Abstract
Objective:
To determine the association of conventional cardiovascular risk factors, markers of platelet activation, and thrombogenic blood-borne microvesicles with white matter hyperintensity (WMH) load and progression in recently menopausal women.
Methods:
Women (n = 95) enrolled in the Mayo Clinic Kronos Early Estrogen Prevention Study underwent MRI at baseline and at 18, 36, and 48 months after randomization to hormone treatments. Conventional cardiovascular risk factors, carotid intima-medial thickness, coronary arterial calcification, plasma lipids, markers of platelet activation, and thrombogenic microvesicles were measured at baseline. WMH volumes were calculated using a semiautomated segmentation algorithm based on fluid-attenuated inversion recovery MRI. Correlations of those parameters with baseline WMH and longitudinal change in WMH were adjusted for age, months past menopause, and APOE ε4 status in linear regression analysis.
Results:
At baseline, WMH were present in all women. The WMH to white matter volume fraction at baseline was 0.88% (0.69%, 1.16%). WMH volume increased by 122.1 mm3 (95% confidence interval: −164.3, 539.5) at 36 months (p = 0.003) and 155.4 mm3 (95% confidence interval: −92.13, 599.4) at 48 months (p < 0.001). These increases correlated with numbers of platelet-derived and total thrombogenic microvesicles at baseline (p = 0.03).
Conclusion:
Associations of platelet-derived, thrombogenic microvesicles at baseline and increases in WMH suggest that in vivo platelet activation may contribute to a cascade of events leading to development of WMH in recently menopausal women.
White matter hyperintensities (WMH) observed in the aging brain are associated with small-vessel disease.1,2 Although high WMH load may lead to mild cognitive impairment3–6 and increase the risk of stroke,7,8 little is known regarding the source or nature of cellular factors mediating their formation.9
Conventional cardiovascular risk factors such as hypertension, increasing age, smoking, hypercholesterolemia, and a history of cerebrovascular disease are the most consistent risk factors for the load and progression of WMH in elderly persons.2,10–12 In addition, thrombogenic risk factors including activated cell-derived microvesicles may contribute to formation of WMH, ischemic brain disease, and cognitive decline.13–16 In recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS), numbers of platelet-derived and thrombogenic (annexin V–positive) microvesicles correlated with carotid artery intima-medial thickness (CIMT).17,18 Furthermore, CIMT correlated positively with the prevalence of WMH and poor cognitive performance.19 Taken together, these studies suggest that cardiovascular risk factors, which may activate platelets to shed thrombogenic microvesicles, affect brain microstructure.
The objective of this study was to investigate the relationship between conventional cardiovascular risk factors and numbers of platelet-derived, thrombogenic microvesicles with WMH. Because most studies of WMH were conducted in elderly and predominantly male cohorts, in this longitudinal study, we evaluated WMH load expressed as WMH volume referenced to total white matter (WM) volume in healthy, recently menopausal women without a history of cardiovascular or cerebrovascular disease.
METHODS
Subjects.
KEEPS (NCT000154180) was a multicenter, randomized, double-blinded, placebo-controlled clinical trial in healthy, recently menopausal women (n = 728) without a history of cardiovascular disease. The study was designed to test the “critical period hypothesis” that hormone therapy started early in menopause (ages 42–59 years, within 6 months to 3 years past last menses) would reduce the progression of subclinical atherosclerosis.20 Progression of atherosclerosis was measured by CIMT and coronary arterial calcification (CAC). All women meeting inclusion and exclusion criteria for KEEPS20 at Mayo Clinic in Rochester, MN, were invited to participate in the current ancillary MRI study. KEEPS exclusion criteria briefly were CAC score of >50 Agatston units, smoking >10 cigarettes per day, body mass index (BMI) >35 kg/m2, history of cardiovascular disease, low-density lipoprotein (LDL) cholesterol >190 mg/dL, triglycerides >400 mg/dL, diagnosis of diabetes, uncontrolled hypertension (systolic blood pressure >150 mm Hg and/or diastolic blood pressure >95 mm Hg), and current or recent (6 months) use of cholesterol-lowering medications (statins, fibrate, or >500 mg/d niacin). It should be noted that women included in the study refrained from aspirin 2 weeks before blood collection. Additional exclusion criteria for enrollment in the KEEPS-MRI study were as follows: 1) contraindications for MRI for safety such as an MRI-incompatible implant or claustrophobia; 2) neurologic diseases that would have an impact on the magnetic resonance measurements such as multiple sclerosis, brain tumors, or epilepsy.
After meeting eligibility for enrollment into the KEEPS-MRI study at Mayo Clinic (n = 118), 104 women consented to participate in the study. Of these, 3 women were excluded because of neurologic disorders; thus, of the 101 women included in the KEEPS-MRI study, 95 had repeat MRI examinations at 18 months (n = 92), 36 months (n = 85), and 48 months (n = 79) after randomization to 1 of the following: 1) oral conjugated equine estrogen (Premarin, 0.45 mg/d); 2) transdermal 17β-estradiol (skin patch, Climera, 50 μg/d); or 3) placebo pill and patch. To protect the lining of the uterus, progesterone was given orally (Prometrium; micronized progesterone, 200 mg/d) for 12 days each month to both active treatment groups. The KEEPS treatment assignments were blinded at the writing of this manuscript. Each KEEPS participant underwent a medical examination, including body morphometrics (BMI, waist/hip ratio measurements), an MRI scan, standard blood chemistries, high-resolution B-mode ultrasound for the assessment of CIMT and CT for the assessment of CAC.21,22 All CAC and CIMT results were read centrally by individuals blinded to participant demographics.22 None of the women in the Mayo Clinic KEEPS-MRI study developed a neurologic or psychiatric disorder such as stroke or depression throughout the duration of the study.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Clinic Institutional Review Board (IRB protocol #2241). All participants gave written informed consent.
Magnetic resonance imaging.
MRI studies were performed on a single 1.5-tesla system, with an 8-channel phased-array head coil (GE Healthcare, Milwaukee, WI) at baseline and at 18, 36, and 48 months. Fluid-attenuated inversion recovery (FLAIR) MRI with repetition time = 11,000 milliseconds, echo time = 147 milliseconds, inversion time = 2,250 milliseconds, 256 × 192 matrix, 1 repetition, bandwidth 16 kHz, 24-cm field of view, and 3-mm interleaved images of the whole head was performed to quantify WMH volume. A 3-dimensional magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence with repetition time/echo time/inversion time of 7/3/900 milliseconds; flip angle 8°; in-plane resolution of 1.0 mm, and a slice thickness of 1.2 mm was used for the segmentation of WM. All MRIs underwent preprocessing corrections for gradient nonlinearity and intensity nonuniformity.
WMH volumes were derived from a semiautomated segmentation of FLAIR images. All MPRAGE and FLAIR images obtained during the same examination period were coregistered and segmented using Statistical Parametric Mapping.23 The MPRAGE image was resampled in the FLAIR space and MPRAGE segmentation was used to generate a WM mask to reduce the number of false positives in the WMH segmentation from FLAIR. WMH on FLAIR images were segmented using an automated slice-based seed initialization and region-growing method. The seeds were determined through empirically derived 1.45 times the mode in 1.5-tesla images. The region-growing step consisted of morphologic dilation of the seeds in 3 dimensions followed by k-means clustering of voxels into 5 classes: CSF, gray matter, WM, WMH, and partial-volume averaged WMH. The WMH and partial-volume averaged WMH was added to the seed-initiated regions as WMH. This step of image dilation using k-means clustering to include new WMH voxels was repeated until no more new WMH voxels were added at dilation or until the maximum number of 50, empirically determined, iterations was reached. Finally, the segmented WMH voxels were multiplied with the WM mask.
A trained image analyst (S.W.), blinded to the timeline of the follow-up scans, inspected the segmented WMH mask overlaid on the FLAIR image. Every segmented slice was visually compared with the unprocessed FLAIR images and all false-positive WMH labels that resulted from artifacts were edited to be excluded from the WMH mask. The inspection of WMH masks at 18, 36, and 48 months was performed by visually comparing each new time point with the baseline WMH mask for consistent editing of the artifacts. The volume of the baseline WMH was referenced to the WM volume and expressed as percentage WMH to account for the variations in WM volume at risk of WMH across participants. Longitudinal change in WMH was calculated by subtracting the WMH volume at 18, 36, or 48 months from the baseline WMH volume and was expressed in cubic millimeters of volume change for each follow-up time point.
Blood chemistries.
Blood samples were collected from the participants at baseline before randomization to study treatments as previously described.17 Total cholesterol, LDL cholesterol, and high-density lipoprotein cholesterol, triglycerides, blood glucose, and 17β-estradiol were measured by Kronos Science Laboratories (Phoenix, AZ) and the Mayo Clinic Department of Laboratory Medicine and Pathology (Rochester, MN).
Platelet count and activation assays.
Platelet count was determined using a Coulter counter, as previously described.24 Expression of activated platelet membrane glycoprotein IIb/IIIa complex binding to PAC-1 antibody (an indirect measure of the fibrinogen receptor complex) was measured by flow cytometry.17,21,24
Blood-borne microvesicles.
Numbers of activated platelet-derived microvesicles and total numbers of thrombogenic (phosphatidylserine-positive defined by annexin V binding) microvesicles were obtained at baseline using binding of fluorophore-conjugated, platelet-specific CD42a antibody and recombinant annexin V, respectively, by flow cytometry.17,25
Statistical analysis.
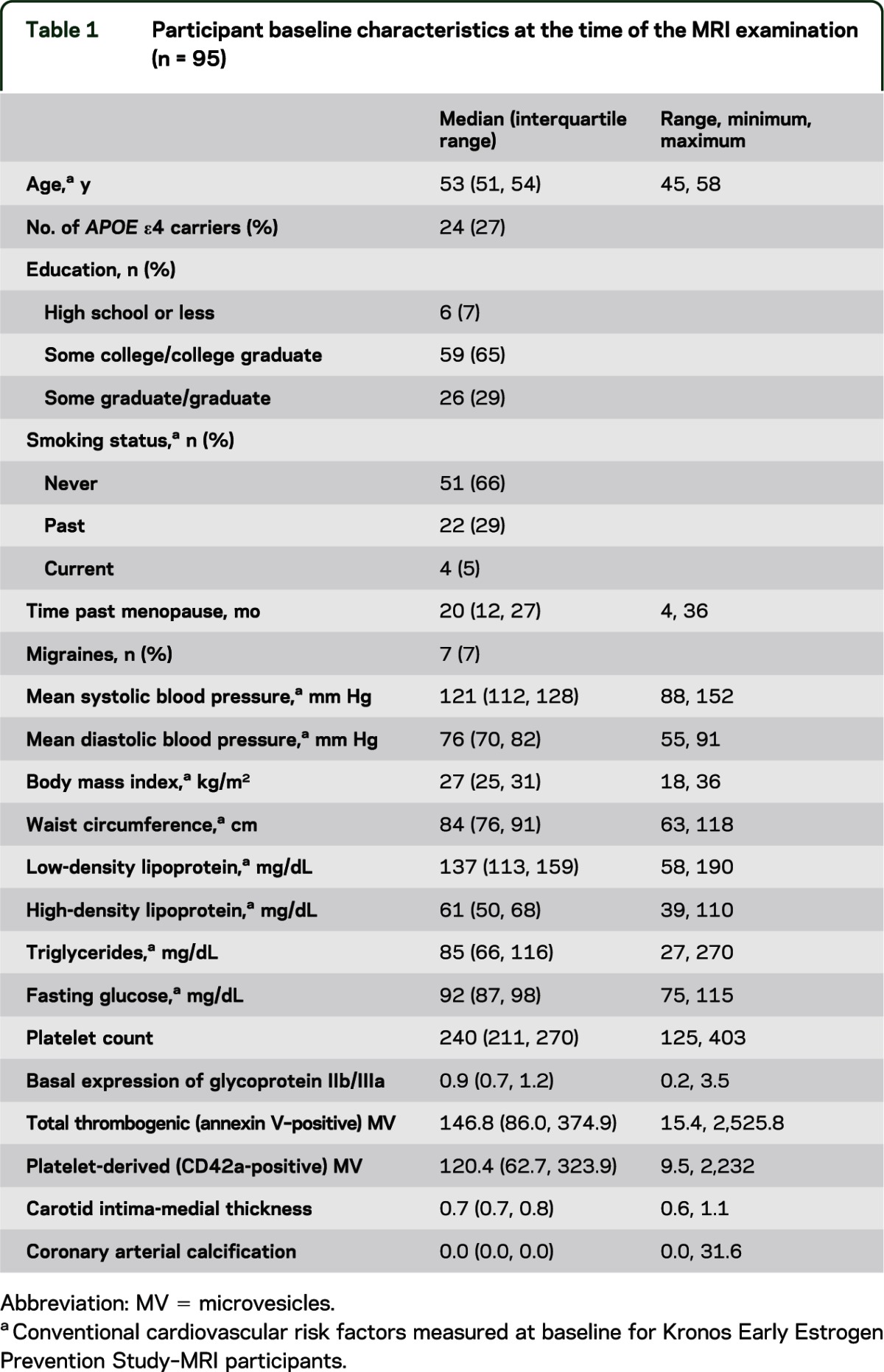
Participant characteristics were summarized at the time of the baseline MRI using counts and percentages for categorical variables, and medians, interquartile ranges, and ranges for continuous variables. Trends in WMH were described over time using means and 95% bootstrap confidence intervals. A series of linear regression models were run to examine and test associations of cardiovascular disease markers with 2 outcomes: baseline WMH to WM volume ratio and change in WMH volume. Log transformations were performed on skewed data, as necessary. Regression coefficients, their associated 95% confidence intervals, and p values were estimated for each model. Given 95 subjects and 4 adjustment variables, there was 80% power in the regression models to detect partial R2 values as small as 0.06 to 0.12 for individual cardiovascular markers. That is, there should be adequate power to detect cardiovascular markers explaining as little as 6% to 12% of the variability in the WMH outcome variables. Both unadjusted and adjusted models were performed for comparison purposes. The adjustment variables included age, time past menopause (TPM) (in months), and apolipoprotein APOE ε4 status. These statistical adjustments were considered important because age has been previously reported as a risk factor for WMH progression13,26 and the APOE ε4 gene has been most consistently identified as a genetic risk factor for cognitive impairment and Alzheimer disease.27 Studies have also implicated TPM28 as an influential factor and potential confounder of cardiovascular risk. Outcome measures of atherosclerosis for the KEEPS were CIMT and CAC. These factors were dichotomized such that the categories were as follows: CAC: 0 for CAC = 0 (no CAC at baseline, n = 90), 1 for CAC >0 and <50 (CAC present, n = 11); CIMT: 0 for CIMT ≤0.7 mm, 1 for CIMT >0.7 mm. The CIMT value of 0.7 mm was selected because it represents the median value of the distribution at baseline.
RESULTS
Characteristics of participants.
The demographics of the KEEPS-MRI group at Mayo Clinic reflected the inclusion criteria with conventional cardiovascular risk factors within normative ranges with 66% being never smokers (table 1). Seven participants had a history of migraines at the onset of the study, and all 7 had their migraines controlled by medication. Of the measures of atherosclerosis, only 11 of the 101 women (12%) had CAC scores >0 and <50 at baseline; CIMT ranged from 0.65 to 0.76 mm (median 0.69 mm). At baseline, the median (interquartile range) WMH was 1,909 mm3 (1,537; 2,655). The WMH to WM volume fraction at baseline was 0.88% (0.69%, 1.16%).
Table 1.
Participant baseline characteristics at the time of the MRI examination (n = 95)
Longitudinal change in WMH.
An example of WMH change over 48 months in one of the participants is demonstrated in figure 1. The volume of WMH (reported as median [interquartile range], in mm3) increased at 18, 36, and 48 months from baseline volumes (figure 2) by 63 mm3 (−145, 307) at 18 months (p = 0.08), 122 mm3 (−164, 539) at 36 months (p = 0.003), and 155 mm3 (−92, 599) at 48 months (p < 0.001). Volume changes at 18 months were not different from 0; therefore, this time point was excluded from further analysis. Neither smoking status nor the history of medication-controlled migraines modified the WMH load and longitudinal change in WMH volume at the specified time points (p > 0.05).
Figure 1. Progression of white matter hyperintensities of a KEEPS-MRI study participant on FLAIR MRI.
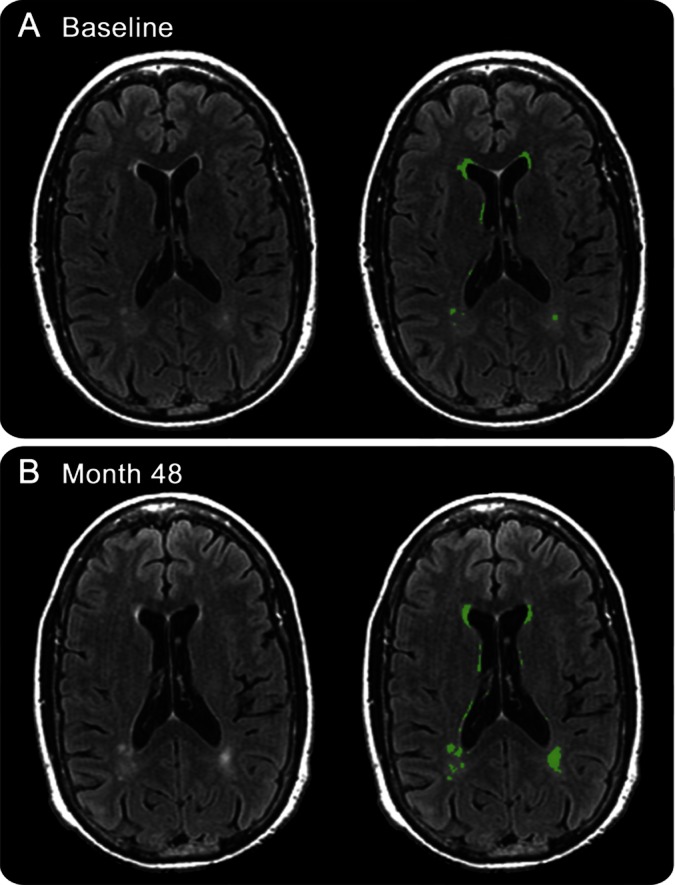
An example of changes in white matter hyperintensities (WMH) on fluid-attenuated inversion recovery (FLAIR) MRI is shown on the left panel. FLAIR MRI with WMH mask in green is shown on the right panel. The WMH volume was 340 mm3 at baseline and 584 mm3 at 48 months in this Kronos Early Estrogen Prevention Study (KEEPS) participant. The FLAIR images displayed are unprocessed, therefore not corrected for bias field for the comparison of the WMH mask with the unprocessed FLAIR image. However, all MRIs underwent preprocessing corrections for gradient nonlinearity and intensity nonuniformity during the automated WMH segmentation procedure.
Figure 2. Longitudinal changes in white matter hyperintensities in cubic millimeters.
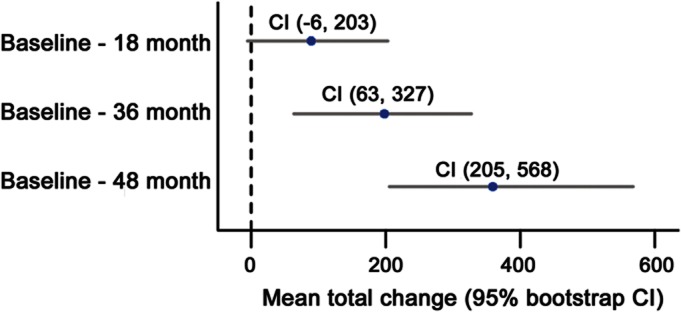
Mean white matter hyperintensity (WMH) change (baseline – 18 months, baseline – 36 months, and baseline – 48 months), with 95% bootstrap confidence intervals (CIs). Blue dots represent mean of the change. X-axis shows the total change in WMH from baseline in cubic millimeters. Numbers in the figure show the minimum and maximum confidence intervals from the bootstrap.
Associations between WMH and risk factors for cardiovascular disease.
There were no associations of either WMH volume at baseline or change in WMH volume at 36 and 48 months with baseline markers of conventional cardiovascular disease risk (BMI, systolic and diastolic blood pressure, triglycerides, high-density lipoprotein, LDL, fasting glucose, smoking status; p > 0.45). Platelet count, total numbers of CD42a and annexin V microvesicles, and KEEPS outcome measures (CIMT and CAC) did not correlate significantly with WMH fraction at baseline (table 2). Basal expression of platelet glycoprotein IIb/IIIa complex measured by PAC-1 antibody binding significantly associated with WMH load at baseline after adjustments for covariates of age, APOE ε4, and TPM (p = 0.04).
Table 2.
Adjusted regression coefficients of associations of white matter hyperintensity load and progression of white matter hyperintensity with baseline platelet characteristics, numbers of microvesicles, and measures of atherosclerosisa
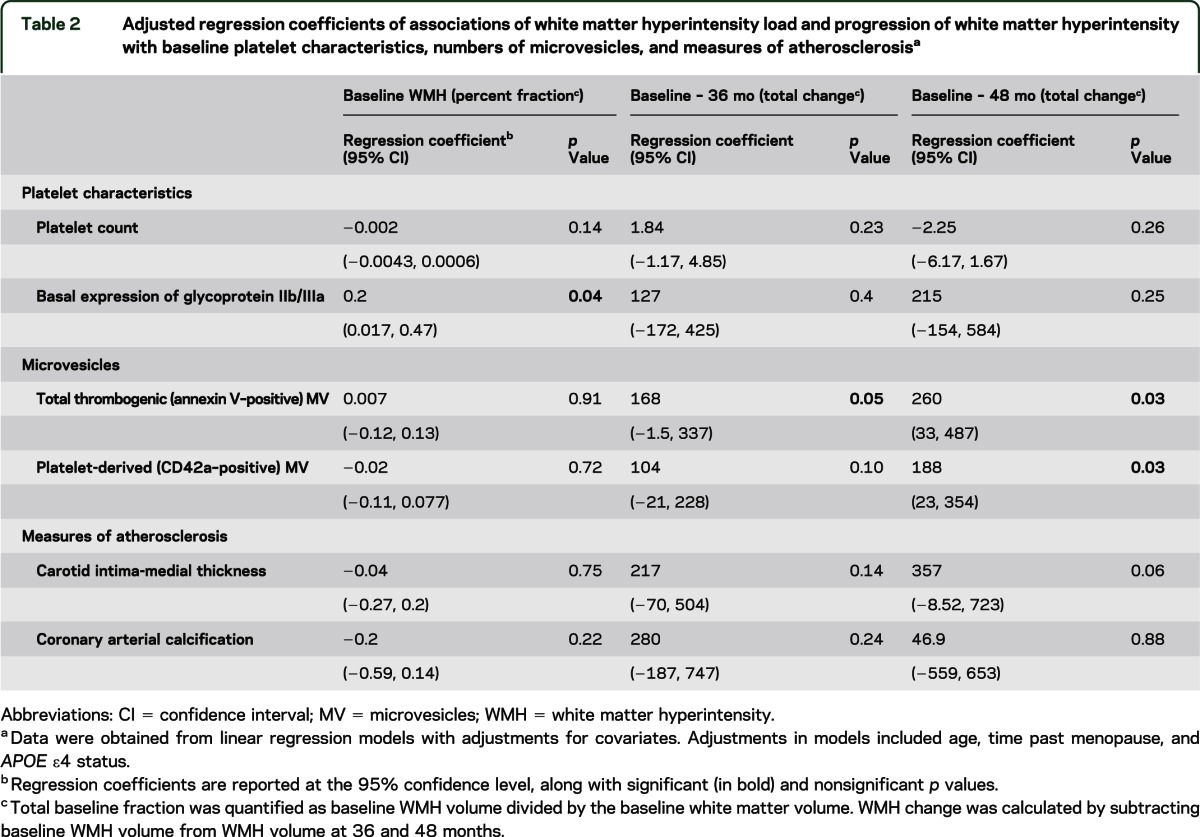
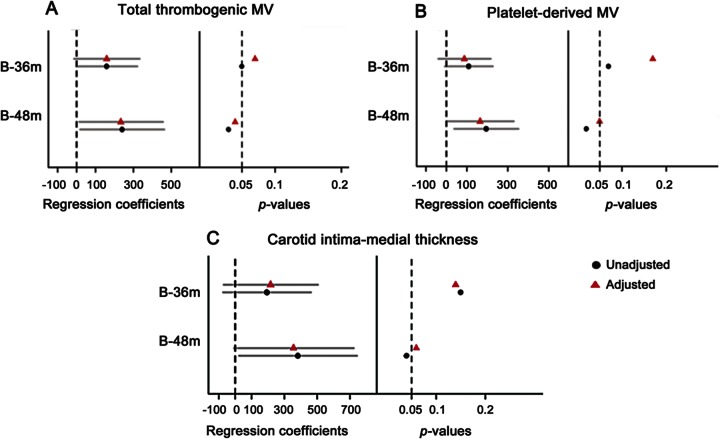
The change in WMH volume at 36 and 48 months associated (p = 0.052 and p = 0.03, respectively; figure 3A) with numbers of microvesicles expressing surface phosphatidylserine (as measured by binding to annexin V) at baseline after adjusting for covariates. Similarly, an association was observed between platelet-derived microvesicles (those positive for CD42a antibody) and change in WMH at 48 months (p = 0.03; figure 3B). The plots demonstrating these associations are included as figure e-1 on the Neurology® Web site at www.neurology.org.
Figure 3. Associations between white matter hyperintensity change and platelet-derived microvesicles and carotid intima-medial thickness.
Total thrombogenic (annexin V–positive) microvesicles (MV) (A), platelet-derived (CD42a-positive) MV (B), carotid intima-medial thickness (CIMT) (C), and regression coefficients of total white matter hyperintensity (WMH) volume change, in cubic millimeters, at the 95% confidence interval at 36 and 48 months. The dotted line on the left panel is set at 0 and represents the estimated mean of total WMH change. The right panel represents p values at p = 0.05 significance level. Circles represent results from unadjusted and triangles represent results from adjusted (age, time in months past menopause, and APOE ε4 status) linear regression models. B = baseline; m = months.
The change in WMH at 48 months was associated with CIMT in an unadjusted model (p = 0.04), but this association only showed a trend after adjusting for covariates (p = 0.06; figure 3C).
DISCUSSION
Findings of this study demonstrate that 1) WMH load increases with age in healthy, recently menopausal women in their 40s and 50s, and 2) nonconventional cardiovascular risk factors within the blood, in particular, expression of fibrinogen receptor complex on platelets, platelet-derived microvesicles, and thrombogenic (annexin V–positive) microvesicles, are associated with the progression of WMH. These findings are consistent with previous studies conducted on older males and sex-mixed populations indicating that hemostatic factor VIIc activity and fibrinogen, significantly and independently, associate with the prevalence of WMH.13 Observations from the present study extend these findings to a younger population of healthy, recently menopausal women using thrombogenic microvesicles as markers of potential hemostatic activity.
Female sex is an independent risk factor for development of WMH.13,29 Associations between WMH load and cardiovascular risk factors were reported in participants of the Women's Health Initiative who were older than 65 years and had moderate risk of cardiovascular disease.30 The current study extends these observations by evaluating the WMH load in a cohort of younger and recently menopausal women whose cardiovascular risk profile was low. In this cohort of healthy women of an age closer to the menopausal transition, no associations were found between WMH and conventional cardiovascular risk factors including systolic and diastolic blood pressure, BMI, waist circumference, cholesterol, smoking, and fasting blood glucose levels at baseline. These findings are consistent with findings in healthy, sex-mixed populations, which also did not detect associations of cholesterol levels with WMH volume11 but in contrast to studies of older populations, which found associations of WMH with blood pressure, BMI, and fasting blood glucose.10,19,31,32 These discrepancies may relate to the fact that none of the women of the KEEPS-MRI study had uncontrolled hypertension, their BMI was <30 kg/mm2, and fasting glucose was within the normative range.
Platelet activation measured by glycoprotein IIb/IIIa expression, numbers of platelet-derived microvesicles, and total numbers of thrombogenic microvesicles18 at baseline associated with WMH load and progression in the recently menopausal women. These findings suggest a subsequent and sustained effect of an activated intravascular compartment on WMH. It is noteworthy that the relationship between WMH and platelet-derived microvesicles in the peripheral circulation was only significant with data adjusted for age, because age is associated with increased WMH load in older women.2,32 Moreover, the observed variability in changes of WMH load in the KEEPS-MRI study population may be the result of biological variability and hormonal effects of treatment that could not be analyzed because of blinding to treatment randomization. An activated intravascular compartment may lead to a cascade of events that predicate development of arterial lesions in the periphery with subsequent damage to the heart or kidneys.33,34 The findings of the current study provide evidence of a similar role for platelet-derived and thrombogenic microvesicles in the brain.
There was an association between CIMT and the longitudinal change in WMH at 48 months in unadjusted models, where the effects of aging are statistically preserved. Although an association between CIMT and WMH load has been reported in older and sex-mixed populations,19,35 age was an important modifier of CIMT progression and stroke risk.36 Previous evaluation of the KEEPS women found a relationship between CIMT, markers of platelet activation, and blood-borne thrombogenic microvesicles, supporting a link between intravascular platelet activation and carotid intima-medial thickening.17,37 Although an activated intravascular compartment might appear to influence both CIMT and WMH, the relationship between CIMT and WMH seems to be weaker and dependent on age.
These results should be interpreted with several limitations in mind. First, there may be several mechanisms underlying the pathophysiology of WMH, which were not addressed in this study, such as genetic modifiers of WMH load and progression.11,38 Second, the Mayo Clinic KEEPS consisted of healthy, well-educated women, most of whom were nonsmokers, and may not reflect the general population of recently menopausal women. However, an advantage of a homogeneous population is that findings may reflect physiologic processes without the confounding effect of manageable cardiovascular risk factors. Finally, the observed associations of WMH with platelet activation factors must be confirmed in future studies of longer duration with a larger sample size.
The findings of the present study indicate that activated platelets are associated with WMH load and progression in recently menopausal women, and thus, modifying activation state of platelets may serve as a therapeutic target to prevent progression of WMH in the brain. The associations between platelet-derived and thrombogenic microvesicles at baseline and WMH progression after 3 to 4 years might suggest that prothrombotic characteristics of the blood contribute to structural changes in the WM of healthy postmenopausal women. As the clinical significance of WMH in younger populations remains controversial, additional research is needed to determine the underlying mechanisms of WMH development and progression in recently menopausal women.
Supplementary Material
ACKNOWLEDGMENT
The authors gratefully acknowledge the KEEPS participants; study coordinator Teresa G. Zais; and Robert D. Litwiller for his technical assistance collecting the blood and performing the platelet functional assays.
GLOSSARY
- BMI
body mass index
- CAC
coronary arterial calcification
- CIMT
carotid intima-medial thickness
- FLAIR
fluid-attenuated inversion recovery
- KEEPS
Kronos Early Estrogen Prevention Study
- LDL
low-density lipoprotein
- MPRAGE
magnetization-prepared rapid acquisition gradient echo
- TPM
time past menopause
- WM
white matter
- WMH
white matter hyperintensity
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Raz: design or conceptualization of the study, analysis and interpretation of the data, drafting the manuscript. Dr. Jayachandran, Ms. Tosakulwong, Mr. Lesnick, Ms. Wille, Dr. Murphy, Mr. Senjem, Dr. Gunter, Dr. Vemuri, Dr. Jack: analysis or interpretation of the data, revising the manuscript. Dr. Miller: design or conceptualization of the study, revising the manuscript. Dr. Kantarci: design or conceptualization of the study, analysis or interpretation of the data, revising the manuscript.
STUDY FUNDING
This study was funded by grants from the NIH R21 NS66147 and AG040042 to Kejal Kantarci, HL90639 and AG044170 to Virginia M. Miller, the Aurora Foundation to the Kronos Longevity Research Institute, HD065987, UL1 TR000135, and UL1 RR024150, and the Mayo Foundation. Grant UL1 RR024150 was sponsored by the National Center for Research Resources (NCRR), and grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), both components of the NIH, and the NIH Roadmap for Medical Research. This publication was made possible by CTSA grant UL1 TR000135 from NCATS, a component of the NIH. Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
DISCLOSURE
L. Raz is supported as a Building Interdisciplinary Research Careers in Women's Health (BIRCWH) Scholar by K12 HD065987. M. Jayachandran, N. Tosakulwong, T.G. Lesnick, S.M. Wille, M.C. Murphy, M.L. Senjem, and J.L. Gunter report no disclosures. P. Vemuri is funded by the NIH (K99/R00 AG37573) and Alzheimer's Association New Investigator Research grant. C.R. Jack serves as a consultant for Janssen, Bristol-Meyer-Squibb, General Electric, and Johnson & Johnson and is involved in clinical trials sponsored by Allon and Baxter, Inc. He receives research funding from the NIH (R01-AG011378, RO1-AG037551, U01-HL096917, U01-AG032438, U01-AG024904), and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. V.M. Miller is funded by the Aurora Foundation, the Mayo Foundation, NIH HL90639, and R21 NS066147 HD065987. K. Kantarci serves on the data safety monitoring board for Takeda Global Research & Development Center, Inc. She is funded by the NIH R01AG040042 (PI), R21 NS066147 (PI), Mayo Clinic Alzheimer's Disease Research Center/Project 1 P50 AG16574/P1 (PI), and R01 AG11378 (Co-I). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan Study. Stroke 2008;39:2712–2719 [DOI] [PubMed] [Google Scholar]
- 3.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 2001;58:643–647 [DOI] [PubMed] [Google Scholar]
- 4.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology 2009;72:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008;71:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swartz RH, Stuss DT, Gao F, Black SE. Independent cognitive effects of atrophy and diffuse subcortical and thalamico-cortical cerebrovascular disease in dementia. Stroke 2008;39:822–830 [DOI] [PubMed] [Google Scholar]
- 7.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke 2009;40:S48–S52 [DOI] [PubMed] [Google Scholar]
- 8.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke 2004;35:1857–1861 [DOI] [PubMed] [Google Scholar]
- 9.Fernando MS, O'Brien JT, Perry RH, et al. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol 2004;30:385–395 [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raz N, Yang Y, Dahle CL, Land S. Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta 2012;1822:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silbert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: white matter hyperintensity progression matters. Neurology 2009;73:120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology 1994;44:1246–1252 [DOI] [PubMed] [Google Scholar]
- 14.Horstman LL, Jy W, Bidot CJ, et al. Potential roles of cell-derived microparticles in ischemic brain disease. Neurol Res 2009;31:799–806 [DOI] [PubMed] [Google Scholar]
- 15.Aukes AM, de Groot JC, Aarnoudse JG, Zeeman GG. Brain lesions several years after eclampsia. Am J Obstet Gynecol 2009;200:e501–e505 [DOI] [PubMed] [Google Scholar]
- 16.Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol 2007;197:e361–e366 [DOI] [PubMed] [Google Scholar]
- 17.Jayachandran M, Litwiller RD, Lahr BD, et al. Alterations in platelet function and cell-derived microvesicles in recently menopausal women: relationship to metabolic syndrome and atherogenic risk. J Cardiovasc Transl Res 2011;4:811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller VM, Jayachandran M, Hashimoto K, Heit JA, Owen WG. Estrogen, inflammation, and platelet phenotype. Gend Med 2008;5:S91–S102 [DOI] [PubMed] [Google Scholar]
- 19.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham Study. Stroke 2009;40:1590–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric 2005;8:3–12 [DOI] [PubMed] [Google Scholar]
- 21.Jayachandran M, Litwiller RD, Owen WG, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ Physiol 2008;295:931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller VM, Black DM, Brinton EA, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Transl Res 2009;2:228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851 [DOI] [PubMed] [Google Scholar]
- 24.McBane RD, II, Karnicki K, Tahirkheli N, Miller RS, Owen WG. Platelet characteristics associated with coronary artery disease. J Thromb Haemost 2003;1:1296–1303 [DOI] [PubMed] [Google Scholar]
- 25.Jayachandran M, Miller VM, Heit JA, Owen WG. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J Immunol Methods 2012;375:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostrup E, Gouw AA, Vrenken H, et al. The spatial distribution of age-related white matter changes as a function of vascular risk factors: results from the LADIS study. Neuroimage 2012;60:1597–1607 [DOI] [PubMed] [Google Scholar]
- 27.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923 [DOI] [PubMed] [Google Scholar]
- 28.Matthews KA, Santoro N, Lasley B, et al. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab 2006;91:1789–1795 [DOI] [PubMed] [Google Scholar]
- 29.Saji N, Shimizu H, Kawarai T, Tadano M, Kita Y, Yokono K. Increased brachial-ankle pulse wave velocity is independently associated with white matter hyperintensities. Neuroepidemiology 2011;36:252–257 [DOI] [PubMed] [Google Scholar]
- 30.Kuller LH, Margolis KL, Gaussoin SA, et al. Relationship of hypertension, blood pressure, and blood pressure control with white matter abnormalities in the Women's Health Initiative Memory Study (WHIMS): MRI trial. J Clin Hypertens 2010;12:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging Study. Stroke 2002;33:26–30 [DOI] [PubMed] [Google Scholar]
- 32.Inaba M, White L, Bell C, et al. White matter lesions on brain magnetic resonance imaging scan and 5-year cognitive decline: the Honolulu-Asia Aging Study. J Am Geriatr Soc 2011;59:1484–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller VM, Jayachandran M, Owen WG. Ageing, oestrogen, platelets and thrombotic risk. Clin Exp Pharmacol Physiol 2007;34:814–821 [DOI] [PubMed] [Google Scholar]
- 34.Xiong J, Miller VM, Li Y, Jayachandran M. Microvesicles at the crossroads between infection and cardiovascular diseases. J Cardiovasc Pharmacol 2012;59:124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kearney-Schwartz A, Rossignol P, Bracard S, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke 2009;40:1229–1236 [DOI] [PubMed] [Google Scholar]
- 36.Rosenkranz M, Thomalla G, Havemeister S, et al. Older age and greater carotid intima-media thickness predict ischemic events associated with carotid-artery stenting. Cerebrovasc Dis 2010;30:567–572 [DOI] [PubMed] [Google Scholar]
- 37.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol 1995;57:791–804 [DOI] [PubMed] [Google Scholar]
- 38.Fornage M, Debette S, Bis JC, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol 2011;69:928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.