A, Serra-Majem L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev 2008;9:582–93 [DOI] [PubMed] [Google Scholar]
- 18.McManus K, Antinoro L, Sacks F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord 2001;25:1503–11 [DOI] [PubMed] [Google Scholar]
- 19.Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr 2003;90:717–27 [DOI] [PubMed] [Google Scholar]
- 20.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics 2008;122(suppl 2):S28–35 [DOI] [PubMed] [Google Scholar]
- 21.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol 2001;154:1089–99 [DOI] [PubMed] [Google Scholar]
- 22.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73 [DOI] [PubMed] [Google Scholar]
- 23.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608 [DOI] [PubMed] [Google Scholar]
- 24.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute of Medicine and National Research Council Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academy Press, 2009 [PubMed] [Google Scholar]
- 26.Lemieux S, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, Despres JP. Seven-year changes in body fat and visceral adipose tissue in women. Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care 1996;19:983–91 [DOI] [PubMed] [Google Scholar]
- 27.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA 1998;280:1843–8 [DOI] [PubMed] [Google Scholar]
- 28.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006;368:1164–70 [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Warden BA, Paeratakul S, Bray GA. Healthy Eating Index and obesity. Eur J Clin Nutr 2004;58:1580–6 [DOI] [PubMed] [Google Scholar]
- 30.Kant AK, Graubard BI. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J Am Coll Nutr 2005;24:294–303 [DOI] [PubMed] [Google Scholar]
- 31.Boardley DJ, Sargent RG, Coker AL, Hussey JR, Sharpe PA. The relationship between diet, activity, and other factors, and postpartum weight change by race. Obstet Gynecol 1995;86:834–8 [DOI] [PubMed] [Google Scholar]
- 32.Rowland ML. Self-reported weight and height. Am J Clin Nutr 1990;52:1125–33 [DOI] [PubMed] [Google Scholar]
- 33.Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. Am J Public Health 1993;83:1100–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Department of Commerce, Economics and Statistics Administration, US Census Bureau. Overview of race and Hispanic origin. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau, 2010 [Google Scholar]