Abstract
Introduction
PTEN (phosphatase and tensin homolog deleted on chromosome 10) plays a pivotal role in controlling intracellular signaling for cell survival and proliferation by inhibiting the PI3K/Akt pathway, and its dysfunction is associated with several neoplastic diseases. PTEN is frequently found mutated in many pathological conditions highlighting its importance in normal physiological function. Unlike several cellular proteins which are activated by phosphorylation, PTEN is inactivated upon phosphorylation by specific kinases which phosphorylate serine and threonine residues in its C-terminal region. Therefore, development of therapeutic agents that specifically target kinases and kinase-domain-containing proteins affecting PTEN would lead to the treatment of PTEN-related diseases.
Areas covered
With increasing evidence on the role of PTEN in many human diseases, the present review focuses on the clinical relevance of PTEN with a comprehensive list of currently identified modulators of PTEN, and proposes potentially novel molecular targets which could aid in the development of drug candidates for the treatment of PTEN-related diseases such as cardiovascular diseases, diabetes, obesity, cancer, autism, Parkinson’s and Alzheimer’s diseases.
Expert opinion
This review describes several target sites that could help in the development of novel drug candidates to regulate or restore the normal physiological functions of PTEN and are essential in the treatment of human diseases where PTEN plays a pivotal role.
Keywords: cancer, cardiovascular diseases, kinases, modulators, obesity, phosphatase, PTEN, transcription factors
1. Introduction
PTEN (phosphatase and tensin homolog deleted on chromosome 10), first identified as a tumor suppressor, is among the most mutated genes discovered thus far and was found frequently mutated or deleted in several neoplastic diseases, including glioblastoma, endometrial cancer, prostate cancer, and small-cell lung cancer. Specific mutations in the PTEN gene were attributed to hereditary diseases, such as Cowden syndrome, Bannayan-Zonana syndrome (Bannayan–Riley–Ruvalcaba syndrome), Lhermitte-Duclos syndrome, Proteus syndrome, and Proteus-like syndrome, which are clinically referred to as Hamartoma-Tumor Syndromes. Besides its genetic alterations, PTEN expression is also affected by DNA methylation, mRNA degradation, post-translational modifications, transcriptional and translational regulation inside the cell indicating that PTEN is a highly targeted protein in many human diseases [1,2]. Numerous mutations ranging from point mutations to allelic loss have been detected at high frequency in PTEN gene in different malignancies, and cancer genome sequencing further confirms that PTEN is one of the most commonly mutated genes identified [3,4].
PTEN expression has been observed in most tissue types from embryonic to adulthood, hence it is presumed to be expressing constitutively in the cells. However, its stability is regulated by its interaction with other cellular proteins. PTEN gene (NCBI Acc # NM_000314.4, also known as BZS; DEC; GLM2; MHAM; TEP1; MMAC1; PTEN1; 10q23del) contains nine exons with a variable exon 5b; in addition, the 3′ end of exon 8 was reported to generate splice variants. This 5.572 kb gene that was mapped onto human chromosome 10q23 region encodes for a 403 aa, 47.17 kDa protein with distinct N-terminal and C-terminal regions. The first six exons encode for a 185 aa N-terminal domain with phosphatase activity, which catalyzes dephosphorylation of phospholipids, and the rest three exons code for the 218 aa C-terminal domain which has C2 subdomain, two PEST sequences and PDZ binding domain [5]. Molecular structure of PTEN shows characteristic regions of a phosphatase protein having P-loop, WPD-loop and the TI-loop regions (Figure 1A). The N-terminal domain is reported to exert phosphatase activity while the C-terminal tail region contains several phosphorylation sites (S362, T366, S370, S380, T382, T383, and S385) which, upon phosphorylation, modulate PTEN functions that are vital to normal cellular activities. Phosphorylation of these amino acid residues by specific kinases along with a predicted structure for the C-terminal tail region is shown in Figure 1B. The functional activity of PTEN is regulated by phosphorylation, oxidation, acetylation and protein–protein interactions, and cellular functions that are regulated by PTEN include cell proliferation, cell cycle progression, chemotaxis, angiogenesis, apoptosis, aging, muscle contractility and DNA damage response. Membrane binding and activation of PTEN which leads to inhibition of the cellular pathways associated with PTEN-related diseases is illustrated in Figure 1C. Also, three of the common cellular signaling pathways that are associated with PTEN are shown in Figure 2.
Figure 1.
A. Represents the Chain A structure of PTEN with PDB id “1D5R” with P-loop, WPD-loop and TI-loops, as highlighted, which are phosphatase characteristic regions. B. Shows specific residues that are phosphorylated by different kinases in the C-terminal tail region of PTEN. The panel B also shows the possible β-sheet structural features of the C-terminal tail region. C. Illustrates membrane binding and activation of PTEN which leads to inhibition of cellular pathways that are associated with specific human diseases, as described.
Figure 2. PTEN inhibits different cellular pathways.
The inhibitory role of PTEN is highlighted to indicate its molecular targets in cellular signaling pathways that affect cell migration, proliferation, survival and protein synthesis.
2. Kinases and kinase-domain-containing proteins that modulate PTEN
Unlike many cellular proteins, PTEN becomes functionally inactive upon phosphorylation at different serine and threonine residues, especially in the C-terminal region. This phosphorylation of PTEN is mediated by specific kinases, which affect PTEN function to dephosphorylate PIP3 [6]. CK2 is a major protein kinase that phosphorylates PTEN at Thr366, Ser370, Ser380, Thr382, Thr383 and Ser385, and inhibits its stability and activity [7,8]. The kinase LKB1 was also reported to phosphorylate PTEN at residue S385. Although this phosphorylation at S385 residue renders PTEN inactive, subsequent phosphorylation by LKB1 at S380, T382 or T383 residues on PTEN was observed, suggesting a cooperative dependence of S385 phosphorylation with S380, T382 or T383 residues which was confirmed using site-directed mutagenesis. In addition, Src kinase has been specifically found to phosphorylate tyrosine residues in the C-terminal region of PTEN [9]. GSK3β phosphorylates PTEN at Ser362 and Thr366, which greatly reduces its phosphatase activity [10]. The kinases JNK and JNKK were also reported to inhibit PTEN activity. Besides kinases PICT1 and FRK, the kinase-domain-containing proteins, MAGI and MAST, were also reported to stabilize or activate PTEN [11–15]. RhoA-associated kinase (ROCK) activates PTEN by phosphorylating it at Ser229, Thr232, Thr319 and Thr321 residues [16]. Figure 3 shows some of the kinases and proteins containing kinase domains which either inhibit or enhance PTEN activity, thus modulating its cellular functions.
Figure 3. PTEN modulation by kinases or kinase-domain-containing proteins.
Different intracellular proteins that target PTEN to inhibit or activate its cellular functions are shown. This inhibition or activation of PTEN functions is primarily mediated through phosphorylation of specific residues in its C-terminal region.
JNK and PTEN signaling pathways crosstalk and regulate progression of prostate neoplasia to invasive adenocarcinoma with increased activity of JNK signaling in the absence of PTEN. Notably, PTEN and JNK deficiency caused reduced luminal cell–cell interactions and increased luminal cell apoptosis compared to PTEN-deleted mice [17]. Although apparent crosstalk between JNK and PTEN signaling is evident, the exact molecular interactions that connect the two signaling pathways are still unclear. Erythropoietin receptor-mediated activation of Jak2 upregulates Src, which in turn activates CK2a and inhibits PTEN [18]. The specific kinases and kinase-domain-containing proteins that have direct or indirect effects on PTEN are shown in Table 1.
Table 1.
Specific kinases and kinase-domain-containing proteins as potential targets for the development of therapeutic drugs which can restore normal PTEN functions.
| Potential target | Effect on PTEN | Refs. |
|---|---|---|
| CK2 (casein kinase 2) | Inactivates PTEN by phosphorylating at S370 and S385 | [8] |
| GSK3β (glycogen synthase kinase) | Inhibits PTEN activity by phosphorylation at S362 and T366 | [10] |
| ROCK (RhoA-associated Kinase) | Activates PTEN and targets it to the plasma membrane; phosphorylates at S229, T232, T319, T321 | [16] |
| MKK4 (JNKK or SEK1) | Suppresses PTEN transcription via direct binding of NFκB to PTEN promoter | [36] |
| Src Kinases | Inhibits PTEN by phosphorylation at tyrosine residues | [39] |
| IKK | Transient expression of PTEN suppressed IKK activation and TNF-induced NFκB DNA binding and transactivation | [40] |
| JNK | Loss of PTEN upregulates JNK indicating, PTEN inhibits JNK | [41] |
| Jak2 | Upregulates Src which activates CK2 and leads to inhibition of PTEN | [18] |
| PICT1 | Upregulates PTEN levels and phosphorylates at S380 | [14] |
| PLK-1 | Plk1 phosphorylates PTEN at Thr366 and/or Ser370 and promotes its association with chromatin. | [42] |
| STK11 (LKB-1) | Binds to the N-terminal end of C2 domain of PTEN and forms a cytoplasmic complex | [9] |
| RAK (FRK, Fyn-related kinase) | Activates PTEN by phosphorylation at Tyr366 and regulates PTEN stability and function | [15] |
| MAGI-2 & 3 (membrane-associated guanylate kinase inverted) | Stabilizes PTEN through interactions with vinculin and β-catenin | [11,12,43] |
| MAST1, MAST3 (microtubule-associated serine-threonine kinase) | Stabilizes PTEN by phosphorylation | [13] |
| PDK1 (phosphoinositide-dependent protein kinase) | A target of PTEN that induces neoplasia in PTEN null conditions and in elevated levels of Akt and S6K | [44] |
3. Pathological significance of PTEN
3.1 Role of PTEN in the treatment of type 2 diabetes and obesity
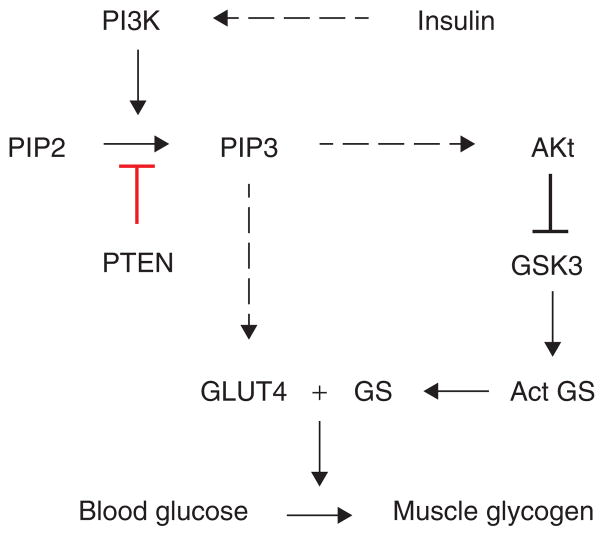
Some of the kinases expressed upstream in the pathway can inhibit PTEN. Thus, indirect methods for restoring PTEN function would be to strategically block the upstream kinases using specific inhibitors or antibodies. An effective strategy to address the inhibition of PTEN in type 2 diabetes is to regulate the downstream pathway that converts blood glucose to muscle glycogen. Akt is a key enzyme in insulin signaling that regulates glucose uptake. Since PTEN inhibits formation of PIP3, which is a substrate for Akt, inhibition of PTEN is being considered as a possible drug target to treat type 2 diabetes. In this regard, bisperoxovanadium compounds (bpV), even in the nanomolar range, are reported to be effective as PTEN inhibitors [19]. In hepatocyte-specific conditional PTEN null mice, PTEN is an important regulator of lipogenesis, glucose metabolism, hepatocyte homeostasis, and tumorigenesis in liver [20]. Leptin promotes phosphorylation of PTEN at multiple sites in the C-terminal region and thus inhibiting the phosphatase activity of PTEN. Specific inhibitors of CK2 and GSK3 block this leptin-mediated phosphorylation of PTEN. Interestingly, GSK3 activity is inhibited by insulin which phosphorylates its N-terminal region [21]. These reports, though still are in the preliminary stage, elucidate a clear role for PTEN in diabetes and obesity. The role of PTEN in type 2 diabetes is summarized in Figure 4.
Figure 4. Role of PTEN in type 2 diabetes.
Figure shows the intracellular insulin regulation pathway and the interference of PTEN through the regulation of glucose metabolism.
3.2 PTEN inhibition to prevent heart failure
Inhibition of PTEN signaling in the heart may present a novel approach to slow the progression of heart failure in response to pathologically induced biomechanical stress. In PTEN knockout mice that were subjected to aortic banding, a mild reduction in systolic function with minimal ventricular dilation and loss of heart function was observed, indicating that loss of PTEN prevents the development of heart failure. Thus, inhibition of PTEN expression could be clinically significant in the prevention of heart failure [22].
3.3 Genetic role of PTEN in autism
Three genetic mutations in the PTEN gene (H93R in exon 4 and D252G and F241S in exon 7) have been identified which leads to macrocephaly/autism syndrome [23]. Targeted inactivation of PTEN in the differentiated neurons of the cerebral cortex in mice resulted in increased response to sensory stimuli with neuronal hypertrophy, including hypertrophic and ectopic dendrites and axon tracts with increased synapses. This suggests that defects in PTEN cause macrocephaly and autistic syndrome in mice [24].
3.4 Other diseases in which PTEN could be a therapeutic target
Besides the obvious role of PTEN in preventing neoplastic diseases, recent evidence also suggests its role in several other diseases, such as Parkinson’s, Alzheimer’s and allergy/inflammation.
In adipose tissue, binding of insulin to insulin receptors activates PI3K signaling. Since PTEN inhibits PI3K pathway, it acts as a negative regulator of insulin signaling and insulin sensitivity. Interestingly in neuronal tissues, PTEN regulates neuronal insulin signaling and resistance through its phosphatase activity that inhibits FAK/ERK signaling which is critical for negative regulation of neuronal insulin. With increasing evidence that impaired insulin signaling in the brain acts as a mediator for chronic neurodegenerative disorder, Alzheimer’s disease, it is clear that regulating PTEN could be a therapeutic approach in treating the disease [25].
Mutation in the PTEN-induced putative kinase 1 (PINK1) gene has been identified to cause early onset of autosomal recessive Parkinson’s disease which was linked to PARK6 locus on chromosome 1p [26,27]. This study supports that haploinsufficiency of PINK1 may indicate a susceptibility factor toward Parkinson’s disease and therefore modulation of PTEN activity could be an effective treatment strategy.
Intratracheal administration of adenovirus-expressing PTEN reduces eosinophilic inflammation and airway hyperresponsiveness in allergen-challenged mice, indicating that PTEN inhibits allergic inflammation and that PTEN gene therapy could be beneficial in treating allergic/inflammatory disorders in the airways [28].
4. Therapeutic inhibitors used in PTEN-associated pathologies
Receptor tyrosine kinases are predominantly growth factor receptors that mediate outside-in signaling through cell survival pathways, mostly through PI3K/Akt or Ras/MEK axis, and regulate cell proliferation, migration and intracellular metabolism. Targeting these kinases is a logical approach to achieve anticipated therapeutic benefits of PTEN expression or inhibition in the treatment of specific diseases. Since PTEN plays a prominent role in inhibiting PI3K/Akt-mediated cell survival pathway, use of its agonists or mimetics to inhibit cell growth, cell division, cell proliferation and/or cell migration could prove to be an effective approach in the treatment of neoplastic diseases. Overexpression of PTEN with subsequent activation of IGF-1 affects cell cycle progression in saphenous vein smooth muscle cells. Also, overexpression of PTEN in smooth muscle cells inhibits IGF-1-induced cellular proliferation mediated by PI3K–Akt axis, and PTEN inactivation leads to sustained levels of increased temporal expression of pAkt in smooth muscle cells [29,30]. The inhibitors and other therapeutic drugs that are currently used To Whom It May Concern: treat various PTEN-associated diseases are listed in Table 2.
Table 2.
List of therapeutic drugs that enhance PTEN activity.
| Drug | Target | Effect on PTEN | Patentee/provider (Refs.) |
|---|---|---|---|
| Ublituximab | NFκB-Snail-RKIP | Induces PTEN and sensitizes TRAIL apoptotic pathway | LFB Biotechnologies US20120100133; [45,46] |
| Trastuzumab (Herceptin) | HER2 | Increases PTEN activity through Src inhibition | Genentech & UCLA US20110165155A1 [47,48] |
| Pertuzumab | HER2 dimerization | Activates PTEN, similar to Trastuzumab | Genentech US20110044977A1 [49,50] |
| Rituximab | PI3K-Akt pathway | Induces PTEN | IDEC Pharmaceuticals EP1974747B1 [51,52] |
| Cetuximab | EGFR | PTEN loss is associated with nonresponsiveness to cetuximab | Yeda & Sanofi-Aventis EP0667165 [53,54] |
| Sunitinib (Sutent, SU11248) | PDGFR signaling | Induces PTEN expression | Sugen-Pfizer US6573293 [55,56] |
| Gefitinib (ZD1839, Iressa) | EGFR | Loss of PTEN results in gefitinib resistance | AstraZeneca EP1509230B1 [57,58] |
| Erlotinib | EGFR | PTEN loss contributes to erlotinib resistance | OSI Pharmaceuticals US6900221 [59,60] |
| Resistin (cytokine) | p38MAPK and ATF2 | Increases PTEN expression by activating p38MAPK and ATF2, and leads to decreased Akt and eNOS | Identified in University of Pennsylvania [61] |
| Simvastatin | NFκB | Increases PTEN expression level by inhibiting NFκB | Apotex, Inc. US5393893 [62,63] |
| Lovastatin (Mevacor) | PPAR-γ | Increases PTEN expression | MERCK US4231938 [64,65] |
| Rosiglitazone (Avandia, PPAR-γ agonist) | PPAR-γ | Upregulates PTEN expression | GlaxoSmithKline (GSK) US7358366 [64,66] |
| NVP-AEW541 (IGF-1R inhibitor) | IGF-1R | Induces radiosensitization in a PTEN-dependent manner | Novartis [67] |
| PP1, Herbimycin (Src inhibitors) | Src | Reduces pervanadate-induced tyrosine phosphorylation of PTEN | EMD Biosciences [39] |
| γ-Secretase inhibitor (NOTCH1 inhibitors) | NOTCH1 | Upregulates PTEN expression | SelleckBio [32] |
| DMAT (CK2 inhibitor) | CK2-Leptin | Prevents leptin from phosphorylating PTEN at Ser-370 and Ser-385 residues but not at Thr-366 residue | Sigma [21] |
| CT99021 (GSK3 inhibitor) | GSK3 | Prevents leptin from phosphorylating only Thr-366 residue of PTEN | SelleckChem [21] |
| Salinosporamide A (NPI-0052) | PTEN & RKIP | Induces expression of PTEN and RKIP | Univ of California Oakland & Nereus Pharmaceuticals US20090148445 [68] |
| Sodium Selenite | Thioredoxin, Akt, CK2 | Increases PTEN activity | [69] |
5. Possible therapeutic targets that affect PTEN expression
5.1 Transcription factors
Aside from post-translational modifications, PTEN expression can be regulated at both the transcriptional and translational levels under various pathological conditions. Thus, targeting transcription factors that regulate PTEN is a promising strategy for developing therapeutic drugs.
Complete loss of PTEN has been shown to induce activation of p53-dependent cellular senescence, and combined inactivation of PTEN and Trp53 shows unconstrained tumor growth, indicating that mutants with partial PTEN function may have a selective advantage for the treatment of PTEN deficiency in cancer [31]. Active NOTCH1 increases PTEN expression through CBF-1 and MYC transcription factors. In addition, activation of NOTCH1 represses PTEN expression through the HES-1 transcription factor. This is indicative of the many roles played by NOTCH1. Thus, the regulation of PTEN by NOTCH1 could be a therapeutic strategy in the use of NOTCH1 inhibitors in leukemia [32]. RAS-mediated PTEN suppression is mediated by TGF-β-dependent mechanism, and the oncogenic RAS-RAF-MEK-ERK pathway suppresses PTEN levels through the transcriptional factor, c-Jun [33–35]. The stress kinases, including MEKK4 and JNK, promote resistance to apoptosis by suppressing transcription of PTEN mediated by NF-κB [36]. The aforementioned reports demonstrate a strong transcriptional regulation of PTEN mediated by different cellular factors. Table 3 lists some of the known transcription factors that regulate PTEN expression. Since several synthetic compounds are known to affect these transcription factors, their application in the therapy of PTEN-associated diseases is becoming increasingly significant.
Table 3.
Transcription factors that regulate PTEN expression.
| Transcription factor | Effect on PTEN expression | Refs. |
|---|---|---|
| p53 | Activates PTEN by binding in the promoter region | [70] |
| Egr-1 | Activates PTEN and induces apoptosis in irradiation-induced signaling by binding to the 5’ UTR of PTEN | [71] |
| Evi1 | Represses PTEN transcription in bone marrow by binding to polycomb group of proteins | [72] |
| E2F1 | Activates PTEN by binding to the 5’ UTR and induces p53-independent apoptosis | [73] |
| PPAR-γ | Activates PTEN by binding to PPRE1 and PPRE2 elements in the promoter | [66] |
| JUN | Suppresses PTEN expression by binding to AP-1 site in the PTEN promoter | [33] |
| GATA2 | Inhibits PTEN expression and stimulates Akt phosphorylation | [74] |
| SNAIL | Binds to the PTEN promoter and prevents the association of p53 with the PTEN promoter region | [75] |
| NOTCH1 | Inhibits PTEN through HES-1 transcription factor; increases PTEN transcription by MYC or CBF1 | [32] |
| NFκB | NFκB downregulates PTEN expression through IKKβ pathway, and this regulation is mediated by the p65 subunit of NFκb | [76] |
5.2 Cellular proteins that regulate PTEN expression
Several cellular proteins, including phosphatases, either directly or indirectly affect PTEN expression primarily via protein–protein interactions. These proteins interact with PTEN by binding to its N-terminal region, C-terminal region and the PDZ binding domain. Some of these proteins also help in the translocation of PTEN across the cytoplasm.
Another approach is to prevent the degradation of PTEN mediated by ubiquitin proteasomal pathway using proteasome inhibitor “MG132,” which has proven to be effective. NEDD4-1, which is E3 ubiquitin-protein ligase, interacts with PTEN and the overexpression of NEDD4-1 leads to ubiquitination of PTEN. Therefore, inhibition of NEDD4-1 may help in the development of a strategic therapeutic drug that can upregulate PTEN in cancer [37]. WWP2 has recently been identified as an E3 ubiquitin ligase for PTEN, which mediates its ubiquitination-dependent degradation. Although the associated kinase has yet to be identified, it is evident that phosphorylation at Tyr155 in PTEN is essential for WWP2-assisted PTEN ubiquitination [38]. The cellular proteins that interact with PTEN are summarized in Table 4.
Table 4.
Cellular proteins that interact with PTEN and regulate its functions.
| Target protein | Therapeutic implications | Refs. |
|---|---|---|
| p17 (HIV-1 matrix protein) | Activates PTEN by binding to its C-terminal tail region, and suppresses Akt signaling mediated by ROCK | [77] |
| MAG (myelin-associated glycoprotein) | Activates PTEN and decreases phosphorylated Akt in the neurons | [78] |
| SHP-1 (SH2 domain containing tyrosine phosphatase) | Selectively binds and dephosphorylates PTEN making it inactive | [39] |
| NEP | Electrostatically binds to and phosphorylates PTEN in the C-terminal tail region and enhances its stability and phosphatase activity besides recruiting it in the plasma membrane | [79] |
| RhoA | Activates ROCK which upregulates PTEN | [80,81] |
| YSV tripeptide | Increased expression of PTEN and other tumor suppressor genes. Activates PTEN and inhibits Akt by dephosphorylating both | [82] |
| SPARC | Overexpression increases PTEN activity and suppresses Akt both in vitro and in vivo | [83] |
| Semaphorin 4D | Activates PTEN by dephosphorylation at Ser-380. | [84] |
| CSIG | Interacts with PTEN mRNA in the 5′ UTR and negatively regulates PTEN expression promoting cell proliferation | [85] |
| β-arrestin | Controls activation of PTEN and also inhibits the antimigratory effects of PTEN | [86] |
| Tissue transglutamase-2 | Promotes degradation of PTEN by ubiquitin-proteasomal pathway and activates FAK/Akt pathway | [87] |
| PGE synthase-1 | Inhibits expression of PTEN by activating EGFR–PI3K–AKT–mTOR signaling | [88] |
| p110δ (catalytic subunit of PI3K) | Inhibits PTEN through RhoA pathway | [89] |
| P-REX2a | Inhibits PTEN lipid phosphatase activity | [90] |
| PCAF (p300/CBP-associated factor) | Negative regulator of PTEN, acetylates Lys125 and Lys128 | [91] |
| SIPL1 (Shank interacting protein-like1) | Negative regulator of PTEN inhibits its phosphatase activity | [92] |
| MAN2C1 (α-Mannosidase 2C1) | A negative regulator of PTEN which inhibits PTEN function by binding to PTEN and prevents its phosphatase activity | [93] |
| ROS (reactive oxygen species) | Inactivate PTEN by oxidizing Cys124 | [94] |
| NHERF-1, 2 (Na+/H+ exchanger regulatory factor) | Interacts with PDZ domain of PTEN and restricts PI3K activation effects | [95] |
| SPRY2 | Increases PTEN levels and activity by decreasing PTEN phosphorylation | [96] |
| NEDD4-1 | Polyubiquitylates PTEN and promotes its degradation in the cytoplasm | [37] |
6. Conclusions
Under various pathological conditions, PTEN has been a primary target for the derailment of the normal cellular physiology. Thus, the protection of PTEN from the cellular proteins that affect its expression and functions is a promising approach for restoring normal cellular function. Along these lines, several therapeutic drugs have been developed that assist proper functioning of PTEN through indirect mechanisms and several inhibitors of specific kinases that directly modulate PTEN functions have also been developed. From the therapeutic perspective, the present review identifies potential targets for the development of drugs to help in the treatment of diseases in which PTEN expression is affected. In addition to the list of current drugs used in the treatment of PTEN-associated diseases, this review identifies specific kinases, cellular proteins and transcription factors that act as modulators of PTEN, which should garner them strong consideration as promising candidates to develop patentable therapeutic drugs.
7. Expert opinion
PTEN is a vital protein phosphatase. Strategic approaches to inhibit or activate PTEN could be employed in the treatment of several human diseases. Accordingly, several kinases, transcription factors and cellular proteins have been identified that directly or indirectly act on PTEN. This indicates transcriptional, translational and post-translational regulations as the key mechanisms by which PTEN functions are regulated. It is noteworthy that the absence of the C-terminal tail region of PTEN did not affect its cellular functions or its stability, but phosphorylation of specific residues in its C-terminal tail region would lead to its functional inactivation. However, its catalytic activity toward lipid substrates was found decreased when phosphorylated at other residues. A possible auto-inhibitory mechanism was proposed which suggests that the phosphorylation of PTEN in its C-terminal tail region would lead to conformational changes in its structure, as a result, the C-terminal tail region directly interacts with C2 domain leading to masking of its PDZ binding region. These structural changes could lead to functional inactivation of PTEN.
Functional attributes of PTEN are regulated by its phosphorylation at different serine and threonine residues in the C-terminal region, and this phosphorylation is mediated by specific kinases both upstream and downstream to PTEN. The kinases CK2, GSK3 and Src have a direct effect on PTEN by phosphorylating it and rendering it inactive, and the kinases MKK4, IKK and STK11 impede PTEN activity mediated by other cellular proteins. Interestingly, kinases, including ROCK, PICT1 and RAK, activate PTEN by phosphorylation; and other proteins, including MAGI and MAST, stabilize PTEN through indirect mechanisms.
Expression of PTEN is also regulated by several transcription factors, notably p53, Egr-1, E2F1, and PPAR-γ, which activate PTEN expression by binding to different sites in its promoter region. Contrary to this, the transcription factors, Evi1, JUN, GATA2, SNAIL, NOTCH1 and NFκB, prevent expression of PTEN. Several cellular proteins have also been identified which regulate PTEN. Among these, MAG, SHP-1, NEP, RhoA, SPARC, β-arrestin, Semaphorin 4D, NHERF-1, NHERF-2, SPRY2, the p17 matrix protein from HIV-1 and the YSV tripeptide activate or increase PTEN expression levels while Src, CSIG, p110δ subunit of PI3K, tissue transglutamase-2, PGE synthase-1, P-REX2a, PCAF, SIPL1, MAN2C1, NEDD4-1 and reactive oxygen species suppress or negatively regulate PTEN expression in the cells.
Additionally, several monoclonal antibodies, protein inhibitors, synthetic and natural compounds have been identified which modulate PTEN. With high significance of PTEN in establishing normal cellular functions, PTEN modulators are specifically explored. In this regard, the present review highlights numerous possibilities for the development of novel therapeutic interventions with promising commercial interests. In addition, this review summarizes several potential targets for the treatment of major human diseases in which PTEN plays a pivotal role, such as cancer, type 2 diabetes, obesity, cardiovascular diseases, Alzheimer’s, autism, and Parkinson’s disease.
Article highlights.
PTEN regulates normal physiological functions in the cell by inhibiting biochemical pathways that induce cell proliferation, cell survival, protein synthesis, and other cellular activities.
Some of the PTEN-related diseases in human include cardiovascular diseases, diabetes, obesity, cancer, autism, Parkinson’s and Alzheimer’s diseases.
At the genetic level in several pathological conditions, PTEN gene is deleted, mutated or methylated. At the protein level, PTEN gene is modified due to phosphorylation, acetylation and oxidation, indicating that it is a highly targeted gene/protein whose loss of function leads to many pathological conditions in human.
Several transcription factors and cellular proteins also affect PTEN expression.
Specific kinases and kinase-domain-containing proteins phosphorylate PTEN at several amino acid residues in its C-terminal tail region.
Development of novel PTEN agonists or mimetic compounds could lead to restoration of its normal cellular functions.
Development and use of monoclonal antibodies, peptide or inhibitors specific to kinases, transcription factors and cellular proteins that affect PTEN could be of high therapeutic value in the treatment of PTEN-related diseases.
This box summarizes key points contained in the article.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest
The authors declare no conflict of interest. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers R01HL090580, R01HL104516, and R01HL112597.
Bibliography
Papers of special note have been highlighted as either of interest
(•) or of considerable interest
(••) to readers.
- 1.Wiencke JK, Zheng S, Jelluma N, et al. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neurol Oncol. 2007;9(3):271–9. doi: 10.1215/15228517-2007-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120(Pt 23):4071–9. doi: 10.1242/jcs.015230. Good commentary describing regulations of PTEN and its role in human diseases. [DOI] [PubMed] [Google Scholar]
- 3.COSMIC: catalog of somatic mutations in cancer. Welcome Trust, Sanger Institute; Cambridge, UK: 2011. Available from: http://www.sanger.ac.uk/perl/genetics/CGP/cosmic?action=gene&ln=PTEN [Cited 11 November 2012] • This interactive website shows different mutations that are identified in the PTEN gene. [Google Scholar]
- 4.Tumorscape: copy number alterations across multiple cancer types. The Broad Institute of MIT and Harvard; US: Available from: http://www.broadinstitute.org/tumorscape/pages/portalHome.jsf# [Cited 14 November 2012] [Google Scholar]
- 5.Govender D, Chetty R. Gene of the month: PTEN. J Clin Pathol. 2012;65(7):601–3. doi: 10.1136/jclinpath-2012-200711. [DOI] [PubMed] [Google Scholar]
- 6.Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7•.Miller SJ, Lou DY, Seldin DC, et al. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528(1–3):145–53. doi: 10.1016/s0014-5793(02)03274-x. Identified the role of CK2 in the oncogenesis by inhibiting PTEN. [DOI] [PubMed] [Google Scholar]
- 8.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276(2):993–8. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 9.Mehenni H, Lin-Marq N, Buchet-Poyau K, et al. LKB1 interacts with and phosphorylates PTEN: a functional link between two proteins involved in cancer predisposing syndromes. Hum Mol Genet. 2005;14(15):2209–1. doi: 10.1093/hmg/ddi225. [DOI] [PubMed] [Google Scholar]
- 10.Al-Khouri AM, Ma Y, Togo SH, et al. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem. 2005;280(42):35195–202. doi: 10.1074/jbc.M503045200. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Dowbenko D, Spencer S, et al. Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. J Biol Chem. 2000;275(28):21477–85. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Hepner K, Castelino-Prabhu S, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci USA. 2000;97(8):4233–8. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valiente M, Andres-Pons A, Gomar B, et al. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J Biol Chem. 2005;280(32):28936–43. doi: 10.1074/jbc.M504761200. [DOI] [PubMed] [Google Scholar]
- 14.Okahara F, Itoh K, Nakagawara A, et al. Critical role of PICT-1, a tumor suppressor candidate, in phosphatidylinositol 3,4,5-trisphosphate signals and tumorigenic transformation. Mol Biol Cell. 2006;17(11):4888–95. doi: 10.1091/mbc.E06-04-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yim EK, Peng G, Dai H, et al. Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell. 2009;15(4):304–1. doi: 10.1016/j.ccr.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Dong X, Wang Z, et al. Regulation of PTEN by rho small GTPases. Nat Cell Biol. 2005;7(4):399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 17••.Hubner A, Mulholland DJ, Standen CL, et al. JNK and PTEN cooperatively control the development of invasive adenocarcinoma of the prostate. Proc Natl Acad Sci USA. 2012;109(30):12046–51. doi: 10.1073/pnas.1209660109. First report indicating a link between JNK and PTEN in regulating prostate cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang K, Esteva FJ, Albarracin C, et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell. 2010;18(5):423–35. doi: 10.1016/j.ccr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566(1–3):35–8. doi: 10.1016/j.febslet.2004.03.102. Bisperoxovanadium compounds are used as PTEN inhibitors for the treatment of type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 20.Horie Y, Suzuki A, Kataoka E, et al. Hepatocyte-specific pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113(12):1774–83. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning K, Miller LC, Laidlaw HA, et al. Leptin-dependent phosphorylation of PTEN mediates actin restructuring and activation of ATP-sensitive K+ channels. J Biol Chem. 2009;284(14):9331–40. doi: 10.1074/jbc.M806774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oudit GY, Kassiri Z, Zhou J, et al. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc Res. 2008;78(3):505–14. doi: 10.1093/cvr/cvn041. [DOI] [PubMed] [Google Scholar]
- 23.Butler MG, Dasouki MJ, Zhou XP, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42(4):318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Kwon CH, Luikart BW, Powell CM, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50(3):377–88. doi: 10.1016/j.neuron.2006.03.023. Defects in PTEN were found associated with macrocephaly and autistic syndrome in the mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Gupta A, Dey CS. PTEN, a widely known negative regulator of insulin/PI3K signaling, positively regulates neuronal insulin resistance. Mol Biol Cell. 2012;23(19):3882–98. doi: 10.1091/mbc.E12-05-0337. This study concludes that FAK/ERK signaling, in the absence of PTEN, is critical to prevent neurodegeneration that could result in Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–60. doi: 10.1126/science.1096284. The kinase PINK1 expression that was linked to PARK6 locus on chromosome 1p, is induced by PTEN expression and lack of PTEN indicates susceptibility to Parkinson’s disease. [DOI] [PubMed] [Google Scholar]
- 27.Valente EM, Salvi S, Ialongo T, et al. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004;56(3):336–41. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 28••.Kwak YG, Song CH, Yi HK, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111(7):1083–92. doi: 10.1172/JCI16440. PTEN expression reduces allergen-induced eosinophilic inflammation in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Jia G, Mitra AK, Gangahar DM, Agrawal DK. Regulation of cell cycle entry by PTEN in smooth muscle cell proliferation of human coronary artery bypass conduits. J Cell Mol Med. 2009;13(3):547–54. doi: 10.1111/j.1582-4934.2008.00384.x. Identified that PTEN overexpression inhibits IGF-1-induced proliferation of vascular smooth muscle cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitra AK, Jia G, Gangahar DM, Agrawal DK. Temporal PTEN inactivation causes proliferation of saphenous vein smooth muscle cells of human CABG conduits. J Cell Mol Med. 2009;13(1):177–8. doi: 10.1111/j.1582-4934.2008.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of pten-deficient tumorigenesis. Nature. 2005;436(7051):725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Palomero T, Sulis ML, Cortina M, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13(10):1203–10. doi: 10.1038/nm1636. Suggests the use of NOTCH1 inhibitors in the treatment of leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hettinger K, Vikhanskaya F, Poh MK, et al. C-jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14(2):218–29. doi: 10.1038/sj.cdd.4401946. [DOI] [PubMed] [Google Scholar]
- 34•.Chow JY, Quach KT, Cabrera BL, et al. RAS/ERK modulates TGFbeta-regulated PTEN expression in human pancreatic adenocarcinoma cells. Carcinogenesis. 2007;28(11):2321–7. doi: 10.1093/carcin/bgm159. Elucidates RAS/RAF/MEK/ERK pathway that suppresses PTEN through TGFβ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasudevan KM, Burikhanov R, Goswami A, Rangnekar VM. Suppression of PTEN expression is essential for antiapoptosis and cellular transformation by oncogenic ras. Cancer Res. 2007;67(21):10343–50. doi: 10.1158/0008-5472.CAN-07-1827. [DOI] [PubMed] [Google Scholar]
- 36.Xia D, Srinivas H, Ahn YH, et al. Mitogen-activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression through an NF kappa B-dependent pathway. J Biol Chem. 2007;282(6):3507–19. doi: 10.1074/jbc.M610141200. [DOI] [PubMed] [Google Scholar]
- 37••.Wang X, Trotman LC, Koppie T, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128(1):129–3. doi: 10.1016/j.cell.2006.11.039. This study suggests that the use of proteasome inhibitors, such as “MG132,” would prevent PTEN degradation through ubiquitin proteasome pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddika S, Kavela S, Rani N, et al. WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol. 2011;13(6):728–33. doi: 10.1038/ncb2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y, Yu Q, Liu JH, et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem. 2003;278(41):40057–66. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- 40••.Gustin JA, Maehama T, Dixon JE, Donner DB. The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor kappa B activity. J Biol Chem. 2001;276(29):27740–4. doi: 10.1074/jbc.M102559200. Identified that PTEN inhibits NFκB. [DOI] [PubMed] [Google Scholar]
- 41•.Vivanco I, Palaskas N, Tran C, et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11(6):555–69. doi: 10.1016/j.ccr.2007.04.021. Studied the role of PTEN in JNK signaling. [DOI] [PubMed] [Google Scholar]
- 42.Choi BH, Lee KS, Dai W. Phosphorylation and regulation of PTEN by polo-like kinase 1. Am Assoc Cancer Res; Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; March 31 – April 4; Chicago, IL, Philadelphia (PA). 2012. p. abstract # 2048. [Google Scholar]
- 43.Subauste MC, Nalbant P, Adamson ED, Hahn KM. Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein beta-catenin with the scaffolding protein MAGI-2. J Biol Chem. 2005;280(7):5676–81. doi: 10.1074/jbc.M405561200. [DOI] [PubMed] [Google Scholar]
- 44.Bayascas JR, Leslie NR, Parsons R, et al. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/−) mice. Curr Biol. 2005;15(20):1839–46. doi: 10.1016/j.cub.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 45.Baritaki S, Militello L, Malaponte G, et al. The anti-CD20 mAb LFB-R603 interrupts the dysregulated NF-kappaB/snail/RKIP/PTEN resistance loop in B-NHL cells: role in sensitization to TRAIL apoptosis. Int J Oncol. 2011;38(6):1683–94. doi: 10.3892/ijo.2011.984. [DOI] [PubMed] [Google Scholar]
- 46.Fisson S, Fridman C, Urbain R, inventors. LFB Biotechnologies, assignee. US20120100133. Use of anti-CD20 antibody for treating primary intraocular lymphoma. 2012
- 47••.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–12. doi: 10.1016/j.ccr.2004.06.022. Trastuzumab works in conjunction with PTEN in cancer patients. [DOI] [PubMed] [Google Scholar]
- 48.Agresta S, Klencke B, inventors. Genentech I, assignee. US20110165155A1. Methods of treating metastatic breast cancer with trastuzumab-MCC-DM1. 2011
- 49.Alder M, Grauschofp U, Mahler HC, Stauch OB, inventors. Genentech I, assignee. US20110044977A1. Subcutaneous anti-Her2 antibody formulations and uses thereof. 2011
- 50.Goltsov A, Faratian D, Langdon SP, et al. Features of the reversible sensitivity-resistance transition in PI3K/PTEN/AKT signalling network after HER2 inhibition. Cell Signal. 2012;24(2):493–504. doi: 10.1016/j.cellsig.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 51••.Suzuki E, Umezawa K, Bonavida B. Rituximab inhibits the constitutively activated PI3K-akt pathway in B-NHL cell lines: involvement in chemosensitization to drug-induced apoptosis. Oncogene. 2007;26(42):6184–93. doi: 10.1038/sj.onc.1210448. Rituximab induces PTEN and inhibits PI3K/Akt pathways. [DOI] [PubMed] [Google Scholar]
- 52.Lopez AG, inventor. Biogen Idec, Inc, assignee. EP1974747B1. Combination therapies for B-cell lymphomas comprising administration of anti-CD20 antibody. 2012
- 53.Frattini M, Saletti P, Romagnani E, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97(8):1139–45. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francoise B, David G, Richard MK, Joseph S, inventors. RHONE-POULENC RORER INTERNATIONAL (HOLDINGS), INC, assignee. EP0667165B1. Therapeutic compositions containing monoclonal antibodies specific to human epidermal growth factor receptor. 2002
- 55••.Abouantoun TJ, Castellino RC, MacDonald TJ. Sunitinib induces PTEN expression and inhibits PDGFR signaling and migration of medulloblastoma cells. J Neurooncol. 2011;101(2):215–26. doi: 10.1007/s11060-010-0259-9. Identifies novel functions of sunitinib as a PTEN inducer in preventing tumor cell migration through PDGFR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang CT, Miller TA, Li X, et al., inventors. Sugen, Inc., & Pharmacia Upjohn Co, assignee. US6573293. Pyrrole substituted 2-indolinone protein kinase inhibitors. 2003
- 57.She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/akt pathway signaling. Clin Cancer Res. 2003;9(12):4340–6. [PubMed] [Google Scholar]
- 58.Agus BD, inventor. Cedars-Sinai Medical Center, assignee. EP1509230B1. Gefitinib (iressa) for the treatment of cancer. 2007
- 59•.Sos ML, Koker M, Weir BA, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69(8):3256–61. doi: 10.1158/0008-5472.CAN-08-4055. Indicates PTEN-dependent functions of erlotinib in preventing lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norris T, Ferry G, Raggon JW, et al., inventors. OSI Pharmaceuticals I, assignee. US6900221. Stable polymorph on N-(3-ethynylphenyl)-6, 7-bis(2methoxyethoxy)-4-quinazolinamine hydochloride, methods of production, and pharmaceutical uses thereof. 2005
- 61.Shen YH, Zhang L, Gan Y, et al. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281(12):7727–36. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- 62••.Ghosh-Choudhury N, Mandal CC, Ghosh-Choudhury N, Ghosh Choudhury G. Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cell Signal. 2010;22(5):749–58. doi: 10.1016/j.cellsig.2009.12.010. Simvastatin inhibits NFκB and thus prevents the repression of PTEN expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubela R, Radhakrishnan J, inventors. Apotex I, assignee. US5393893. Process for producing simvastatin and analogs thereof. 1995
- 64.Teresi RE, Shaiu CW, Chen CS, et al. Increased PTEN expression due to transcriptional activation of PPARgamma by lovastatin and rosiglitazone. Int J Cancer. 2006;118(10):2390–8. doi: 10.1002/ijc.21799. [DOI] [PubMed] [Google Scholar]
- 65.Monaghan RL, Alberts AW, Hoffman CH, Schonberg GA, inventors. Merck & Co, assignee. US4231938. Hypocholesteremic fermentation products and process of preparation. 1980
- 66.Patel L, Pass I, Coxon P, et al. Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11(10):764–8. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 67.Isebaert SF, Swinnen JV, McBride WH, Haustermans KM. Insulin-like growth factor-type 1 receptor inhibitor NVP-AEW541 enhances radiosensitivity of PTEN wild-type but not PTEN-deficient human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2011;81(1):239–47. doi: 10.1016/j.ijrobp.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 68.Bonavida B, Palladino MA, inventors. The Regents of the University of California & Nereus Pharmaceuticals, Inc, assignee. US20090148445A1. Methods of sensitizing cancer to therapy-induced cytotoxicity. 2009
- 69.Berggren M, Sittadjody S, Song Z, et al. Sodium selenite increases the activity of the tumor suppressor protein, PTEN, in DU-145 prostate cancer cells. Nutr Cancer. 2009;61(3):322–31. doi: 10.1080/01635580802521338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Stambolic V, MacPherson D, Sas D, et al. Regulation of PTEN transcription by p53. Mol Cell. 2001;8(2):317–25. doi: 10.1016/s1097-2765(01)00323-9. First report describing the role of p53 in regulating PTEN transcription. [DOI] [PubMed] [Google Scholar]
- 71.Virolle T, Adamson ED, Baron V, et al. The egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3(12):1124–8. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimi A, Goyama S, Watanabe-Okochi N, et al. Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood. 2011;117(13):3617–28. doi: 10.1182/blood-2009-12-261602. [DOI] [PubMed] [Google Scholar]
- 73.Olson MV, Alonso MM, Gomez-Manzano C, et al. E2F1 transcriptionally activates PTEN [abstract #2808]. Proc Am Assoc Cancer Res. Cellular and Molecular Biology 38: oncogenic pathways in cancer; Philadelphia (PA) Am Assoc Cancer Res. 2005;46(1):661. [Google Scholar]
- 74.Wang Y, He X, Ngeow J, Eng C. GATA2 negatively regulates PTEN by preventing nuclear translocation of androgen receptor and by androgen-independent suppression of PTEN transcription in breast cancer. Hum Mol Genet. 2012;21(3):569–76. doi: 10.1093/hmg/ddr491. [DOI] [PubMed] [Google Scholar]
- 75.Escriva M, Peiro S, Herranz N, et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol. 2008;28(5):1528–40. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vasudevan KM, Gurumurthy S, Rangnekar VM. Suppression of PTEN expression by NF-kappa B prevents apoptosis. Mol Cell Biol. 2004;24(3):1007–21. doi: 10.1128/MCB.24.3.1007-1021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giagulli C, Marsico S, Magiera AK, et al. Opposite effects of HIV-1 p17 variants on PTEN activation and cell growth in B cells. PLoS One. 2011;6(3):e17831. doi: 10.1371/journal.pone.0017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78•.Perdigoto AL, Chaudhry N, Barnes GN, et al. A novel role for PTEN in the inhibition of neurite outgrowth by myelin-associated glycoprotein in cortical neurons. Mol Cell Neurosci. 2011;46(1):235–44. doi: 10.1016/j.mcn.2010.09.006. Describes MAG-mediated inhibition of neuronal growth by PTEN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sumitomo M, Iwase A, Zheng R, et al. Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell. 2004;5(1):67–78. doi: 10.1016/s1535-6108(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 80.Yang S, Kim HM. The RhoA-ROCK-PTEN pathway as a molecular switch for anchorage dependent cell behavior. Biomaterials. 2012;33(10):2902–15. doi: 10.1016/j.biomaterials.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 81.Vemula S, Shi J, Hanneman P, et al. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;115(9):1785–96. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu Z, Jia J, Lu R, et al. Expression of PTEN, p27, p21 and AKT mRNA and protein in human BEL-7402 hepatocarcinoma cells in transplanted tumors of nude mice treated with the tripeptide tyroservatide (YSV) Int J Cancer. 2006;118(6):1539–44. doi: 10.1002/ijc.21501. [DOI] [PubMed] [Google Scholar]
- 83.Bhoopathi P, Gorantla B, Sailaja GS, et al. SPARC overexpression inhibits cell proliferation in neuroblastoma and is partly mediated by tumor suppressor protein PTEN and AKT. PLoS One. 2012;7(5):e36093. doi: 10.1371/journal.pone.0036093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Oinuma I, Ito Y, Katoh H, Negishi M. Semaphorin 4D/plexin-B1 stimulates PTEN activity through R-ras GTPase-activating protein activity, inducing growth cone collapse in hippocampal neurons. J Biol Chem. 2010;285(36):28200–9. doi: 10.1074/jbc.M110.147546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma L, Chang N, Guo S, et al. CSIG inhibits PTEN translation in replicative senescence. Mol Cell Biol. 2008;28(20):6290–301. doi: 10.1128/MCB.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lima-Fernandes E, Enslen H, Camand E, et al. Distinct functional outputs of PTEN signalling are controlled by dynamic association with beta-arrestins. EMBO J. 2011;30(13):2557–68. doi: 10.1038/emboj.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verma A, Guha S, Wang H, et al. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res. 2008;14(7):1997–2005. doi: 10.1158/1078-0432.CCR-07-1533. [DOI] [PubMed] [Google Scholar]
- 88.Lu D, Han C, Wu T. Microsomal prostaglandin E synthase-1 inhibits PTEN and promotes experimental cholangiocarcinogenesis and tumor progression. Gastroenterology. 2011;140(7):2084–9. doi: 10.1053/j.gastro.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89••.Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J. 2007;26(13):3050–61. doi: 10.1038/sj.emboj.7601763. First report on the feedback loop controlling PI3K where p110δ negatively regulates PTEN by inhibiting RhoA, and suggests RhoA/ROCK pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fine B, Hodakoski C, Koujak S, et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science. 2009;325(5945):1261–5. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okumura K, Mendoza M, Bachoo RM, et al. PCAF modulates PTEN activity. J Biol Chem. 2006;281(36):26562–8. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- 92.He L, Ingram A, Rybak AP, Tang D. Shank-interacting protein-like 1 promotes tumorigenesis via PTEN inhibition in human tumor cells. J Clin Invest. 2010;120(6):2094–108. doi: 10.1172/JCI40778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He L, Fan C, Kapoor A, et al. Alpha-mannosidase 2C1 attenuates PTEN function in prostate cancer cells. Nat Commun. 2011;2:307. doi: 10.1038/ncomms1309. [DOI] [PubMed] [Google Scholar]
- 94.Seo JH, Ahn Y, Lee SR, et al. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/akt pathway. Mol Biol Cell. 2005;16(1):348–57. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi Y, Morales FC, Kreimann EL, Georgescu MM. PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J. 2006;25(4):910–12. doi: 10.1038/sj.emboj.7600979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edwin F, Singh R, Endersby R, et al. The tumor suppressor PTEN is necessary for human sprouty 2-mediated inhibition of cell proliferation. J Biol Chem. 2006;281(8):4816–22. doi: 10.1074/jbc.M508300200. [DOI] [PubMed] [Google Scholar]