Abstract
Understanding when and how multipotent progenitors segregate into diverse fates is a key question during embryonic development. The neural crest (NC) is an exemplary model system with which to investigate the dynamics of progenitor cell specification, as it generates a multitude of derivatives. Based on ‘in ovo’ lineage analysis, we previously suggested an early fate restriction of premigratory trunk NC to generate neural versus melanogenic fates, yet the timing of fate segregation and the underlying mechanisms remained unknown. Analysis of progenitors expressing a Foxd3 reporter reveals that prospective melanoblasts downregulate Foxd3 and have already segregated from neural lineages before emigration. When this downregulation is prevented, late-emigrating avian precursors fail to upregulate the melanogenic markers Mitf and MC/1 and the guidance receptor Ednrb2, generating instead glial cells that express P0 and Fabp. In this context, Foxd3 lies downstream of Snail2 and Sox9, constituting a minimal network upstream of Mitf and Ednrb2 to link melanogenic specification with migration. Consistent with the gain-of-function data in avians, loss of Foxd3 function in mouse NC results in ectopic melanogenesis in the dorsal tube and sensory ganglia. Altogether, Foxd3 is part of a dynamically expressed gene network that is necessary and sufficient to regulate fate decisions in premigratory NC. Their timely downregulation in the dorsal neural tube is thus necessary for the switch between neural and melanocytic phases of NC development.
Keywords: Ednrb2, Foxd3, Melanocyte, Mitf, Peripheral nervous system, Pigment cells, Neural tube, Roof plate, Schwann cells, Snail2, Sox9, Chick, Mouse
INTRODUCTION
The neural crest (NC) is a highly suitable system for investigating mechanisms of lineage segregation during embryogenesis, owing to the variety of derivatives issued from an initially discrete progenitor population. These derivatives range from peripheral nervous system (PNS) components such as sensory and autonomic neurons, satellite glia and Schwann cells (SC), to endocrine cells, pigment and mesectoderm, all produced in various combinations and in a stereotypic, axial-specific fashion (García-Castro and Bronner-Fraser, 1999; Le Douarin and Kalcheim, 1999).
Of particular interest is the separation between neural and non-neural phenotypes, such as melanocytes. In vitro clonal analysis revealed the existence of single cells with melanocyte-glial potential (Dupin and Le Douarin, 2003), reminiscent of a common ancestor. Furthermore, attenuating expression of the melanocyte regulator microphtalmia-associated transcription factor (MITF) in culture facilitated the advent of glial markers (Thomas and Erickson, 2008; Thomas and Erickson, 2009). In addition, in vivo analysis showed that a late subset of melanocytes is normally produced from an initial population of Schwann cell precursors (Adameyko et al., 2009), and some diseases of the NC-derived nervous system are frequently associated with abnormal pigmentation (Adameyko and Lallemend, 2010).
Neural and melanocyte progenitors are sequentially produced in the flank of avian embryos, where NC cells emigrate over a period of about 48 hours. The early emerging cells migrate ventrally through the rostral sclerotome to generate first progeny in the sympatho-adrenal primordium, then SC of the peripheral nerves, and neurons and glia of the dorsal root ganglia (DRG), respectively (Krispin et al., 2010a; Krispin et al., 2010b; Serbedzija et al., 1989). A day after the onset of NC delamination, late-emerging cells migrate dorsolaterally between the dissociating dermomyotome and ectoderm, and give rise to melanocytes (Erickson and Reedy, 1998; Krispin et al., 2010a; Krispin et al., 2010b). This progressive ventral to dorsal order of colonization of NC derivatives is accounted for by a dynamic spatiotemporal fate map in the dorsal neural tube (NT). For instance, the dorsalmost cells in the NT are the first to delaminate and generate the ventralmost derivative, sympathetic ganglia (SG); and the ventralmost cells of the dorsal NT domain emigrate last and produce melanocytes. Progressive cell exit is compensated for by a corresponding ventral to dorsal relocation of progenitors towards the dorsal area of the NT, which therefore acts as a transition zone for the progressive influx and departure of cells (Krispin et al., 2010a; Krispin et al., 2010b).
Furthermore, discrete lineage analysis of cells in the dorsal NT generated progeny in single derivatives, suggesting fate restriction of NC progenitors before departure from the NT (Krispin et al., 2010a; Krispin et al., 2010b). Consistently, when early delaminating neural progenitors were forced to migrate dorsolaterally, they ectopically upregulated neural markers, yet failed to activate melanocyte traits, further substantiating the notion that at least part of the premigratory NC progenitors are fate restricted (Krispin et al., 2010a; Krispin et al., 2010b). The molecular mechanisms responsible for segregating neural from melanocytic lineages remain, however, largely unknown.
We recently reported a molecular distinction between both lineages, apparent already in the dorsal NT before cell emigration. Whereas neural progenitors express Foxd3, Sox9 and Snail2, prospective melanoblasts are negative for the above (Dottori et al., 2001; Kos et al., 2001; Krispin et al., 2010b). This suggests that lineage segregation occurs earlier than previously proposed, but direct evidence for the exact timing and mechanisms is still lacking.
Next, when melanoblasts leave the NT, they upregulate Mitf and endothelin receptor type B2 (Ednrb2; Harris and Erickson, 2007; Harris et al., 2008). The Mitf isoform M is the earliest marker for melanocytes and is considered to be a master regulator of the lineage (Goding, 2000; Levy et al., 2006; Thomas and Erickson, 2008). During development, expression of Foxd3 is largely exclusive to that of Mitf, which is upregulated following cell emigration and only after Foxd3 downregulation (Kos et al., 2001; Krispin et al., 2010b; Thomas and Erickson, 2009). Consistent with this, Foxd3 represses Mitf expression (Curran et al., 2010; Curran et al., 2009; Ignatius et al., 2008). Ednrb2 is expressed in melanoblasts after delamination and during dorsolateral migration (Dupin and Le Douarin, 2003; Harris and Erickson, 2007). Misexpression of Ednrb2 in early-migrating neural progenitors is sufficient to induce premature colonization of this path (Harris and Erickson, 2007; Krispin et al., 2010b; Pla et al., 2005). No direct connection between Ednrb2 and Mitf expression has yet been established.
Here we show that in avians, prospective melanocytes are initially part of the Foxd3-positive premigratory epithelium, yet segregate from the neural lineages, which persistently transcribe Foxd3, at a stage preceding emigration. If forced to continue Foxd3 expression, melanocyte progenitors fail to upregulate Mitf and Ednrb2. In this context, Foxd3 acts downstream of Snail2 and Sox9, all comprising a dynamic network of dorsal NT genes that negatively regulate melanocyte specification and pathfinding. Furthermore, the latter processes are separable experimentally, as Mitf is sufficient to induce melanocytic traits in neural progenitors without altering their ventral migratory pathways, whereas Ednrb2 drives subectodermal migration without altering cell fate [see also Krispin et al. (Krispin et al., 2010b)]. In addition, mice mutants lacking Foxd3 in the NC exhibit ectopic upregulation of melanogenic traits in the dorsal NT, in migrating cells, and in sensory ganglia at the expense of neural development, suggesting lineage reprogramming. Altogether, Foxd3 is part of a network of dynamically expressed genes that operate in the dorsal NT and is necessary and sufficient to modulate neural versus melanocyte development from the NC.
MATERIALS AND METHODS
Embryos
Avian embryos
Chick (Gallus gallus) eggs were from commercial sources. Experiments were conducted at the flank level (somites 20-25) or in the prospective flank in embryos younger than 20 somite pairs.
Mouse embryos
The Foxd3 conditional and null alleles (Foxd3flox and Foxd3−) were described previously (Hanna et al., 2002; Teng et al., 2008). The Wnt1-Cre transgenic line was used to delete Foxd3flox in the NC (Foxd3flox/−; Wnt1-Cre mutant embryos) and to lineage map NC the R26RYFP reporter strain was used (Mundell and Labosky, 2011). Mouse lines were handled in accordance with Association for Assessment and Accreditation of Laboratory Animal Care standards and protocols with approval from the Vanderbilt University Institutional Animal Care and Use Committee.
Expression vectors and electroporation
Expression vectors were: pCAGGS-AFP (Krispin et al., 2010b), pCAGG-qEdnrb2 (Pla et al., 2005), Mitf cloned into pCAGGS (Planque et al., 2004), cFoxd3 (Dottori et al., 2001), pCAGGS-cSnail2-IRES-nls-GFP, pCAGGS-Sox9-IRES-nls-GFP and pCAGGS-Sox9-EnR-IRES-nlsGFP (Cheung et al., 2005), and Snail2-BD cloned into pCAGG (Sasai et al., 2001). DNA (2-5 mg/ml) was microinjected into the lumen of the NT at the flank level of the axis and at specific stages as detailed for each experiment. For hemi-tube electroporations (EP), 0.5 mm tungsten electrodes were placed on either side of the embryo. A square wave electroporator (BTX, San Diego, CA) was used to deliver 1-3 pulses of current at 15-25 volts for 10 mseconds. Embryos were then incubated for various times, ranging from 6 to 48 hours as indicated.
Lineage analysis of Foxd3-expressing cells
Plasmids containing the hs168 and hs169 enhancers from the Vista enhancer browser (http://enhancer.lbl.gov) driving expression of Cre recombinase were co-electroporated along with a reporter plasmid in which a floxed transcriptional STOP module was inserted between the CAGG enhancer/promoter module and the green fluorescent protein (GFP) gene (pCAGG::LoxP-STOP-LoxP-GFP) as previously detailed (Avraham et al., 2009; Timmer et al., 2001).
Immunohistochemistry and in situ hybridization
Antibodies against HNK1 (CD57, BD Biosciences), GFP (Invitrogen), MC/1 (Krispin et al., 2010b; Mochii et al., 1988), islet 1, Melem, P0 (Hybridoma Bank), Fabp7 (from C. Birchmeier) (Kurtz et al., 1994), Tuj1 and Brn3a (Chemicon) were used as described (Burstyn-Cohen and Kalcheim, 2002). Cell nuclei were visualized with Hoechst. In situ hybridization was as described (Shoval et al., 2007). The following probes were employed: Foxd3 (Dottori et al., 2001; Kos et al., 2001), Sox9 (Cheung and Briscoe, 2003), Snail2 (Nieto et al., 1994), neurofilament (Ernsberger et al., 2005), Kit (BBSRC clone ID chEST583a5), Mitf (Mochii et al., 1998), Fabp7 (Kang et al., 2012) and Mafb (Lecoin et al., 2004). The Ednrb2 probe was cloned from a cDNA library of E4 chick embryo using the following primers: 5′-ATGACTCGAGGC-CTCTGAACAGAAATCCAG-3′ and 5′-TATCGGATCCTCTCATCTGTTGGCGTGATG-3′. Mouse probes used were Mitf, Kit, Trp2 (Stolt et al., 2008).
Data analysis and statistics
The percentage of dorsolaterally migrating MC/1+, or P0+ cells out of total GFP+ NC-derived cells was monitored in serial sections of chick embryos. In mouse DRG, the percentage of Brn3a+, Fabp7+ and Mitf+ cells out of total labeled cells was quantified. Results represent mean ± s.e.m. of six to 40 sections counted in four to nine embryos per experimental treatment. Data were subjected to statistical analysis using the nonparametric Mann-Whitney and Kruskal-Wallis tests. All tests applied were two-tailed with P≤0.05.
RESULTS
Temporal analysis of the Foxd3 lineage suggests that neural and melanocyte progenitors segregate before cell emigration
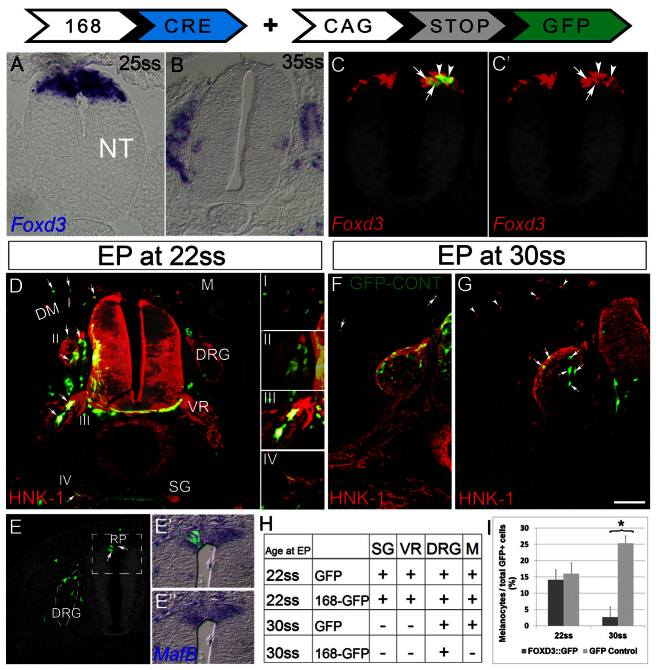
We previously showed that Foxd3 is dynamically expressed in the dorsal NT of avian embryos during NC emigration. At flank levels of embryos aged 23-35 somites (ss), when emigration of neural progenitors is underway Foxd3 is widely transcribed, and its expression is gradually restricted as a result of emigration of the Foxd3-positive cells. Later, at 35 ss, when neural progenitors have emigrated and only melanoblasts remain in the epithelium, Foxd3 expression is no longer detected (Fig. 1A,B) (see Krispin et al., 2010b). Furthermore, ventrally migrating NC progenitors destined to become neurons or glia continue expressing Foxd3, whereas dorsolaterally migrating cells are negative (Fig. 1B; supplementary material Fig. S1A). As neural, but not melanogenic, progenitors express Foxd3 at both pre- and postmigratory stages, we asked whether melanoblasts were ever part of the Foxd3 lineage. This notion was directly tested by lineage tracing the progeny of Foxd3-expressing neuroepithelial progenitors.
Fig. 1.
Melanocyte progenitors segregate from neural lineages of the NC before emigration from the NT. (A,B) In situ hybridization showing a wide expression domain of Foxd3 in the dorsal NT before NC emigration (A), and complete absence of mRNA signal following emigration of neural progenitors (B). At this stage (35 ss, flank level), melanocyte progenitors are still confined to the NT. (C,C′) Progenitors expressing the #168 reporter (green) are a subset of Foxd3-transcribing NC (red), in both the NT (arrows) and emigrating cells (arrowheads). (D-E″) EP of the 168 Foxd3 reporter (168::Cre/CAGG-LoxP-STOP-LoxP-GFP) at early premigratory stage (22 ss) generates labeled progeny in all NC derivatives (arrows in D, and see insets I-IV) and also in RP cells that co-express MafB (arrows in E; E′ and E″ are higher magnifications of boxed area in E), when monitored 2 days later. In E, DRG is marked by dashed lines and nuclei were stained with Hoechst. Note also reporter + cells in dI2 interneurons, DRG and melanocytes. (F,G) EP at 30 ss produces reporter+ cells (green) only in DRG but not melanocytes (G, arrows and arrowheads, respectively), whereas EP of control-GFP produces labeled cells in both sites (F). HNK-1 marks NC cells. (H) Summary showing that initially (22s), melanocyte progenitors are part of the Foxd3+ lineage, yet by 32 ss they already segregated. (I) At early stages, the reporter labels a similar proportion of melanocytes as control GFP; at later stages, when control GFP is enriched in melanocytes (as EP is done after emigration of most neural progenitors), the reporter no longer marks melanocytes (*P<0.05). Scale bars: 33 μm in A,C,C′; 50 μm in B; 65 μm in D,E; 40 μm in E′,E″; 75 μm in F,G. VR, ventral root of spinal nerve.
Large-scale transgenic mouse screens of highly conserved noncoding sequences in the human genome revealed several hundred enhancer elements that target β-galactosidase reporter gene expression to specific cell types in transgenic mice at E11.5 (Pennacchio et al., 2006). Two enhancers (#168, #169) located upstream of the Foxd3 gene and conserved throughout evolution were found to drive expression of a reporter gene in subpopulations of central nervous system (CNS) or PNS cells of transgenic mice at E11.5 (Vista enhancer browser, http://enhancer.lbl.gov/; supplementary material Fig S1B,C). Whereas in mice expressing β-galactosidase under the regulation of the #169 enhancer a positive signal was detected only in the CNS, enhancer #168 triggered expression in both CNS and PNS populations (supplementary material Fig. S1C).
To drive robust and stable expression in avian embryos, the enhancer elements were cloned upstream of Cre recombinase and electroporated into hemi-tubes of embryos aged 22-30 ss along with a Cre-dependent GFP plasmid (enhancer::Cre + pCAGG::LoxP-STOP-LoxP-GFP). This paradigm enables stable lineage tracing of the enhancer-expressing cells (Avraham et al., 2009; Zisman et al., 2007). The #169 sequence drove expression of GFP only in specific CNS progenitors, whereas control RFP was broadly expressed in both the transfected hemi-NT and in NC derivatives (supplementary material Fig. S1D). By contrast, enhancer #168 triggered GFP expression in both PNS and CNS populations (Fig. 1D,G; supplementary material Fig. S1E), comparable to the patterns observed in mouse embryos. We then focused on the #168 enhancer to further analyze NC-derived lineages.
First, we determined the identity of reporter-expressing cells 6 hours after electroporation (EP) of 22-25 ss embryos, when robust fluorescence was first observed. GFP expression under the control of #168 was restricted to Foxd3+ cells that comprised presumptive premigratory and emigrating NC (arrows and arrowheads, respectively, Fig. 1C,C′). Quantification revealed that 87% of GFP+ cells co-expressed Foxd3 (n=8 embryos). Performing the experiment at later stages also revealed co-expression of #168/Foxd3 in V1 and dI2 CNS interneurons (supplementary material Fig. S1D) (Dottori et al., 2001; Helms and Johnson, 2003). As these patterns recapitulate expression of endogenous Foxd3, we suggest that #168 is a specific Foxd3 enhancer.
Next, we followed the fate of Foxd3-reporter-GFP-expressing cells after EP to the NT at the flank level of 22 ss or 30 ss embryos and fixation at E4. When electroporated at 22 ss, GFP expression was observed in all trunk NC derivatives, including SG, SC precursors, DRG and melanocytes (n=6/6, Fig. 1D, insets I-IV, 1E,H) as well as in definitive roof plate (RP) cells that co-expressed the RP marker MafB (Lecoin et al., 2004; Liem et al., 1997) (Fig. 1E-E″). By contrast, when given at 30 ss, the progeny of Foxd3-expressing cells was restricted to DRG, and GFP was not detected in melanocytes or RP (Fig. 1G-I, n=7/7). This, despite the fact that melanocyte delamination follows that of DRG progenitors, and that at the time of EP, melanocyte progenitors still reside in the NT, as monitored by transfection of similarly staged embryos with control GFP (Fig. 1F) (see Krispin et al., 2010b). Indeed, the proportion of melanocytes out of total NC derivatives increased upon transfection of control GFP at 30 ss when compared with 22 ss, as expected. By contrast, the proportion of Foxd3-reporter-expressing melanocytes significantly decreased (Fig. 1I, P<0.05). As anticipated, no labeling in SG or SC precursors was apparent at 30 ss, as at this stage the respective progenitors had already emigrated (Fig. 1F-H) (see Krispin et al., 2010b). Hence, whereas all NC progenitors initially express Foxd3 in the dorsal NT, neural progenitors continuously transcribe the gene upon emigration, whereas prospective melanocytes segregate from the Foxd3-positive lineage before delamination, a result consistent with the observed mRNA patterns. These results highlight for the first time a molecular difference between prospective neurogenic and melanogenic NC lineages in the dorsal NT. These data also suggest that RP and NC cells initially share Foxd3 before segregating from each other.
Foxd3, Sox9 and Snail2 act as a switch between the production of neural and melanocyte lineages
Continuous expression of Foxd3 in prospective melanocytes inhibits expression of melanocyte markers and of Ednrb2
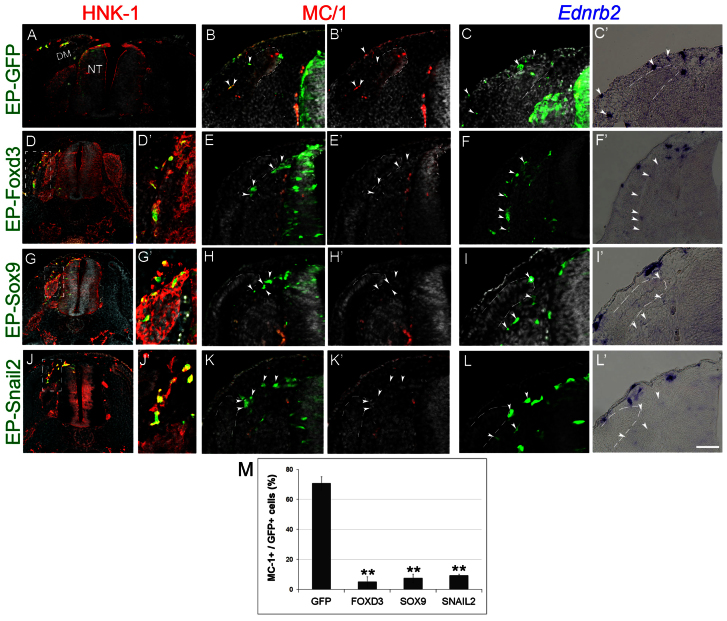
To directly examine whether the temporal coincidence between loss of Foxd3 and melanocyte development are functionally related, we prevented the downregulation of Foxd3 by electroporating Foxd3 or control-GFP at 35 ss, when neural progenitors are already delaminated and only melanocytes still reside in the NT (Krispin et al., 2010b). This protocol differs from previous experiments in which Foxd3 was overexpressed in the entire NC population (Dottori et al., 2001; Kos et al., 2001).
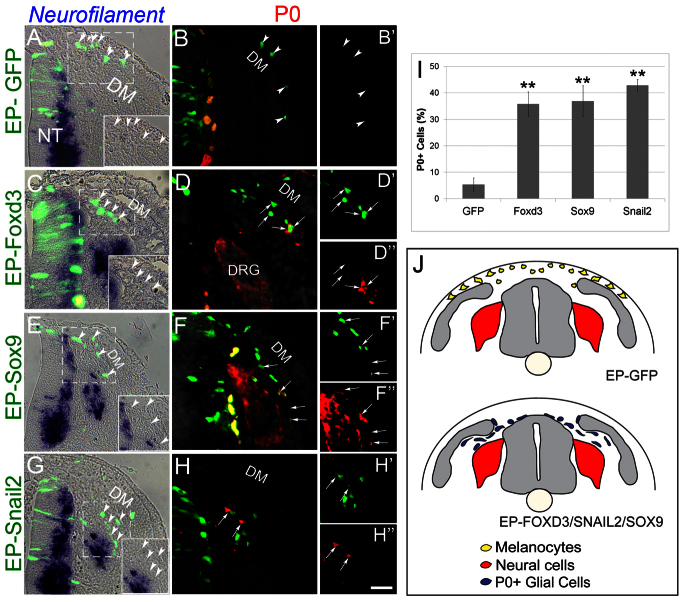
A day following EP, cells that received control-GFP had delaminated and invaded the dorsolateral pathway. These cells co-expressed the melanocyte markers MC/1 and Ednrb2 (Fig. 2A-C′, arrowheads). By contrast, not only did Foxd3-misexpressing cells fail to upregulate MC/1 (Fig. 2E,E′, arrowheads, 2M; n=4, P=0.01), they also failed to upregulate Ednrb2 and invade the dorsolateral pathway (n=7, P<0.01). Instead, they migrated ventrally between the DRG and myotome (Fig. 2D-F′ arrowheads; Fig. 3C-D″; supplementary material Fig. S2). As these cells did not generate melanocytes, we examined whether they upregulated neural markers. No expression of tyrosine hydroxylase, islet 1 or neurofilament was detected (Fig. 3C arrowheads and data not shown), but transfected cells upregulated the peripheral glial/Schwann cell markers P0 (n=5, P<0.01, Fig. 3D-D″ compared with 3B,B′,I; supplementary material Fig. S2) and Fabp7 (supplementary material Fig. S3). Notably, the Foxd3-induced migratory defect was rescued by co-EP with Ednrb2 (n=7, P<0.005 compared to Foxd3 alone, supplementary material Fig. S2) yet the dorsolaterally migrating cells still expressed P0 rather than melanocyte traits (supplementary material Fig. S2F,F′; data not shown). Thus, continuous expression of Foxd3 in late premigratory NC that normally shuts off its transcription, respecifies melanoblasts into glial/Schwann cells and shifts cell migration from a dorsolateral into a ventral path via regulation of Ednrb2.
Fig. 2.
Foxd3, Sox9 and Snail2 inhibit melanocyte specification and dorsolateral migration. (A-L′) Late plasmid EP (35 ss) performed before melanoblast emigration in the flank. Thirty hours later, control labeled cells were present in the dorsolateral pathway, and expressed MC/1 and Ednrb2 (A-C′). By contrast, Foxd3-, Sox9- or Snail2-transfected cells were found in the ventral pathway, negative for MC/1 and Ednrb2 (arrowheads, D-L′). (M) Quantification of the percentage of MC/1+/GFP+ cells (**P=0.01, P<0.01, P=0.01 for Foxd3, Sox9 and Snail2, respectively). Scale bars: 100 μm in A; 120 μm in D,G,J; 60 μm in E,D′,G′,J′; 75 μm in B-C′,H,H′,K. DM, dermomyotome.
Fig. 3.
Foxd3, Sox9 and Snail2 upregulate P0 expression in prospective melanocytes. (A-B′) Late-emigrating NC progenitors that received control GFP at 35 ss, flank level, migrate dorsolaterally and are negative for neurofilament or P0 (arrowheads). (C-H″) EP with Foxd3, Sox9 or Snail2 upregulates P0 (arrows) but not neurofilament (arrowheads) and cells are diverted ventrally (see also Fig. 2). (I) Quantification of the percentage of P0-expressing cells in all treatments (**P<0.01). (J) Schematic representation of the results suggesting that putative melanoblasts lose neurogenic ability but keep the potential to develop into glia under these experimental conditions. Orange and yellow cells in B,D,F represent autofluorescence of blood cells. Scale bars: 70 μm in A,B,B′,E,G; 60 μm in F-F″; 55 μm in C; 50 μm in D-D″,H-H″. DM, dermomyotome.
Continuous expression of Sox9 and Snail2 recapitulates the effects of Foxd3 on melanocyte specification and dorsolateral migration
Snail2 and Sox9 exhibit a similar, dynamic pattern of expression to that of Foxd3 in the dorsal NT, as they are transcribed during delamination of neural but not of melanoblast progenitors (Krispin et al., 2010b). Hence, we hypothesized that Snail2 and Sox9 may similarly affect melanoblast development. When transfected at 35 ss, each factor prevented the normal upregulation of MC/1 (Fig. 2G-H′,J-K′,M, arrowheads; n=4 for Sox9 and n=4 for Snail2, P<0.01 and P=0.01, respectively), and electroporated cells generated P0+ glia (Fig. 3E-H′,I,J; n=4 and 5 for Sox9 and Snail2, P=0.01 and P<0.01, respectively). Thus, the effects of Foxd3, Sox9 and Snail2 on MC/1 and P0 are similar and statistically indistinguishable (P-values ranging from 0.3 to 0.9). In addition, the late progenitors transfected with either Sox9 or Snail2 failed to activate expression of Ednrb2 or to invade the dorsolateral pathway (Fig. 2I-L′; Fig. 3E-H″,J).
Altogether, the timely downregulation of Foxd3, Snail2 and Sox9 in the NT is necessary both to specify NC cells to the melanocyte lineage and to allow them to migrate dorsolaterally. Hence, activity of these genes in the early dorsal NT might serve to inhibit the premature advent of melanocyte traits by linking the regulation of cell specification with pathfinding.
Snail2 and Sox9 control the expression of Foxd3 in neural crest progenitors
As described above, Snail2, Sox9 and Foxd3 exhibit comparable expression patterns in the dorsal NT (Krispin et al., 2010b) and similarly affect melanogenic and glial traits in late emigrating NC cells (Figs 2, 3). Therefore, we examined whether there is an interaction between these genes in vivo. To this end, each factor was separately misexpressed in the late NT, when transcription of none of the above was detected any longer. One day later, transcription of the other two genes was monitored in migrating progenitors. First, we confirmed that none of the genes is indeed expressed in migrating cells following transfection with control-GFP (Fig. 4A, arrows; data not shown). Next, we observed that continuous expression of Snail2 upregulated both Sox9 and Foxd3 mRNAs in cells whose migration was now diverted ventrally (Fig. 4B; data not shown). By contrast, transfection of Sox9 upregulated Foxd3 but did not stimulate expression of Snail2 (Fig. 4C; data not shown). Finally, EP of Foxd3 did not upregulate either Snail2 or Sox9 (Fig. 4D,E). These data suggest that Snail2 and Sox9 are both sufficient to maintain Foxd3 expression. Next we examined whether Snail2 or Sox9 activities are necessary for Foxd3 expression. In early EPs of control GFP Foxd3 expression was evident in the dorsal NT. By contrast, EP of dominant-negative versions of Snail2 (Snail2-BD), a Snail2 construct lacking the N-terminal domain, or of Sox9 (Sox9-EnR) in which the C-terminal transactivation domain was replaced with the engrailed repressor domain, inhibited Foxd3 transcription (Fig. 4G-H′). Notably, both Snail2-BD and Sox9-EnR were effective only in early-stage EPs (15 ss), yet had no effect at 20-25 ss (not shown). Thus, Snail2 and Sox9 may be necessary for initial Foxd3 transcription but not for its maintenance.
Fig. 4.
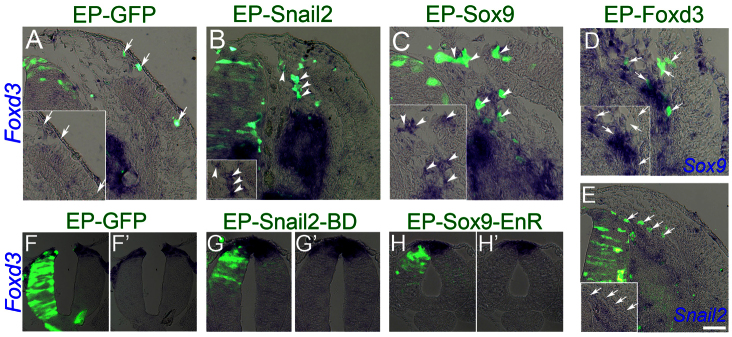
Interaction between Foxd3, Sox9 and Snail2. (A) EP of late NTs (35 ss, flank level) with control GFP results in GFP+/Foxd3-NC cells migrating along the dorsolateral pathway (A, arrows). (B,C) Electroporation of Snail2 or Sox9 upregulates Foxd3 in migrating NC cells whose migration is diverted to a ventral path (arrowheads). (D,E) EP of Foxd3 does not induce transcription of either Snail2 or Sox9, and transfected cells migrate ventrally. Arrows point to GFP+ cells negative for the inspected gene. (F-H′) Sixteen hours after EP of young NT (15 ss, flank level) with control GFP, Foxd3 expression is evident in the transfected side (F-F′). EP of a truncated version of Snail2 (Snail2-BD) or of Sox9 fused to the engrailed repressor domain (Sox9-EnR), results in downregulation of Foxd3 in transfected progenitors (G-H′). Scale bars: 50 μm in A,B; 30 μm in C; 40 μm in D; 80 μm in E; 60 μm in F-H′.
As these three factors are present in the dorsal NT during the ‘neural’ phase of NC ontogeny, they may be part of a molecular network underlying specification of neural lineages at the expense of melanocytes.
Specification to the melanocyte fate and choice of migratory pathways are separable events
Transcription of both Mitf and Ednrb2 begins after melanocyte progenitors emigrate from the NT (Harris et al., 2008; Krispin et al., 2010b; Pla et al., 2005). As shown above, Sox9, Snail2 and Foxd3 inhibit expression of both MC/1 and Ednrb2 thus linking cell specification with guidance. This observation raised the question whether Mitf-dependent melanoblast specification regulates Ednrb2 downstream of Foxd3, Sox9 and Snail2, and consequently, migration through the dorsolateral pathway, and/or vice-versa. Alternatively, these two processes can be experimentally separated. The latter scenario would suggest that cell specification and guidance are independently regulated.
To examine these possibilities, Mitf or Ednrb2 were misexpressed in the early NT. A day later, embryos were analyzed to trace prospective ventrally migrating ‘neural’ progenitors, the only cells that exited the NT by this stage. In control GFP-treated embryos, migration was restricted to the ventral pathway where labeled cells co-expressed islet 1 or neurofilament; these were never observed in the dorsolateral pathway under normal conditions at early or late stages. The ventrally migrating progenitors, however, did not express melanocyte markers such as MC/1, Kit or Melem (Fig. 5A-C,M; supplementary material Fig. S4A,B) (see Krispin et al., 2010b). Misexpression of Mitf in ‘neural’ precursors upregulated MC/1 (Fig. 5D, short arrows), Kit and Melem (supplementary material Fig S4C,D, arrows), and prevented the advent of neural markers (Fig. 5E,F, dashed arrows). Furthermore, in the dorsal NT, Mitf misexpression inhibited transcription of Foxd3, Sox9 and Snail2, which mostly characterize the neural phase of NC development (supplementary material Fig. S5). Importantly, in spite of reprogramming early neural progenitors into a melanocyte fate, Mitf did not alter their original ventral migratory pathway (n=7, P=0.8, Fig. 5D-F,M; supplementary material Fig. S4).
Fig. 5.
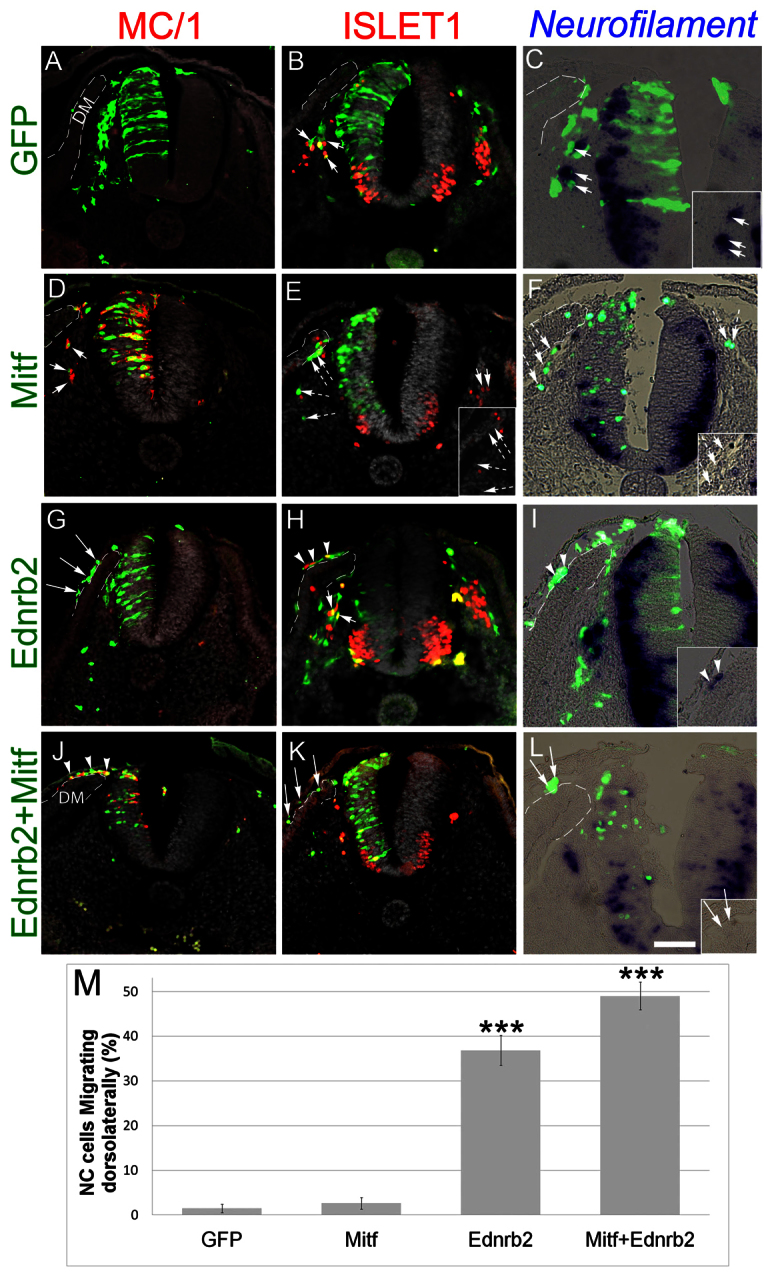
The combination of Mitf and Ednrb2 is sufficient for respecification of early NC progenitors into melanocytes. (A-C) EP of control GFP in the early NT reveals the presence of labeled cells along the ventral migratory path that co-express Islet1 (arrows in B) or neurofilament (arrows in C) but are negative for MC/1 (A). (D-F) Transfection of Mitf-GFP causes the loss of Islet1 and neurofilament and the upregulation of MC/1. Labeled cells still migrate ventrally (dashed arrows in E and F). Note the loss of neuronal markers in more ventral areas of the NT. (G-I) Ednrb2 switches migration towards the dorsolateral path and cells upregulate Islet1 or neurofilament (arrowheads in H,I), yet fail to express MC/1 (arrows in G). (J-L) Co-EP of Ednrb2 and Mitf reprograms neural cells into melanocytes. Transfected cells express MC/1 but not neural markers, and migrate dorsolaterally. Arrows point to GFP+ cells that are negative for additional markers. Arrowheads point to double+ cells. (M) Quantification of the percentage of NC cells that migrate along the dorsolateral (DL) path (***P<0.005 and 0.001, for Ednrb2 or Ednrb2+Mitf, respectively). Scale bars: 70 μm in A,G,H,J,K; 80 μm in B; 50 μm in C,F,I,L; 60 μm in D,E.
Misexpression of Ednrb2 in the early NT diverted migrating crest cells into the dorsolateral pathway, confirming previous findings (n=5, P<0.005; Fig. 5G-I,M) (Harris et al., 2008; Krispin et al., 2010b). However, these cells failed to upregulate MC/1 (Fig. 5G) and instead induced expression of islet 1 or neurofilament (Fig. 5H,I). Moreover, in contrast to Mitf, Ednrb2 did not alter expression of Foxd3, Sox9 or Snail2 (supplementary material Fig. S5G-I″) in the dorsal NT. So, whereas the latter genes inhibit both Mitf and Ednrb2 mRNAs, only Mitf negatively impacts their expression. Altogether, Mitf and Ednrb2 control different aspects of melanocyte development. We then examined whether combining both genes would induce melanocytes that migrate prematurely through the dorsolateral pathway. Indeed, misexpression of both genes in the NT during the neural phase of NC production resulted in labeled cells in the dorsolateral pathway that expressed MC/1, but not islet 1 or neurofilament (n=9, P<0.001 for the percentage of cells in dorsolateral pathway, Fig. 5J-M). Thus, expression of both factors in vivo is sufficient to reprogram ventrally migrating neural progenitors into laterally migrating melanocytes.
Next, we asked whether Mitf and Ednrb2 regulate each other. When Ednrb2 was misexpressed at 20 ss, labeled cells that prematurely entered the dorsolateral pathway did not upregulate Mitf, similar to control-GFP transfected cells that, as expected, migrated ventrally (supplementary material Fig. S6A-C′). Conversely, MITF only mildly stimulated expression of Ednrb2 in emigrating progenitors compared with the contralateral sides and to control-GFP, to levels far lower than those in normal melanocytes (supplementary material Fig. S6F,F′ compared with S6D) and clearly insufficient to redirect cell migration dorsolaterally (see also Fig. 5). Therefore, in spite of being expressed together in normally emigrating melanoblasts (supplementary material Fig. S6A,D), Mitf and Ednrb2 are likely to act via parallel pathways to regulate melanocyte specification and guidance, respectively.
Neural progenitors in the mouse NC lacking Foxd3 respecify into melanocytes
Here we showed that targeted overexpression of Foxd3 in avian melanocyte progenitors inhibits their differentiation while promoting a glial fate. Conversely, attenuation of Foxd3 in vitro with antisense morpholinos enhanced melanogenesis (Kos et al., 2001). As in avians and other species, Foxd3 is one of the earliest molecular markers of the murine NC lineage. It is expressed in premigratory and migrating NC and is downregulated as the cells differentiate into neurons in peripheral ganglia or into melanocytes (Dottori et al., 2001; Labosky and Kaestner, 1998). Earlier work demonstrated that loss of Foxd3 in the NC resulted in a cell-autonomous bias in lineage selection away from neural and glial cell fates towards a mesenchymal fate (myofibroblast) (Mundell and Labosky, 2011). However, the question remained open whether Foxd3 gene function is necessary for the balance between development of neural versus melanocyte lineages in vivo. To examine this issue, we analyzed mouse embryos carrying a conditional deletion of Foxd3 in the NC. We compared Foxd3flox/−; Wnt1-Cre; R26R (mutant) embryos to Foxd3flox/+; Wnt1-Cre; R26R (control) embryos.
To evaluate changes in melanocyte development embryos were stained for Mitf at different stages. At thoracic levels of 9.5 days post coitum (dpc) embryos, NC cells emigrated and similarly migrated through the ventral pathway in both controls and mutants but no Mitf mRNA signal was yet detectable (not shown). At axial levels caudal to the hind limbs of 10.5 dpc controls, many YFP+ NC cells were found along the ventral migratory pathways, and the first Mitf+ cells were already apparent in a dorsolateral position outside the NT (Fig. 6A,A′). Strikingly, at the same age in mutant embryos, Mitf was ectopically expressed in subsets of dorsal NT progenitors and a few Mitf + cells were found just outside the NT (Fig. 6B,B′). These ectopic premigratory MITF+ cells were no longer detected at later stages.
Fig. 6.
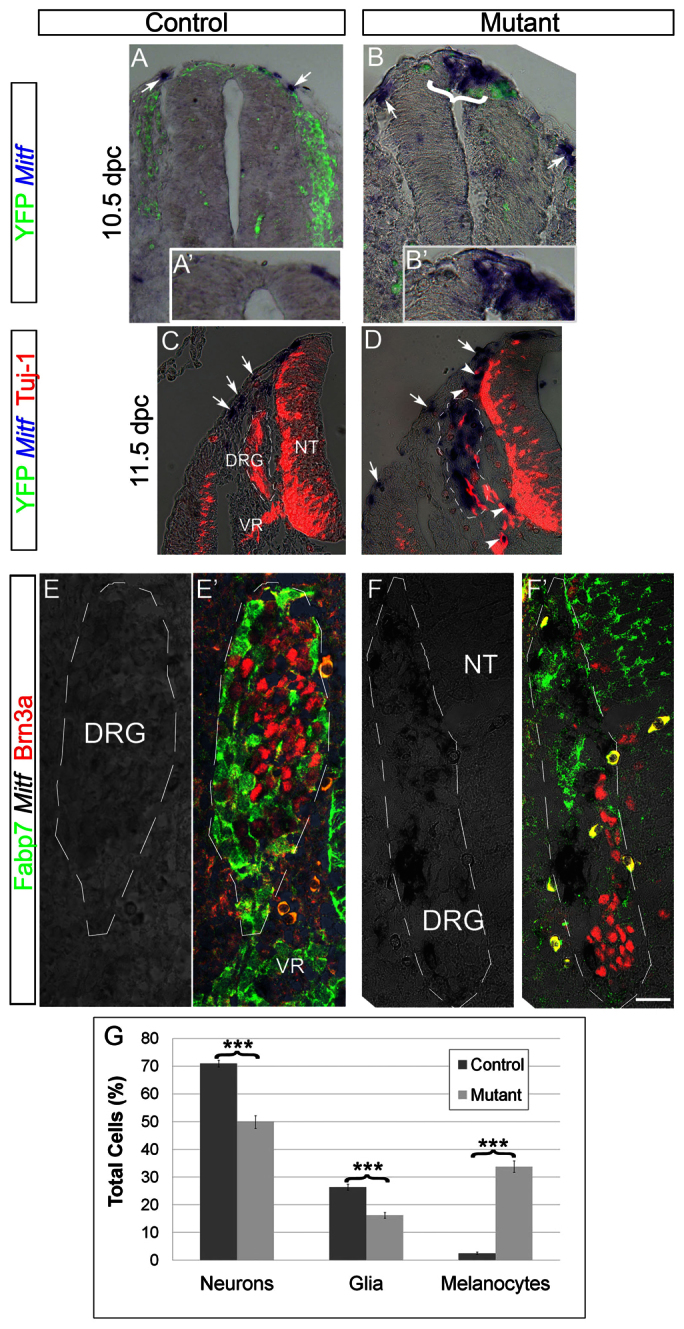
Ectopic expression of Mitf at the expense of neural traits in mutant mouse embryos lacking Foxd3 in the NC. (A-B′) Caudal to the hind limbs of 10.5 dpc controls, YFP+ NC migrate ventrally, and few Mitf+ cells are apparent in a dorsolateral position outside the NT (A,A′, arrows). Notably, in mutants, ectopic expression of Mitf is also seen in the dorsal NT (bracket in B, higher magnification in B′) in addition to emigrating NC (arrow in B). (C,D) In 11.5 dpc embryos Mitf+ melanocytes migrate ventral to the ectoderm in both controls and mutants (C,D, arrows). In controls, DRG cells express Tuj1 (C), whereas in mutants Mitf transcription is observed with a concomitant decrease in Tuj1 (D). Dorsal and ventral roots also contain Mitf+ cells in mutants (D, arrowheads). (E-F′) Expression of Mitf mRNA in mutant, but not control DRG, in comparison with staining for Brn3a and Fabp7. (G) Quantification of the percentage of melanocytes (Mitf+), neurons (Brn3a+) and glia (Fabp7+) of total labeled cells. Note melanocyte development at the expense of both neurons and glia (***P<0.005, n=40 and 37 sections counted in 12 ganglia of control and mutants, respectively). Scale bars: 35 μm in A,B; 20 μm in A′,B′; 130 μm in C; 100 μm in D; 30 μm in E-F′. VR, ventral root.
In the trunk of 11.5 dpc embryos Mitf+ melanocytes migrated subectodermally in both controls and mutants (Fig. 6C,D, arrows). Furthermore, in control embryos, well-established DRG expressing neuronal tubulin (Tuj1) were apparent (Fig. 6C). In striking contrast, substantial Mitf transcription was observed in the mutant DRG with a concomitant loss of both neuronal (Tuj1+, Brn3a+) and glial (Fabp7+) markers (Fig. 6D-G) altogether, suggesting that a neural to melanocytic shift occurred at the level of NC progenitors. This altered distribution of the various cell types in the mutant DRG may have accounted for their irregular morphologies and size (Fig. 6D,F; Fig. 7B,D) (Teng et al., 2008). Consistently, dorsal and ventral roots also contained numerous Mitf+ cells in mutant but not control embryos (Fig. 6D, arrowheads). In addition to Mitf, ectopic Trp2+ and Kit+ cells were also observed in DRG of the mutant embryos (Fig. 7A-D), further substantiating a fate reallocation into melanocytes. Additionally, a similar upregulation of Mitf was observed at 10.5 dpc in mutant cranial sensory ganglia, yet only a few Mitf+ cells were present in the periphery of control ganglia preferentially abutting the external mesodermal side (Fig. 7E-F′). In contrast to sensory ganglia, no ectopic melanogenesis was observed within sympathetic ganglia (data not shown). Together, these genetic data reveal that Foxd3 is necessary for the maintenance of neural progenitors of the NC in part by repressing premature and ectopic melanogenesis.
Fig. 7.
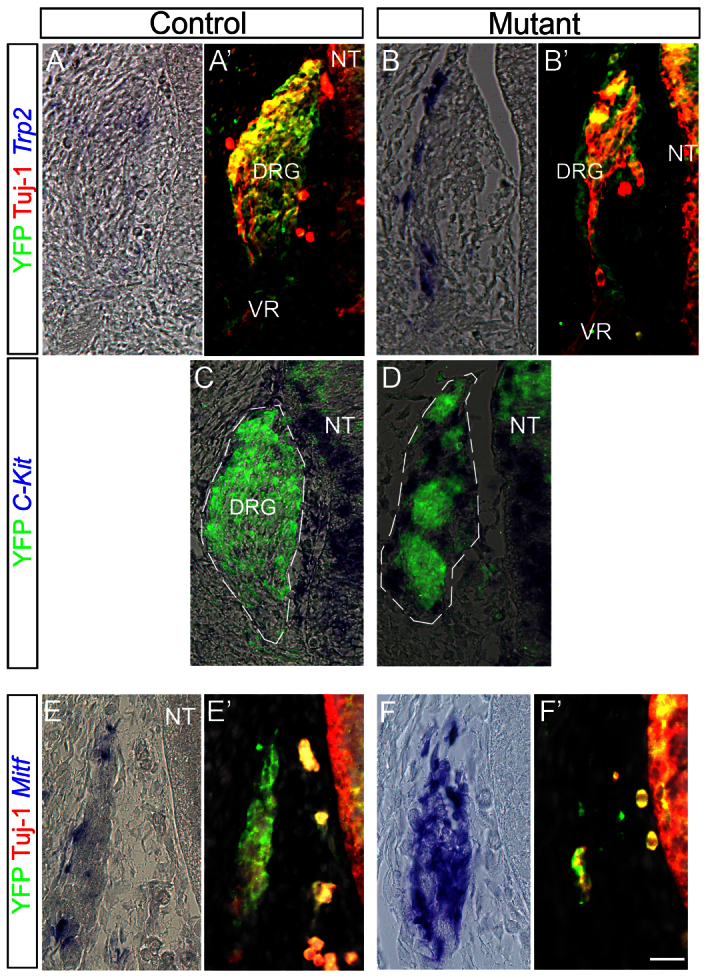
Ectopic expression of melanocyte markers in sensory ganglia lacking Foxd3 gene activity. (A-D) Trp2 (A-B′) and Kit (C,D) are transcribed in mutant but not in control DRG at 12.5 dpc. (E-F′) Expression of Mitf was observed at 10.5 dpc in the facio-acoustic ganglion complex (VII-VIII) of Foxd3 mutants (F), whereas few Mitf+ cells line the periphery of control ganglia (E). Note that Mitf staining masks the YFP signal (D,F). Scale bars: 35 μm in A-B′; 30 μm in C,D; 25 μm in E-F′.
DISCUSSION
We investigated the segregation of neural and melanocyte lineages derived from the NC. Using stable lineage tracing of progenitors expressing a Foxd3 enhancer, we report that in the early NT presumptive dorsal melanoblasts are included within the Foxd3-positive neuroepithelium, but segregate from this lineage before delamination, whereas neural derivatives maintain Foxd3 expression. This is consistent with the late downregulation of Foxd3 in the dorsal NT and absence in melanoblasts and with its persistence in neural progenitors until differentiation (Dottori et al., 2001; Kos et al., 2001; Krispin et al., 2010b; Labosky and Kaestner, 1998). Hence, the progressive narrowing of the domain of Foxd3 transcription observed in the dorsal NT during NC ontogeny stems from two distinct processes. First, the actual delamination of neural precursors that express the gene (Krispin et al., 2010b) and second, its downregulation in presumptive melanoblasts (this study).
In addition, our findings highlight the observation that Foxd3 is also an early marker of RP cells, reported to originate ventral to presumptive melanoblasts in the dorsal NT (Krispin et al., 2010b). Therefore, before the onset of NC departure from the neural primordium, Foxd3 in the dorsal NT is not a NC-specific marker. Although the lineage of Snail2 and Sox9-expressing progenitors was not directly traced, the above findings are also likely to be relevant to these genes, which exhibit similar dynamics and act upstream of Foxd3. Notably, the use of a specific Foxd3 reporter also enabled us to determine that the segregation between RP, melanocytes and neural lineages occurs earlier than previously thought, as both melanoblasts and RP downregulate Foxd3 before delamination of the former. Furthermore, lineage analysis of the dorsal NT by this time generated progeny containing either RP or melanocyte cell types, further supporting the notion of some early fate restrictions (Krispin et al., 2010b).
In this regard, a recent study partly challenged the notion of early fate restrictions by analyzing the outcomes of premigratory progenitors located at various dorsoventral positions in the avian NT (McKinney et al., 2013). They reported that except for sympathetic precursors, the choice of migratory pathways and homing sites does not correlate with the position of origin of NC cells in the NT, thus concluding a lack of fate restrictions for sensory progenitors and melanocytes. A possible difference between this report and that of Krispin may stem from the broad range of stages pooled together (19-35 somites) in McKinney's study. As dorsal NT progenitors progressively relocate ventrodorsally before emigration, as reported by Krispin et al. (Krispin et al., 2010b) and confirmed by McKinney et al. (McKinney et al., 2013), the dorsal NT exhibits a highly dynamic behavior, with different progenitors transiting the area as a function of time. This implies that the fate of relatively ventral progenitors marked at a young stage would be equivalent to that of dorsal precursors labeled at an older stage. In other words, labeling of a dorsal zone at a range of ages instead of a precise stage would lead to multiple fates. In addition, given their interpretation, a clonal approach would have been warranted to truly challenge the existence of fate restrictions in the premigratory NC.
The above findings raise several questions. First, what are the roles of Foxd3 in the early neuroepithelium and in derivatives that exhibit prolonged expression? Second, what are the mechanisms responsible for its downregulation in pigment and RP progenitors? In both zebrafish and mice, loss of Foxd3 function resulted in lack of specific NC derivatives but not in defective NC specification (Arduini et al., 2009; Cooper et al., 2009; Lister et al., 2006; Montero-Balaguer et al., 2006; Mundell and Labosky, 2011; Stewart et al., 2006; Teng et al., 2008). This suggests that Foxd3 regulates maintenance rather than specification of the NC (but see Pohl and Knochel, 2002; Sasai et al., 2001). Kos et al. (Kos et al., 2001) reported that in avians, morpholinos against Foxd3 resulted in early cell entrance into the dorsolateral pathway yet the phenotype of the cells was not monitored; in vitro, an increased proportion of melanocytes was reported but the responsible mechanisms were not addressed. The present results show the advent of melanocyte traits in the dorsal NT and in peripheral ganglia of mouse embryos lacking Foxd3 activity. Our results are consistent with results of loss of Foxd3 function that suggested a shift from neural into mesenchymal fates (Mundell and Labosky, 2011). Hence, in addition to maintaining a progenitor state and being necessary for cell survival, Foxd3 plays a pivotal role in repression of normal melanogenesis, as premature expression of pigment traits appear in prospective melanocytes within the NT of Foxd3 mutants. Hence, Foxd3 activity is necessary for the maintenance of neural progenitors of the NC, and its timely downregulation in the NT may be a prerequisite for melanocyte development (Fig. 8). Along this line, the death of neural progenitors in the Foxd3 mutants (Teng et al., 2008) might result from a misspecification into pigment in ectopic sites, including the premigratory domain, reflecting an independent role of Foxd3 on cell survival. An additional function of Foxd3 is the stimulation of glial/Schwann cell fates, which is consistent with its maintained expression in these lineages (Kelsh et al., 2000; Kos et al., 2001). This notion stems from gain-of-function experiments in avians where prospective melanocytes were able to shift their identity into P0 and Fabp7-expressing cells (this study and Kos et al., 2001) and downregulate Ednrb2 (this study), and by loss-of-function data in mice where DRG glia and SC progenitors along nerves undergo massive melanogenesis and fail to migrate (this study) (E.N. and C.K., unpublished). Hence, Foxd3 activity affects the glia-melanocyte choice both in premigratory NC as well as in DRG and SC precursors consistent with the existence of putative glia-melanocyte bipotent progenitors (see Introduction). By contrast, developing neurons downregulate endogenous Foxd3 (Dottori et al., 2001). We hypothesize that their observed inability to respond to gain of Foxd3 function at the late stage stems from the fact that they segregate from the Foxd3+ lineage earlier than their glial counterparts. Notably, no ectopic melanogenesis was observed within sympathetic ganglia, indicating a differential requirement for Foxd3 activity among the various NC derivatives. Alternatively, sympathoblasts might have undergone premature melanogenesis during migration; as their journey to the target sites is the longest, this might have prevented them from reaching the para-aortic area. Together, these results suggest that Foxd3 plays specific and differential roles at progressive stages of NC development.
Fig. 8.
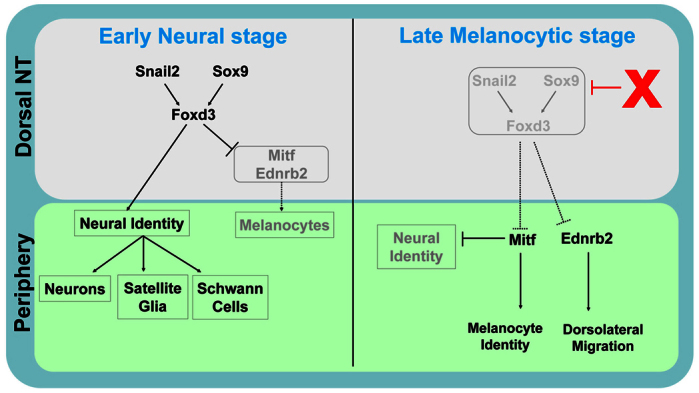
A minimal dynamic regulatory network underlying early neural versus late melanocyte development from the NC. Snail2, Sox9 and Foxd3 are transcribed in the dorsal NT at an early stage where they promote neural development while inhibiting melanogenesis. At a later stage, expression of these genes is lost upon emigration of the neural progenitors. Of these three genes, Foxd3 is both carried by the migrating neural precursors and downregulated in prospective melanoblasts while still resident in the NT (this study). The factors responsible for downregulation of the network (red X) remain to be elucidated. Consequently, inhibition of Mitf and Ednrb2 transcription is removed and melanocyte development (both specification and dorsolateral migration, respectively) begins.
As Sox9 and Snail2: (1) similarly inhibit melanogenesis and proper dorsolateral cell migration; (2) exhibit the same dynamic behavior as Foxd3 in the dorsal NT; and (3) act in this context upstream of Foxd3 to promote its expression, the question of the mechanisms responsible for downregulating Foxd3 could in fact be asked regarding all three factors in the hierarchy (Fig. 8). BMP4 is a candidate for regulating levels of expression of the above genes, as BMP4 maintains their transcription (Sela-Donenfeld and Kalcheim, 1999; Tribulo et al., 2003), and with progressive exit of neural progenitors, the domain of Bmp4 mRNA expression in the dorsal NT becomes restricted to a narrow stripe, suggesting that its effective concentration decreases. Although a lower concentration or activity range of BMP4 may still suffice for sustaining Wnt1 transcription in the dorsal NT (Burstyn-Cohen et al., 2004; Chesnutt et al., 2004), which may affect melanocyte development (Jin et al., 2001), it may not be enough to preserve transcription of Sox9, Snail2 and Foxd3. Another mechanism might involve changes in cell-cell interactions and/or a progressive loss of multipotent features in the NT itself during NC delamination. These might affect transcription of specific genes whose activity is necessary for maintaining an unspecified state. In zebrafish, histone deacetylase1 was found to be required, directly or indirectly, to repress Foxd3. Whether it acts by regulating chromatin structure, which in turn affects gene expression, is not known (Ignatius et al., 2008). It would be interesting to examine whether histone deacetylase1 acts in response to dorsal NT-derived factors such as BMP4 and Wnt1. Likewise, methylation of the Foxd3 promoter might account for its observed downregulation (Cheng et al., 2013).
It is becoming increasingly clear that Foxd3-dependent failure of melanocyte development results from the direct or indirect inhibition of Mitf (Curran et al., 2010; Curran et al., 2009; Ignatius et al., 2008; Thomas and Erickson, 2009). Our data support and further extend these results by placing Foxd3 downstream of Snail2 and Sox9 in this context, thus identifying a minimal functional network (Fig. 8). Reciprocally, we also observed that Mitf inhibits transcription of the three genes, suggesting that once Mitf is expressed, melanocyte differentiation is initiated at the expense of unspecified or of neural progenitors. As lack of Foxd3 is conducive to melanogenic differentiation, the question arises whether the melanocyte state is a default of NC development at certain levels of the axis.
Furthermore, we report that in avian embryos, the migratory phenotype caused by continuous Foxd3 expression results from Foxd3 repressing transcription of Ednrb2. In spite of Foxd3 controlling both Mitf and Ednrb2, we show that the pathways controlled by the latter proteins can be uncoupled. For instance, ectopic expression of Mitf in neural progenitors induced melanocyte markers without altering their original ventral migration. Indeed, Mitf only marginally upregulates Ednrb2 in the NT in contrast to neuroretinal cultures, in which Mitf induced Ednrb2 (Planque et al., 2004). Reciprocally, Ednrb2 diverted neural progenitors into the lateral ‘melanocytic’ pathway, yet they still adopted neural traits and did not upregulate Mitf, further supporting the notion that initial fate acquisition is independent of the migratory environment (Krispin et al., 2010b). Along this line, the premature entrance of NC cells isolated from ‘old’ NT explants into the dorsolateral path of young hosts was interpreted to reflect a commitment to the melanocyte lineage (Erickson and Goins, 1995). In light of the new data, we reinterpret these results to mean that, most likely, the grafted cells expressed at least Ednrb2, which is sufficient to direct dorsolateral migration (Harris et al., 2008; Krispin et al., 2010b). Hence, in spite of becoming expressed simultaneously following Foxd3 downregulation and melanoblast delamination, Mitf and Ednrb2 play different roles (Fig. 8). In the mouse, loss of Foxd3 altered specification with no apparent changes in migration, yet the mechanisms coupling both processes are likely to differ from those in avians because Ednrb2 is avian-specific, and in mice Ednrb is expressed in both ventral and dorsolateral pathways (Dupin and Le Douarin, 2003); this suggests that additional receptors may be relevant in guiding murine melanocyte migration.
Supplementary Material
Acknowledgements
We are grateful to Axel Visel for help with characterization of the enhancers in mice and for sharing the mouse lacZ images. We also thank Tallie Bdolach for statistical analysis and all members of our group for discussions.
Footnotes
Funding
This work was supported by grants from the German Research Foundation (DFG) [UN34/27-1], the Association Francaise Contre les Myopathies (AFM) [15642] and the Israel Science Foundation [11/09] to C.K.; and by grants from the National Institutes of Health (NIH) [HD36720] and the American Heart Association (AHA) [11GRNT7690040] to P.A.L. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.093294/-/DC1
References
- Adameyko I., Lallemend F. (2010). Glial versus melanocyte cell fate choice: Schwann cell precursors as a cellular origin of melanocytes. Cell. Mol. Life Sci. 67, 3037-3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adameyko I., Lallemend F., Aquino J. B., Pereira J. A., Topilko P., Müller T., Fritz N., Beljajeva A., Mochii M., Liste I., et al. (2009). Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 139, 366-379 [DOI] [PubMed] [Google Scholar]
- Arduini B. L., Bosse K. M., Henion P. D. (2009). Genetic ablation of neural crest cell diversification. Development 136, 1987-1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham O. H., Hadas Y., Vald L., Zisman S., Schejter A., Visel A., Klar A. (2009). Transcriptional control of axonal guidance and sorting in dorsal interneurons by the Lim-HD proteins Lhx9 and Lhx1. Neural Dev. 4, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T., Kalcheim C. (2002). Association between the cell cycle and neural crest delamination through specific regulation of G1/S transition. Dev. Cell 3, 383-395 [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T., Stanleigh J., Sela-Donenfeld D., Kalcheim C. (2004). Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development 131, 5327-5339 [DOI] [PubMed] [Google Scholar]
- Cheng A. S., Li M. S., Kang W., Cheng V. Y., Chou J. L., Lau S. S., Go M. Y., Lee C. C., Ling T. K., Ng E. K., et al. (2013). Helicobacter pylori causes epigenetic dysregulation of FOXD3 to promote gastric carcinogenesis. Gastroenterology 144, 122-133e9. [DOI] [PubMed] [Google Scholar]
- Chesnutt C., Burrus L. W., Brown A. M., Niswander L. (2004). Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev. Biol. 274, 334-347 [DOI] [PubMed] [Google Scholar]
- Cheung M., Briscoe J. (2003). Neural crest development is regulated by the transcription factor Sox9. Development 130, 5681-5693 [DOI] [PubMed] [Google Scholar]
- Cheung M., Chaboissier M. C., Mynett A., Hirst E., Schedl A., Briscoe J. (2005). The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 8, 179-192 [DOI] [PubMed] [Google Scholar]
- Cooper C. D., Linbo T. H., Raible D. W. (2009). Kit and foxd3 genetically interact to regulate melanophore survival in zebrafish. Dev. Dyn. 238, 875-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran K., Raible D. W., Lister J. A. (2009). Foxd3 controls melanophore specification in the zebrafish neural crest by regulation of Mitf. Dev. Biol. 332, 408-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran K., Lister J. A., Kunkel G. R., Prendergast A., Parichy D. M., Raible D. W. (2010). Interplay between Foxd3 and Mitf regulates cell fate plasticity in the zebrafish neural crest. Dev. Biol. 344, 107-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottori M., Gross M. K., Labosky P., Goulding M. (2001). The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128, 4127-4138 [DOI] [PubMed] [Google Scholar]
- Dupin E., Le Douarin N. M. (2003). Development of melanocyte precursors from the vertebrate neural crest. Oncogene 22, 3016-3023 [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Goins T. L. (1995). Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development 121, 915-924 [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Reedy M. V. (1998). Neural crest development: the interplay between morphogenesis and cell differentiation. Curr. Top. Dev. Biol. 40, 177-209 [DOI] [PubMed] [Google Scholar]
- Ernsberger U., Esposito L., Partimo S., Huber K., Franke A., Bixby J. L., Kalcheim C., Unsicker K. (2005). Expression of neuronal markers suggests heterogeneity of chick sympathoadrenal cells prior to invasion of the adrenal anlagen. Cell Tissue Res. 319, 1-13 [DOI] [PubMed] [Google Scholar]
- García-Castro M., Bronner-Fraser M. (1999). Induction and differentiation of the neural crest. Curr. Opin. Cell Biol. 11, 695-698 [DOI] [PubMed] [Google Scholar]
- Goding C. R. (2000). Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 14, 1712-1728 [PubMed] [Google Scholar]
- Hanna L. A., Foreman R. K., Tarasenko I. A., Kessler D. S., Labosky P. A. (2002). Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 16, 2650-2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. L., Erickson C. A. (2007). Lineage specification in neural crest cell pathfinding. Dev. Dyn. 236, 1-19 [DOI] [PubMed] [Google Scholar]
- Harris M. L., Hall R., Erickson C. A. (2008). Directing pathfinding along the dorsolateral path – the role of EDNRB2 and EphB2 in overcoming inhibition. Development 135, 4113-4122 [DOI] [PubMed] [Google Scholar]
- Helms A. W., Johnson J. E. (2003). Specification of dorsal spinal cord interneurons. Curr. Opin. Neurobiol. 13, 42-49 [DOI] [PubMed] [Google Scholar]
- Ignatius M. S., Moose H. E., El-Hodiri H. M., Henion P. D. (2008). colgate/hdac1 Repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev. Biol. 313, 568-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin E. J., Erickson C. A., Takada S., Burrus L. W. (2001). Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev. Biol. 233, 22-37 [DOI] [PubMed] [Google Scholar]
- Kang P., Lee H. K., Glasgow S. M., Finley M., Donti T., Gaber Z. B., Graham B. H., Foster A. E., Novitch B. G., Gronostajski R. M., et al. (2012). Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74, 79-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh R. N., Dutton K., Medlin J., Eisen J. S. (2000). Expression of zebrafish fkd6 in neural crest-derived glia. Mech. Dev. 93, 161-164 [DOI] [PubMed] [Google Scholar]
- Kos R., Reedy M. V., Johnson R. L., Erickson C. A. (2001). The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 128, 1467-1479 [DOI] [PubMed] [Google Scholar]
- Krispin S., Nitzan E., Kalcheim C. (2010a). The dorsal neural tube: a dynamic setting for cell fate decisions. Dev. Neurobiol. 70, 796-812 [DOI] [PubMed] [Google Scholar]
- Krispin S., Nitzan E., Kassem Y., Kalcheim C. (2010b). Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development 137, 585-595 [DOI] [PubMed] [Google Scholar]
- Kurtz A., Zimmer A., Schnütgen F., Brüning G., Spener F., Müller T. (1994). The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 120, 2637-2649 [DOI] [PubMed] [Google Scholar]
- Labosky P. A., Kaestner K. H. (1998). The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech. Dev. 76, 185-190 [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Kalcheim C. (1999). The Neural Crest. New York, NY: Cambridge University Press; [Google Scholar]
- Lecoin L., Sii-Felice K., Pouponnot C., Eychène A., Felder-Schmittbuhl M. P. (2004). Comparison of maf gene expression patterns during chick embryo development. Gene Expr. Patterns 4, 35-46 [DOI] [PubMed] [Google Scholar]
- Levy C., Khaled M., Fisher D. E. (2006). MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 12, 406-414 [DOI] [PubMed] [Google Scholar]
- Liem K. F., Jr, Tremml G., Jessell T. M. (1997). A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127-138 [DOI] [PubMed] [Google Scholar]
- Lister J. A., Cooper C., Nguyen K., Modrell M., Grant K., Raible D. W. (2006). Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Dev. Biol. 290, 92-104 [DOI] [PubMed] [Google Scholar]
- McKinney M. C., Fukatsu K., Morrison J., McLennan R., Bronner M. E., Kulesa P. M. (2013). Evidence for dynamic rearrangements but lack of fate or position restrictions in premigratory avian trunk neural crest. Development 140, 820-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochii M., Takeuchi T., Kodama R., Agata K., Eguchi G. (1988). The expression of melanosomal matrix protein in the transdifferentiation of pigmented epithelial cells into lens cells. Cell Differ. 23, 133-141 [DOI] [PubMed] [Google Scholar]
- Mochii M., Mazaki Y., Mizuno N., Hayashi H., Eguchi G. (1998). Role of Mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev. Biol. 193, 47-62 [DOI] [PubMed] [Google Scholar]
- Montero-Balaguer M., Lang M. R., Sachdev S. W., Knappmeyer C., Stewart R. A., De La Guardia A., Hatzopoulos A. K., Knapik E. W. (2006). The mother superior mutation ablates foxd3 activity in neural crest progenitor cells and depletes neural crest derivatives in zebrafish. Dev. Dyn. 235, 3199-3212 [DOI] [PubMed] [Google Scholar]
- Mundell N. A., Labosky P. A. (2011). Neural crest stem cell multipotency requires Foxd3 to maintain neural potential and repress mesenchymal fates. Development 138, 641-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. (1994). Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835-839 [DOI] [PubMed] [Google Scholar]
- Pennacchio L. A., Ahituv N., Moses A. M., Prabhakar S., Nobrega M. A., Shoukry M., Minovitsky S., Dubchak I., Holt A., Lewis K. D., et al. (2006). In vivo enhancer analysis of human conserved non-coding sequences. Nature 444, 499-502 [DOI] [PubMed] [Google Scholar]
- Pla P., Alberti C., Solov'eva O., Pasdar M., Kunisada T., Larue L. (2005). Ednrb2 orients cell migration towards the dorsolateral neural crest pathway and promotes melanocyte differentiation. Pigment Cell Res. 18, 181-187 [DOI] [PubMed] [Google Scholar]
- Planque N., Raposo G., Leconte L., Anezo O., Martin P., Saule S. (2004). Microphthalmia transcription factor induces both retinal pigmented epithelium and neural crest melanocytes from neuroretina cells. J. Biol. Chem. 279, 41911-41917 [DOI] [PubMed] [Google Scholar]
- Pohl B. S., Knochel W. (2002). Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mech. Dev. 103, 93-106 [DOI] [PubMed] [Google Scholar]
- Sasai N., Mizuseki K., Sasai Y. (2001). Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development 128, 2525-2536 [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D., Kalcheim C. (1999). Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development 126, 4749-4762 [DOI] [PubMed] [Google Scholar]
- Serbedzija G. N., Bronner-Fraser M., Fraser S. E. (1989). A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development 106, 809-816 [DOI] [PubMed] [Google Scholar]
- Shoval I., Ludwig A., Kalcheim C. (2007). Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development 134, 491-501 [DOI] [PubMed] [Google Scholar]
- Stewart R. A., Arduini B. L., Berghmans S., George R. E., Kanki J. P., Henion P. D., Look A. T. (2006). Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev. Biol. 292, 174-188 [DOI] [PubMed] [Google Scholar]
- Stolt C. C., Lommes P., Hillgärtner S., Wegner M. (2008). The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 36, 5427-5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L., Mundell N. A., Frist A. Y., Wang Q., Labosky P. A. (2008). Requirement for Foxd3 in the maintenance of neural crest progenitors. Development 135, 1615-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. J., Erickson C. A. (2008). The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 21, 598-610 [DOI] [PubMed] [Google Scholar]
- Thomas A. J., Erickson C. A. (2009). FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development 136, 1849-1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer J., Johnson J., Niswander L. (2001). The use of in ovo electroporation for the rapid analysis of neural-specific murine enhancers. Genesis 29, 123-132 [DOI] [PubMed] [Google Scholar]
- Tribulo C., Aybar M. J., Nguyen V. H., Mullins M. C., Mayor R. (2003). Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 130, 6441-6452 [DOI] [PubMed] [Google Scholar]
- Zisman S., Marom K., Avraham O., Rinsky-Halivni L., Gai U., Kligun G., Tzarfaty-Majar V., Suzuki T., Klar A. (2007). Proteolysis and membrane capture of F-spondin generates combinatorial guidance cues from a single molecule. J. Cell Biol. 178, 1237-1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.